Abstract
Aciculosporium take causes continuous shoot growth but maintains normal leaf-arrangement and branching patterns in the host plant, which eventually resulting in witches’ broom disease of bamboo. An in situ hybridization technique with a species-specific oligonucleotide probe was recently used to demonstrate that endophytic mycelia of A. take is predominantly distributed in the intercellular spaces of the shoot apical meristem of the host. Endophytic hyphae in meristematic tissues, which may produce auxin, are responsible for continuous primordium initiation within the shoot apex. Here I examine another bamboo witches’ broom causal fungus, Heteroepichloë sasae. Both species are biotrophic and belong to family Clavicipitaceae: however, H. sasae does not cause continuous shoot growth. Histological study showed that H. sasae mycelia were distributed superficially, even on shoot apical meristems. These observations suggest that when their stromata develop, endophytic A. take destroys shoot apical meristem and epiphytic H. sasae chokes the shoot apex of the host. Stromata formation consequently causes lateral bud outgrowth because of release from apical dominance. This process repeats and eventually results in the witches’ broom symptoms.
Causal Fungi of Bamboo Witches' Broom
Aciculosporium take (Ascomycota; Clavicipitaceae) is a causal agent of bamboo witches' broom disease in East Asia. Colonized shoots by A. take continue to grow in an acropetal sequence with very thin stems and little leaves, although normal bamboo shoots cease to grow when three to five leaves develop (). The elongating shoot closely resembles a stolon or a vine but not super-elongation diseases such as bakanae disease. When the stroma is formed at the shoot apex, lateral buds grow out. Leaf arrangement and branching patterns are maintained even in colonized shoots. As with Epichloë/Neotyphodium endophytes, external fungal materials other than stromata are not observed on plant surfaces. The location of endophytic mycelia is probably involved in well-regulated symptoms. The author recently demonstrated that the endophytic mycelium of A. take was predominantly distributed in the intercellular spaces of shoot apical meristems of the host bamboo plant.Citation1 Endophytic hyphae were visualized by the in situ hybridization technique with a species-specific oligonucleotide probe.
Heteroepichloë sasae (Ascomycota; Clavicipitaceae) also causes witches' broom in small bamboo plants (e.g., Sasa spp.) in Japan and China.Citation2,Citation3 As with A. take, this fungus is included in the family Clavicipitaceae: their phylogenetic relationships were examined.Citation4 Unlike A. take, H. sasae does not cause continuous shoot elongation. Its stroma encloses undeveloped leaves of the host and does not penetrate the leaf tissue.Citation2 Its habitat before stroma formation, its association with the host, and the mechanisms of symptom development have not been addressed thus far. In this addendum, a comparative study was performed between A. take and H. sasae to observe the development of bamboo witches' broom symptoms.
Distribution of Mycelia in Host Plant
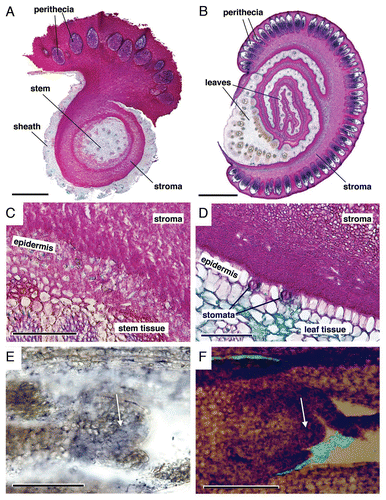
Cross sections of colonized shoots with stromata were observed to examine the distribution of mycelia ( and B). Sections were stained as mentioned in the legend for . Stroma of A. take was formed on the host stem and was connected to the internal mycelia (). Endophytic mycelia were observed in intercellular spaces of the stem tissue, and hyphae emerged between epidermal cells to form stroma (). Stroma of H. sasae enclosed undeveloped leaves and filled in the space between leaves (). No mycelium was observed within leaf tissue, except for stomatal apertures (). It is noted that H. sasae does not seem to intrude even from the stomata.
Longitudinal sections of the colonized shoot without stromata were observed to examine the association of fungi with shoot apex ( and F). Endophytic mycelia of A. take were observed in the intercellular spaces of shoot apical meristem tissue (), as shown previously.Citation1 In contrast, mycelia of H. sasae were observed on the surface of shoot apical meristem (). These observations strongly suggest that H. sasae is entirely an epiphyte. The mycelial distributions of H. sasae are similar to that of Myriogenosora species that is clavicipitaceous fungi and causes dwarfing of host.Citation5,Citation6 Vegetative mycelia of both species may enlarge to develop stromata.
Mechanisms of Bamboo Witches' Broom Symptom Development
The developmental mechanisms responsible for continuous shoot elongation by A. take were discussed previously.Citation1 In brief, colonized shoots may not be able to produce a sufficient amount of endogenous free IAA to expand leaves and stems, whereas endophytic A. take hyphae within the apical meristem may continue to produce exogenous free IAA for inducing primordium initiation and maintaining apical dominance.Citation1,Citation7 This histological study suggests that A. take stroma formation destroys the shoot apical meristem, as a result of which endophytic hyphae pass through the epidermis to form stroma. On the other hand, witches' broom disease caused by H. sasae may develop as follows. Superficial mycelia of H. sasae thicken and become stroma, although it is unclear how nutrients are obtained from the host. The stroma prevents the leaf blade from expanding and eventually chokes the shoot. These observations indicate that both biotrophic fungi finally terminate the function of the host shoot apex when they form stromata. Consequently, lateral buds start growing because of release from apical dominance. Repetition of this sequence may result in symptoms of witches' broom disease.
Endophytic hyphae of A. take appear to regulate shoot development. However, other endophytic clavicipitaceous fungi have an insignificant effect on shoot development. For example, essentially symptomless Neotyphodium endophytes growing entirely in the intercellular spaces also inhabit the shoot apical meristem of the host.Citation8 The study of these host-fungus relationships may provide insight into the shoot development process.
Figures and Tables
Figure 1 Bamboo shoot (Phyllostachys bambusoides) colonized by Aciculosporium take. The colonized shoot continues to grow and finally generates stroma at the shoot apex: it has small leaves from the respective node, although normal shoots have three to five leaves. Bar, 10 cm.

Figure 2 Comparisons between bamboo shoot tissue colonized by Aciculosporium take and Heteroepichloë sasae. Both species cause bamboo witches' broom disease. Samples were fixed, embedded in paraffin, and sectioned as shown previously.Citation1 All colonized bamboos were collected at Kanazawa-shi, Ishikawa, Japan (36°30′25″N, 136°37′59″E). (A–D) Sections stained by Alcian blue (pH 2.5) and periodic acid Schiff reaction methods.Citation9 The magenta color indicates fungal materials. Perithecia with asci were stained blue-purple. (A and B) Several images were combined into one large image because the field of view obtained by a microscope was smaller than the sample dimensions. (A) Cross section of A. take stroma with perithecia. Bar, 500 µm. (B) Cross section of H. sasae stroma with perithecia. Bar, 1 mm. (C) Interface between A. take stroma and the host plant tissue. Endophytic mycelia were observed in the intercellular spaces of plant tissue. Bar, 100 µm. (D) Interface between H. sasae stroma and host plant tissue. Mycelia were observed superficially on the plant surface, except for stomata. Bar, 100 µm. (E) Longitudinal section of an A. take-colonized shoot of P. bambusoides. The blue color indicates fungal mycelia visualized by in situ hybridization with a species-specific oligonucleotide probe.Citation1 Endophytic hyphae were observed in the intercellular spaces of shoot apical meristem (arrow) tissue. Bar, 100 µm. (F) Longitudinal section of an H. sasae-colonized shoot of Sasa palmata. The green color indicates the fungal mycelia of H. sasae stained with fluorescein isothiocyanate-labeled wheat germ agglutinin. Plant tissue was stained with safranine. Mycelia were observed on the surface of the shoot apical meristem (arrow). The bright field image and fluorescent images were combined. Bar, 100 µm.
Addendum to:
References
- Tanaka E. Specific in situ visualization of the pathogenic endophytic fungus Aciculosporium take, the cause of witches' broom in bamboo. Appl Environ Microbiol 2009; 75:4829 - 4834
- Tanaka E, Tanaka C, Gafur A, Tsuda M. Heteroepichloë, gen. nov. (Clavicipitaceae; Ascomycotina) on bamboo plants in East Asia. Mycoscience 2002; 43:87 - 93
- Cheng Y. Identification of Heteroepichloë species on Brachystachyum densiflorum by morphology and phytogeny. Journal of Beijing Forestry University 2009; 31:84 - 90
- Tanaka E, Tanaka C. Phylogenetic study of clavicipitaceous fungi using acetaldehyde dehydrogenase gene sequences. Mycoscience 2008; 49:115 - 125
- Rykard D, Bacon C, Luttrell E. Host relations of Myriogenospora atramentosa and Balansia epichloë (Clavicipitaceae). Phytopathology 1985; 75:950 - 956
- White JF Jr, Glenn A. A study of two fungal epibionts of grasses: structural features, host relationships and classification in the genus Myriogenospora (Clavicipitales). Am J Bot 1994; 81:216 - 223
- Tanaka E, Tanaka C, Ishihara A, Kuwahara Y, Tsuda M. Indole-3-acetic acid biosynthesis in Aciculosporium take, a causal agent of witches' broom of bamboo. J Gen Plant Pathol 2003; 69:1 - 6
- Christensen MJ, Bennett RJ, Ansari HA, Koga H, Johnson RD, Bryan GT, et al. Epichloë endophytes grow by intercalary hyphal extension in elongating grass leaves. Fungal Genet Biol 2008; 45:84 - 93
- Mowry R, Winkler C. The coloration of acidic carbohydrates of bacteria and fungi in tissue sections with special reference to capsules of Cryptococcus neoformans, pneumococci and staphylococci. Am J Pathol 1956; 32:628 - 629