Abstract
Drought stress represents a particularly great environmental challenge for plants. A decreased water availability can severely limit growth, and this jeopardizes the organism’s primary goal – to survive and sustain growth long enough to ensure the plentiful production of viable seeds within the favourable growth season. It is therefore vital for a growing plant to sense oncoming drought as early as possible, and to respond to it rapidly and appropriately in all organs. A typical, fast energy-saving response is the arrest of growth in young organs, which is likely mediated by root-derived signals. A recent publication indicates that three plant hormones (abscisic acid, ethylene and gibberellic acid) mediate the adaptation of leaf growth in response to drought, and that they act at different developmental stages. Abscisic acid mainly acts in mature cells, while ethylene and gibberellic acid function in expanding and dividing leaf cells. This provides the plant with a means to differentially control the developmental zones of a growing leaf, and to integrate environmental signals differently in sink and source tissues. Here we discuss the biological implications of this discovery in the context of long-distance xylem and phloem transport.
Plants have developed an inter-organ communication system to rapidly and dynamically orchestrate developmental changes in response to environmental cues.Citation1,Citation2 Phloem sap contains vast amounts of proteins, mRNAs, hormones, sucrose and other small molecules, all of which have the potential to convey specific information to sink tissues, and many of which are defense- or stress-related.Citation3,Citation4 A well-known example of inter-organ communication that occurs in response to the perception of environmental change is floral induction: lengthening of the photoperiod is perceived in leaves, and this induces the expression of the FT protein in the leaf vasculature. After transport, FT subsequently acts to transform the shoot apical meristem into a floral meristem.Citation5 Lake et al.Citation6 showed that mature leaves are also the source of environment-induced phloemmobile information to young leaves: when only the mature leaves of a plant are exposed to e.g., increased CO2 concentrations, its young leaves will develop with a reduced stomatal index, even though they have not experienced the changed conditions themselves. Long-distance communication between root and shoot is exemplified by the BYPASS1-dependent signal in Arabidopsis. This carotenoid-derived signal molecule, whose identity and mode-of-action remain elusive, is transported from root to shoot through the xylem, and limits the growth of young leaves by acting on both cell division and expansion.Citation7–Citation9 These examples show that xylem and phloem are central circuits for the exchange of information between the different plant organs, with the potential to override the developmental program of the target organ in response to the perception of environmental cues.
Because a major physiological effect of drought is decreased photosynthesis, e.g., due to stomatal closureCitation10 and inhibition of the photosynthetic machinery (Memmi et al., in preparation), the plant's autotrophic production of energy and carbon is threatened. An “appropriate response” to drought—beneficial for growth and survival in the long run—is therefore the immediate conservation of energy, followed by a gradual adaptation to the suboptimal conditions.Citation29 The most prominent sinks are growing organs, in which dividing and expanding cells demand large amounts of energy and resources. A typical response to stress is hence the transient cessation of organ growth until the plant has adapted its energy homeostasis. This growth arrest occurs as rapidly as stomatal closure in leaves (Skirycz A and Inzé D, unpublished results), and long before the water potential in aerial plant parts decreases.Citation11 The responsible signals must therefore originate in the root,Citation12 and they enable the plant to exert tight control over organ growth, depending on the plant's energy status and the environmental challenges it encounters. A recent paper by Skirycz and co-workersCitation13 lifts a tip of the veil, by providing unique information from the receiving end of the signal: the growing leaf itself. Their experiments suggest that the plant systemically sends out three distinct hormonal signals to a young leaf, which each act at different developmental stages to instruct the adaptation to stress.
The first sensing of drought typically occurs underground: roots sense a decrease in water availability, and rapidly send an ABA-signal to the shoot, using the xylem as conduit.Citation14 In mature leaves this ABA signal then activates a cascade of responses that enable the plant to successfully deal with the effects of the oncoming drought, such as rapid stomatal closure and the induction of adaptive molecular mechanisms.Citation15,Citation16
Skirycz et al.Citation13 studied the transcriptome of growing Arabidopsis leaves under mild, growth-limiting osmotic stress conditions. In their assay seedlings were subjected to 25 mM mannitol, and this treatment ultimately led to a 50% reduction in final leaf size. A typical biphasic stress response could be observed in young leaves: a rapid reduction in growth rate, followed by complete recovery and adaptation. An important novelty in this experiment was that the authors managed to separately profile the transcriptome of dividing, expanding and mature leaf cells, thus preventing dilution of developmental-stage-specific transcripts and preserving important developmental information. This approach convincingly demonstrated that there is hardly any overlap in the molecular response of dividing and mature cells to decreased water availability. Remarkably, the ABA-dependent stress response that has been so well characterized in mature cells seems to operate at a much lesser extent in expanding cells, and not at all in dividing cells. Instead, gibberellic acid (GA)- and ethylene-dependent pathways are activated in dividing and expanding cells, in response to stress.
Although it had previously been reported that ABA, ethylene and GA are all involved in the adaptive response of plant growth to stress,Citation16–Citation18 this study thus revealed that the action of these hormones is in fact developmentally separated in a growing leaf: stress-induced ABA appears to target only mature cells, whereas GA and ethylene act in expanding and dividing cells. The rapidity with which a leaf stops growing upon drought perception suggests that—rather than being synthesized de novo in the leaf itself—the hormonal signals are being imported directly from another tissue, to ensure an immediate response. The fact that the genes encoding ethylene and GA biosynthetic enzymes are not upregulated in dividing cells by stressCitation13 supports this view.
All three mentioned phytohormones are certainly amenable to long-distance transport.Citation19 ABA is easily transported from root to shoot in the xylem,Citation20 and it also trafficks in the phloem.Citation21 GAs are translocated via both phloem and xylem, as inactive conjugates which can probably be activated at an appropriate time and location.Citation22,Citation23 The ethylene precursor 1-aminocyclopropane-1-carboxylic acid (ACC) is known to be exported from the root in the xylemCitation24 and it is also present in phloem sap.Citation25
Keeping in mind the biphasic nature of the growth response to drought, it is tempting to speculate that leaf growth is controled by a complex integration of different long-distance signals, sent out by the roots and the mature leaves. This would ensure a dynamic and appropriate growth response to both current water availability and photosynthetic productivity. The rapid cessation of growth is almost certainly dictated by root-derived signals (ABA, ACC, the BYPASS1-dependent molecule, etc.,), which selectively target dividing, expanding or mature cells. During the subsequent recovery of growth, however, we can suspect that also the mature leaves partake in determining the new, adapted growth rate of the sink organs, through a set of phloem-mobile signals. This would give them the potential to limit the sinks' strenght (e.g., reduce the energy demand of dividing cells by reversibly inhibiting the cell cycle) until environmental conditions become stable again to ensure a reliable output from photosynthesis. The nature of this putative phloem-borne signal derived from mature leaves is currently unknown. Apart from ABA, ACC and GAs, mature leaves appear to load a multitude of proteins and transcripts into the phloem sap stream, which results in a highly complex leaf-derived signal.Citation3,Citation4,Citation26,Citation27 At this stage, we can therefore not exclude that proteins or mRNAs in the phloem sap are also involved in the long-distance growth control in response to stress.
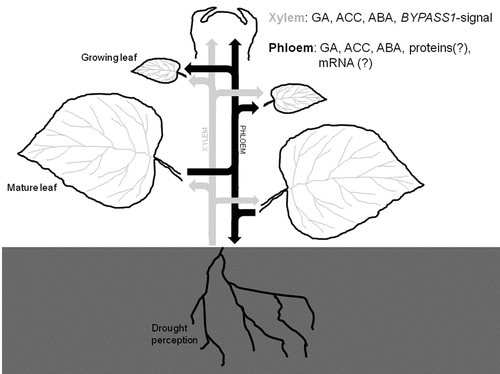
Phloem-mobile signals would not only reach young, growing leaves, but also other sinks. This way the root—as initial source of the signal—also receives feedback from the mature leaves, such that the response to drought can be systemically coordinated, and organ growth can resume at an adapted rate as soon as possible. illustrates how interorgan communication is organized in a plant under drought stress. It will be a very interesting challenge to further dissect the complex long-distance signals with which a plant controls the growth rate of young organs. The experiments of Skirycz et al.Citation13 have provided exciting insight into the molecular adaptations that occur in dividing and expanding cells in response to these signals. Repeating these experiments in e.g., a bps1 mutant background (lacking a functional BYPASS1 gene) could uncover the specific molecular response to the BYPASS1-dependent signal, while the use of ethylene and GA mutants could shed a light upon the role of these hormones in the adaptation of immature cells to stress.
Abbreviations
| ABA | = | abscisic acid |
| ACC | = | 1-aminocyclopropane-1-carboxylic acid |
| GA | = | gibberellic acid |
Figures and Tables
Figure 1 Upon drought perception in the soil, the roots use the transpiration stream of the xylem to send out various (hormonal) signals to the shoot. These signals invoke physiological and molecular responses in both mature and young leaves, and—as revealed by the work of Skirycz et al.Citation13—different hormones act at different developmental stages: ABA in mature leaf cells, and ethylene and GA in expanding and dividing leaf cells. In mature leaves this also prompts the release of secondary signals into the phloem sap, which are transported to sink tissues. In young leaves these phloem-borne signals likely contribute to the dynamic modulation of growth in response to the stress condition.
Addendum to:
References
- Ruiz-Medrano R, Xoconostle-Cázares B, Lucas WJ. The phloem as a conduit for inter-organ communication. Curr Opin Plant Biol 2001; 4:202 - 209
- Lough TJ, Lucas WJ. Integrative plant biology: Role of phloem long-distance macromulecular trafficking. Annu Rev Plant Biol 2006; 57:203 - 232
- Walz C, Giavalisco P, Schad M, Juenger M, Klose J, Kehr J. Proteomics of cucurbit phloem exudate reveals a network of defence proteins. Phytochemistry 2004; 65:1795 - 1804
- Omid A, Keilin T, Glass A, Leshkowitz D, Wolf S. Characterization of phloem-sap transcription profile in melon plants. J Exp Bot 2007; 58:3645 - 3656
- Corbesier L, Vincent C, Jang S, Fornara F, Fan Q, Searle I, et al. FT protein movement contributes to long-distance signaling in floral induction of Arabidopsis. Science 2007; 316:1030 - 1033
- Lake JA, Quick WP, Beerling DJ, Woodward FI. Signals from mature to new leaves. Nature 2001; 411:154
- Van Norman JM, Frederick RL, Sieburth LE. BYPASS1 negatively regulates a root-derived signal that controls plant architecture. Curr Biol 2004; 14:1739 - 1746
- Van Norman JM, Sieburth LE. Dissecting the biosynthetic pathway for the bypass1 root-derived signal. Plant J 2007; 49:619 - 628
- Mouchel CF, Leyser O. Novel phytohormones involved in long-range signaling. Curr Opin Plant Biol 2007; 10:473 - 476
- Flexas J, Medrano H. Drought-inhibition of photosynthesis in C3 plants: stomatal and non-stomatal limitation revisited. Ann Bot 2002; 89:183 - 189
- Saab IN, Sharp RE. Non-hydraulic signals from maize roots in drying soil: inhibition of leaf elongation but not stomatal conductance. Planta 1989; 179:466 - 474
- Davies WJ, Zhang J. Root signals and the regulation of growth and development of plants in drying soil. Ann Rev Plant Physiol Plant Mol Biol 1991; 42:55 - 76
- Skirycz A, De Bodt S, Obata T, De Clercq I, Claeys H, De Rycke R, et al. Developmental stage specificity and the role of mitochondrial metabolism in the response of Arabidopsis leaves to prolonged mild osmotic stress. Plant Physiol 2010; 152:226 - 244
- Wilkinson S, Davies WJ. ABA-based chemical signalling: the co-ordination of responses to stress in plants. Plant Cell Environm 2002; 25:195 - 210
- Assmann SM, Wang X-Q. From milliseconds to millions of years: guard cells and environmental responses. Curr Opin Plant Biol 2001; 4:421 - 428
- Shinozaki K, Yamaguchi-Shinozaki K. Gene networks involved in drought stress response and tolerance. J Exp Bot 2006; 58:221 - 227
- Morgan PW, Drew MC. Ethylene and plant responses to stress. Physiol Plant 2006; 100:620 - 630
- Dugardeyn J, Van der Straeten D. Ethylene: fine-tuning plant growth and development by stimulation and inhibition of elongation. Plant Sci 2008; 175:59 - 70
- Hoad GV. Transport of hormones in the phloem of higher plants. Plant Growth Regul 1995; 16:173 - 182
- Sobeih WY, Dodd IC, Bacon MA, Grierson D, Davies WJ. Long-distance signals regulating stomatal conductance and leaf growth in tomato (Lycopersicon esculentum) plants subjected to partial root-zone drying. J Exp Bot 2004; 55:2353 - 2363
- Zhong W, Hartung W, Komor E, Schobert C. Phloem transport of abscisic acid in Ricinus communis L. seedlings. Plant Cell Environ 2006; 19:471 - 477
- Arteca RN. Plant growth substances: principles and applications 1996; New York, NJ Chapman and Hall 332
- Sponsel VM. Davies PJ. Gibberellin biosynthesis and metabolism. Plant Hormones. Physiology, Biochemistry and Molecular Biology 1995; Dordrecht, The Netherlands Kluwer 66 - 97
- Bradford KJ, Yang SF. Xylem transport of 1-aminocyclopropane-1-carboxylic acid, an ethylene precursor, in waterlogged tomato plants. Plant Physiol 1980; 65:322 - 326
- Morris DA, Larcombe NJ. Phloem transport and conjugation of foliar-applied 1-aminocyclopropane-1-carboxylic acid in cotton (Gossypium hirsutum L). J Plant Physiol 1995; 146:429 - 436
- Haywood V, Yu T-S, Huang N-C, Lucas WJ. Phloem long-distance trafficking of GIBBERELLIC ACID-INSENSITIVE RNA regulates leaf development. Plant J 2005; 42:49 - 68
- Weinl C, Marquardt S, Kuijt SJH, Nowack MK, Jakoby MJ, Hülskamp M, et al. Novel functions of plant cyclin-dependent kinase inhibitors, ICK1/KRP1, can act non-cell-autonomously and inhibit entry into mitosis. Plant Cell 2005; 17:1704 - 1722
- Memmi S, Skirycz A, De Bodt S, Maleux K, Obata T, Fernie AR, et al. A proteomic survey using reciprocal 15N labeling provides new insight into adaptation of Arabidopsis leaf growth to osmotic stress
- Skirycz A, Inzé D. More from less: plant growth under limited water. Curr Opin Biotech 2010; 21:197 - 203