Abstract
Waterlogging or flooding are frequently or constitutively encountered by many plant species. The resulting reduction in endogenous O2 concentration poses a severe threat. Numerous adaptations at the anatomical, morphological, and metabolic level help plants to either escape low oxygen conditions or to endure them. Formation of aerenchyma or rapid shoot elongation are escape responses, as is the formation of adventitious roots. The metabolic shift from aerobic respiration to anaerobic fermentation contributes to a basal energy supply at low oxygen conditions. Ethylene plays a central role in hypoxic stress signaling, and G proteins have been recognized as crucial signal transducers in various hypoxic signaling pathways. The programmed death of parenchyma cells that results in hypoxia-induced aerenchyma formation is an ethylene response. In maize, aerenchyma are induced in the absence of ethylene when G proteins are constitutively activated. Similarly, ethylene induced death of epidermal cells that cover adventitious roots at the stem node of rice is strictly dependent on heterotrimeric G protein activity. Knock down of the unique Gα gene RGA1 in rice prevents epidermal cell death. Finally, in Arabidopsis, induction of alcohol dehydrogenase with resulting increased plant survival relies on the balanced activities of a small Rop G protein and its deactivating protein RopGAP4. Identifying the general mechanisms of G protein signaling in hypoxia adaptation of plants is one of the tasks ahead.
Plant Adaptation to Submergence
Flooding is a major threat to plants, as a flooded environment results in dramatically reduced gas exchange between the plant and its environment. Gases such as CO2, O2 and also the gaseous plant hormone ethylene have a 10,000-fold lower diffusion rate in water as compared to the atmosphere.Citation1 Hence, submergence of plants results in decreased cellular CO2 levels in photosynthetically active shoots in the light,Citation2 or in elevated CO2 levels when no photosynthesis occurs which is the case in roots, in shoots in the dark, or in shoots flooded in turbid water.Citation3 The O2 concentration drops in flooded tissues whereby organs which consume O2 in mitochondrial respiration and which do not produce photosynthetic oxygen such as roots are more apt to suffer from oxygen shortage than tissues that perform photosynthesis. Finally, ethylene levels increase in submerged plant parts not only due to a reduced diffusion from the plant to surrounding flood waters but also because hypoxia stimulates ethylene biosynthesis at least in some plant species. In maize roots, the activities of the two enzymes that are specific to ethylene biosynthesis, 1-aminocyclopropane-1-carboxylic acid (ACC) synthase and of ACC oxidase, increased after a lag phase of about 3 h.Citation4 In roots of rice and tomato, and in rice stems ACC synthase activity was shown to be induced by O2 deficiency.Citation5–Citation7
Ethylene regulates a large number of adaptations that help the plant to cope with submergence. It promotes internodal elongation in rice,Citation8 and petiole growth in Rumex palustris which helps the plant to keep part of the leaves above rising flood levels.Citation9 It promotes growth of adventitious roots e.g., in rice which can replace soil-borne roots and keep the distance short over which gases have to be exchanged.Citation10,Citation11 Cell death is another valuable adaptive mechanism that is likewise regulated by ethylene. Preceding emergence of adventitious roots from the nodes in rice, epidermal cells that cover the root primordia undergo ethylene-regulated cell death.Citation12 Ethylene also promotes death of parenchyma cells which results in formation of gas-filled air spaces (aerenchyma) in shoots and roots of a large number of plant species including maize, rice and Arabidopsis.Citation13–Citation15 These gas-filled air spaces improve gas exchange within the plant and are particular useful when plants retain contact to the oxygenated atmosphere. Finally, hypoxic stress results in metabolic adaptation to ensure maintenance of energy supply. Metabolic changes in submerged plant parts may require the mobilization of carbohydrate reserves to support ATP generation through glycolysis and subsequent fermentation in the absence of mitochondrial respiration. Genes encoding for enzymes of ethanolic fermentation, alcohol dehydrogenase (ADH) and pyruvate decarboxylase (PDC), were shown to be controlled through ethylene signaling.Citation16 The cellular signaling events that take place to mediate and coordinate morphological, anatomical or metabolic adaptation to submergence in a timely fashion are only poorly understood. G proteins are regulatory proteins that have emerged as signaling components in the low oxygen response.
G Proteins and their Modifying Proteins
Heterotrimeric G proteins and small GTPases participate in signaling events in plants. Heterotrimeric G protein subunits are encoded by single or few genes. A unique gene, GPA1 and RGA1 (D1), encodes for the Gα subunit in Arabidopsis and in rice. The Gβ subunit is also encoded by a single-copy gene designated AGB1 in Arabidopsis and RGB1 in rice. Two genes encode the Gγ subunit in Arabidopsis, AGG1 or AGG2, and in rice RGG1 or RGG2. By contrast, small GTPases and GTPase activating proteins (GAP) are encoded by large superfamilies. In Arabidopsis, 93 genes encode small GTPases, and 111 genes were identified in rice.Citation17 The GTPases are classified into six families, Rop, Rab, Ras, Arf, Ran and other GTPase genes. The monomeric RopGTPases regulate cellular processes like H2O2 production, programmed cell death and hormonal responses.Citation18 A total of 65 or 85 GAP genes were identified in the Arabidopsis and the rice genomes. The GAP genes divide into the subgroups RopGAP, RabGAP, ArfGAP, RanGAP and other GAPs. Overall, only little is known about the signaling pathways and resulting cellular and physiological responses in which heterotrimeric G proteins, small GTPases and G protein modifying proteins play a role. Besides hormone signaling and signaling of biotic stress responses, G proteins appear to participate in plant adaptation to abiotic stresses including adaptation to low oxygen conditions. This review highlights recent progress in understanding the input of G proteins in hypoxic stress adaptation.
G Protein Signaling in Hypoxia-Induced Cell Death Responses
A well known response to flooding in many plants is the formation of aerenchyma. In the semi-aquatic rice species, aerenchyma are formed as part of normal development, but the formation is enhanced when rice plants get flooded. Induced aerenchyma formation is observed also in non-aquatic plant species such as maize and was shown to be controlled by ethylene signaling ().Citation4,Citation13 Aside from their main root system, maize plants also form nodal roots. Upon flooding, extensive aerenchyma are formed through death and lysis of cells in the mid-cortex of these nodal roots thus improving gas exchange.Citation13,Citation19 Incubation of maize roots that were kept at normoxic conditions with GTPγS, resulted in cell death and aerenchyma formation. GTPγS is not hydrolyzed by GTPase activity and therefore results in constitutively activated G proteins. Formation of aerenchyma in the presence of GTPγS was accompanied by an increase in cellulase activity which was also observed during hypoxia-induced aerenchyma formation. Cellulases contribute to the degradation of the cell wall and thus to removal of the cell corpse. These results demonstrated a central role for G protein in aerenchyma formation without however revealing the type of G protein that is involved in this response.
As a semi-aquatic plant, rice is well adapted to partial submergence. Hypoxia induces growth of adventitious roots which are present at each node.Citation20 Prior to induction of root growth, epidermal cells that cover the root primordia undergo cell death.Citation12,Citation21 Epidermal cell death is regulated by ethylene. The reactive oxygen species (ROS) hydrogen peroxide acts as a signal transducer downstream of ethylene. Treatment of rice nodes with H2O2 or endogenous accumulation of ROS through inhibition of ROS degradation resulted in enhanced epidermal cell death. Inhibition of the ROS producing NADPH oxidase, in turn, inhibited ethylene-induced cell death.Citation21 A role for a heterotrimeric G protein in epidermal cell death was revealed in a genetic study. Three allelic lines of the unique Gα subunit gene RGA1 (D1) with reduced D1 mRNA levels showed strong inhibition of epidermal cell death.Citation22,Citation23 Neither submergence, nor treatment with ethylene or with H2O2 resulted in significantly elevated epidermal cell death rates in the d1 lines indicating that a heterotrimeric G protein acts downstream of ethylene and H2O2 as a positive regulator of cell death. A function of D1 downstream of H2O2 is supported by observations made in suspension-cultured d1 cell lines of rice cv Taichung 65.Citation24 When treated with the fungal elicitor chitin, both, wt and d1 rice cells displayed identical rates of H2O2 accumulation. Furthermore, expression of pathogenesis related genes encoding for a phenylalanine ammonia lyase, a chitinase, and a β-glucanase were induced in a similar fashion in wt and d1 cv Taichung 65 upon chitin elicitation. Similarly, gene regulation in response to the cell death inducing signals ethylene and H2O2 was not altered in epidermal cells of wt and d1 cv Kinmaze rice plants indicating that gene regulation is not the target of Gα signaling in response to either biotic or hypoxic stress.Citation23
A microarray study revealed that the D1 gene itself was not regulated in epidermal cells by ethylene or H2O2 pointing to post-transcriptional regulation of the D1-dependent G protein activity.Citation23 By contrast, three GTPase genes present in rice, Rac3, Rac7 and Rab11B, were downregulated in epidermal cells above adventitious root primordia as compared to other epidermal cells, and a gene encoding for a GTP-binding domain containing protein was upregulated. It is thus conceivable that additional G proteins are involved in the cell-specific epidermal death response. The small GTPase Rac1 regulates hypersensitive cell death in the innate immune response in rice. Rac1 is part of a protein complex that binds to the N terminus of NADPH oxidase. Binding activates the enzyme and leads to enhanced H2O2 production.Citation25 The expression of Rac1 is regulated by heterotrimeric G protein. Consequently, d1 mutants displayed strongly reduced H2O2 production and a highly reduced hypersensitive cell death response.Citation22
In 12-week-old senescing Arabidopsis plants, aerenchyma were formed in roots and shoots after 7 d of waterlogging with O2 levels of about 4%. Ethylene levels increased, as did H2O2 levels.Citation15 However an involvement of G proteins was not analyzed. In Arabidopsis, aerenchyma are not formed in seedlings or in 4-week-old plants, but were formed in mature plants at the late reproductive stage indicating that aerenchyma formation in Arabidopsis is not an adaptive strategy for long-term survival.
G Proteins are Involved in Metabolic Adaptation to Hypoxia
Oxygen deprivation causes inhibition of mitochondrial respiration and results in the induction of anaerobic fermentation. Ethanolic fermentation is considered advantageous over lactate fermentation because ethanol is readily diffusible in flood waters whereas lactic acid is trapped in plant cells resulting in acidification of the cytoplasm which ultimately leads to acidosis.Citation26 Ethanolic fermentation relies on the enzymatic activities of pyruvate decarboxylase (PDC) and alcohol dehydrogenase (ADH). PDC and ADH genes and enzyme activities are induced by low oxygen.Citation16 GAP proteins accelerate the GTPase activity of small GTPases. GTP hydrolysis inactivates small GTPases. In Arabidopsis, regulation of ADH activity by low O2 was shown to dependent on RopGTPase signaling. RopGAP4 induces H2O2 production through activation of a Ca2+-dependent, DPI-sensitive NADPH oxidase, implicating that regulation of ADH activity at low oxygen conditions may be mediated by a RopGTPase and H2O2.Citation27 The loss-of-function ropgap4-1 mutant displayed hyperinduction of H2O2 accumulation and of ADH activity, whereas expression of the dominant negative form of RopGAP4 resulted in reduced H2O2 production and reduced ADH activity. These results are in accordance with the idea that RopGAP4 is a negative regulator of a RopGTPase which acts as a positive regulator of H2O2 production, and that H2O2 acts as a second messenger in ADH activation. The balancing act between low oxygen tolerance and avoidance of oxidative damage by H2O2 appears to be achieved through attenuation of Rop signaling by feedback induction of RopGAP4 expression by H2O2. Enhanced RopGAP4 expression by H2O2 downregulates Rop and consequently downregulates H2O2 production.
Interestingly, expression of Arabidopsis ADH1 is induced by ethylene pointing to a signaling network that encompasses ethylene, G protein and H2O2. These primary signals and secondary messengers thus regulate not only anatomical and morphological, but also metabolic adaptations to oxygen deficiency.Citation28 It will be interesting to see if and to which extend these mechanisms are unifying within a plant species and to which extent these are conserved in well-adapted and less well-adapted plant species.
Figures and Tables
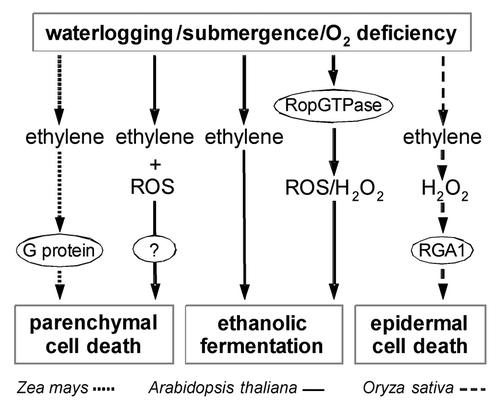
Figure 1 Model of G protein signaling in hypoxia adaptation. A role for G protein signaling in submergence signaling is described for Zea mays, Arabidopsis thaliana and Oryza sativa. The known signaling pathways are summarized in this model. Waterlogging or flooding result in reduced O2 and enhanced ethylene levels. Ethylene promotes parenchymal cell death which results in the formation of aerenchyma. In maize, pharmacological studies implicated a crucial role for G protein signaling in parenchymal cell death. In rice, epidermal cell death precedes emergence of adventitious roots. Cell death is induced by ethylene and is mediated by H2O2. The heterotrimeric Gα subunit RGA1 acts downstream of ethylene and H2O2. Genetic downregulation of RGA1 results in repression of ethylene or H2O2 induced epidermal cell death. G protein signaling in aerenchyma formation in Arabidopsis has not yet been analyzed but is predicted in this model. In Arabidopsis, regulation of ethanolic fermentation is mediated by the activation of a RopGTPase which causes enhanced production of reactive oxygen species, which in turn promote ethanolic fermentation, and enhance low oxygen tolerance.
Acknowledgements
We gratefully acknowledge funding by the Deutsche Forschungsgemeinschaft.
References
- Gibbs J, Greenway H. Review: Mechanisms of anoxia tolerance in plants I. Growth, survival and anaerobic catabolism. Funct Plant Biol 2003; 30:1 - 47
- Mommer L, Visser EJW. Underwater photosynthesis in flooded terrestrial plants: a matter of leaf plasticity. Ann Bot 2005; 96:581 - 589
- Greenway H, Armstrong W, Colmer TD. Conditions leading to high CO2 (>5 kPa) in waterlogged-flooded soils and possible effects on root growth and metabolism. Ann Bot 2006; 98:9 - 32
- He CJ, Finlayson SA, Drew MC, Jordan WR, Morgan PW. Ethylene biosynthesis during aerenchyma formation in roots of Zea mays subjected to mechanical impedance and hypoxia. Plant Physiol 1996a; 112:1679 - 1685
- Cohen E, Kende H. In vivo 1-aminocyclopropane-1-carboxylate synthase activity in internodes of deepwater rice: enhancement by submergence and low oxygen levels. Plant Physiol 1987; 84:282 - 286
- Wang TW, Arteca RN. Effects of low O(2) root stress on ethylene biosynthesis in tomato plants (Lycopersicon esculentum Mill cv Heinz 1350). Plant Physiol 1992; 98:97 - 100
- Zarembinski TI, Theologis A. Anaerobiosis and plant growth hormones induce two genes encoding 1-aminocyclopropane-1-carboxylate synthase in rice (Oryza sativa L.). Mol Biol Cell 1993; 4:363 - 373
- Kende H, van der Knaap E, Cho HT. Deepwater rice: A model plant to study stem elongation. Plant Physiol 1998; 118:1105 - 1110
- Voesenek LA, Benschop JJ, Bou J, Cox MC, Groeneveld HW, Millenaar FF, et al. Interactions between plant hormones regulate submergence-induced shoot elongation in the flooding-tolerant dicot Rumex palustris. Ann Bot 2003; 91:205 - 211
- Suge H. Ethylene and gibberellin: regulation of internodal elongation and nodal root development in floating rice. Plant Cell Physiol 1985; 26:607 - 614
- Bleecker AB, Schuette JL, Kende H. Anatomical analysis of growth and developmental patterns in the internode of deepwater rice. Planta 1986; 69:490 - 497
- Mergemann H, Sauter M. Ethylene induces epidermal cell death at the site of adventitious root emergence in rice. Plant Physiol 2000; 124:609 - 614
- He CJ, Morgan PW, Drew MC. Transduction of an ethylene signal is required for cell death and lysis in the root cortex of maize during aerenchyma formation induced by hypoxia. Plant Physiol 1996; 112:463 - 472
- Colmer TD, Cox MCH, Voesenek LACJ. Root aeration in rice (Oryza sativa L.): evaluation of oxygen, carbon dioxide and ethylene as possible regulators of root acclimatization. New Phytol 2006; 170:767 - 778
- Mühlenbock P, Plaszczyca M, Plaszczyca M, Mellerowicz E, Karpinski S. Lysigenous aerenchyma formation in Arabidopsis is controlled by LESION SIMULATING DISEASE1. Plant Cell 2007; 19:3819 - 3830
- Fukao T, Xu K, Ronald PC, Bailey-Serres J. A variable cluster of ethylene response factor-like genes regulates metabolic and developmental acclimation responses to submergence in rice. Plant Cell 2006; 18:2021 - 2034
- Jiang SY, Ramachandran S. Comparative and evolutionary analysis of genes encoding small GTPases and their activation proteins in eukaryotic genomes. Physiol Genomics 2006; 24:235 - 251
- Yang Z. Small GTPases: versatile signaling switches in plants. Plant Cell 2002; 14:375 - 388
- Drew MC, He CJ, Morgan PW. Programmed cell death and aerenchyma formation in roots. Trends in Plant Science 2000; 5:123 - 127
- Lorbiecke R, Sauter M. Adventitious root growth and cell cycle induction in deepwater rice. Plant Physiol 1999; 119:21 - 29
- Steffens B, Sauter M. Epidermal cell death in rice is confined to cells with a distinct molecular identity and is mediated by ethylene and H2O2 through an auto-amplified signal pathway. Plant Cell 2009a; 21:184 - 196
- Suharsono U, Fujisawa Y, Kawasaki T, Iwasaki Y, Satoh H, Shimamoto K. The heterotrimeric G protein α subunit acts upstream of the small GTPase Rac in disease resistance in rice. PNAS USA 2002; 99:13307 - 13312
- Steffens B, Sauter M. Heterotrimeric G protein signaling is required for epidermal cell death in rice. Plant Physiol 2009; 151:732 - 740
- Tsukuda K, Ishizawa M, Fujisawa Y, Iwasaki Y, Yamaguchi T, Minami E, Shibuya N. Rice receptor for chitin oligosaccharide elicitor does not couple to heterotrimeric G-protein: Elicitor response of suspension cultured rice cells from Daikoku dwarf (d1) mutants lacking a functional G-protein α-subunit. Physiol Plant 2002; 116:373 - 382
- Nakashima A, Chen L, Thao NP, Fijiwara M, Wong HL, Kuwano M, et al. RACK1 functions in rice innate immunity by interacting with the Rac1 immune complex. Plant Cell 2008; 20:2265 - 2279
- Sauter M. Rice in deep water: “how to take heed against a sea of troubles”. Naturwissenschaften 2000; 87:289 - 303
- Baxter-Burrell A, Yang Z, Springer PS, Bailey-Serres J. RopGAP4-dependent Rop GTPase rheostat control of Arabidopsis oxygen deprivation tolerance. Science 2002; 296:2026 - 2028
- Peng HP, Chan CS, Shih MC, Yang SF. Signaling events in the hypoxic induction of alcohol dehydrogenase gene in Arabidopsis. Plant Physiol 2001; 126:742 - 749