Abstract
Jasmonic acid (JA), its metabolites, such as the methyl ester or amino acid conjugates as well as its precursor 12-oxophytodienoic acid (OPDA) are lipid-derived signals. JA, OPDA and JA-amino acid conjugates are known to function as signals in plant stress responses and development. More recently, formation of JA-amino acid conjugates and high biological activity of JA-Isoleucine (JA-Ile) were found to be essential in JA signalling. A breakthrough was the identification of JAZ proteins which interact with the F-box protein COI1 if JA-Ile is bound. This interaction leads to proteasomal degradation of JAZs being negative regulators of JA-induced transcription. Surprisingly, a distinct stereoisomer of JA-Ile, the (+)-7-iso-JA-Ile ((3R,7S) form) is most active. Coronatine, a bacterial phytotoxine with an identical stereochemistry at the cyclopentanone ring, has a similar bioactivity . This was explained by the recent identification of COI1 as the JA receptor and accords well with molecular modelling studies. Whereas over the last two decades JA was quantified to describe any JA dependent process, now we have to take into account a distinct stereoisomer of JA-Ile. Until recently a quantitative analysis of (+)-7-iso-JA-Ile was missing presumable due to its equilibration to (-)-JA-Ile. Now such an analysis was achieved. These aspects will be discussed based on our new knowledge on JA perception and signalling.
Each organism is able to respond to information coming from the environment. Usually this is mediated by small molecules which are perceived by highly specific receptors and the biological information is transduced via a signal transduction cascades leading to specific responses (expression of genes, phenotypic alteration, etc.). Plants have to adjust and to respond to a remarkable high number of various environmental cues due to their sessile life style. At least ten different hormones or hormone-like compounds are involved in responses to environmental changes but are active also in plant growth and development. Among them are jasmonic acid (JA) and its derivatives, collectively called jasmonates. They are known as central regulators in stress responses such as wounding or pathogenic attack but also in root growth or pollen development.
Jasmonates are lipid-derived compounds which originate from α-linolenic acid (α-LeA) of plastid membranes.Citation1,Citation2 JA is formed by at least nine enzymes located in the plastid and the peroxisome. Most of them have been cloned from different plant species. Crystal structures are available for five of them.Citation1,Citation2 The regulatory levels active in JA biosynthesis are similar to that of other plant hormones:
(1) expression of biosynthetic genes, (2) translational and posttranslational control and stability of enzymes, (3) organ- and tissue-specific location of enzymes, (4) substrate (α-LeA) availability, (5) feed forward regulatory loop, (6) proteasomal degradation of regulatory proteins active in JA biosynthesis (e.g. negatively and positively acting factors). Less understood is the role of structural specificity. In JA biosynthesis this aspect became clear by crystal structures of the enzymes.Citation2 But in case of JA perception and action the most active JA compound was missing until recently, and the JA perception was not clear due to lack of knowledge on the JA receptor. Several recent papers highlighted these aspects and will be discussed.
Chemistry and Metabolites of JA
To understand these new aspects few comments are required on chemistry of JA and its metabolites. JA is a cyclopentanone compound formed from its precursor 12-oxo-phytodienoic acid (OPDA), a cyclopentenone compound. In nature only (9S,13S)-OPDA [cis-(+)-OPDA] occurs which is one of the four possible diastereomers.Citation1 This stereospecific structure at the ring is established by the allene oxide cyclase and is kept in the subsequent reactions. The final product is one of four possible distereomers, the (3R,7S)-JA [(+)-7-iso-JA] which is assumed to equilibrate to the more stable (3R,7R)-JA [(−)-JA] (), even a final proof could not be given due to lack of adequate analytical techniques.Citation3 The other two diastereomers, the (3S,7S)-JA and the (3S,7R)-JA do not occur in nature.
Metabolites of JA can be formed by reduction of the keto group, by decarboxylation, conjugation to amino acids or the ethylene precursor ACC, by esterification of the carboxylic acid group with glucose or a methyl group, by hydroxylation of C-12 followed by sulfation, O-glucoside formation or conjugation with amino acids, and finally 12-HOOC-JA formation with subsequent conjugation with amino acids.Citation4 All these metabolites has been detected in plants and some of the corresponding enzymes have been cloned.Citation4,Citation5,Citation6
The Most Bioactive Jasmonate Compound
Since more than 15 years any increase in endogenous levels of JA has been regarded to be indicative for a JA-dependent process. However, the jar1 mutant of A. thaliana, one of the mostly used JA-insensitive mutant known since 1992Citation7 was identified to be affected downstream of JA in a gene which encodes the JA isoleucine conjugate synthaseCitation8 indicating that many JA responses are mediated by JA-Ile. Fortunately, JA-levels correlate tightly with levels of JA-Ile even the former compound accumulates up to ten-fold higher levels in A. thaliana, tomato or tobacco.Citation9,Citation10,Citation11 Surprisingly, the highest biological activity in terms of JA-responses was observed with coronatine (), a phytotoxine which does not occur in plants, but is formed by several strains of Pseudomonas syringae.Citation12 This high activity led to the assumption that the (3R,7S)-configuration at the cyclopentanone ring of coronatine is stabilized by the second ring and might be important for biological activity of jasmonates. Recently this idea was convincingly verified by testing more than 40 jasmonates in vivo (root growth inhibition, anthocyan formation, yeast two hybrid assay) and in vitro.Citation13 Yeast two hybrid assay data and the in vitro data were based on recent and exciting knowledge on proteasomal degradation of negatively acting regulators of transcription factors, so-called JAZ proteins via their interaction with the F-box protein COI1.Citation14
Since the strength of the interaction was dependent on JA-Ile,Citation15,Citation16 40 different jasmonate compounds including their different stereoisomeric forms could be tested by pull down assays using a tagged JAZ protein and crude extracts from plants expressing flag-tagged COI1 under control of the constitutive CaMV 35S-promoter. In this way the (3R,7S)-JA-Ile [(+)-7-iso-JA-Ile] was detected as the most active compound.Citation13,Citation17 The initially detected activity of (−)-JA-Ile could be explained by a residual amount of (3R,7S)-JA-Ile in a (−)-JA-Ile [(3R,7R)-JA-Ile] solution checked by HPLC analysis.Citation13 Since the (3R,7S)-stereoisomer was assumed to equilibrate rapidly under in vivo conditions to the more stable (−)-form (3R,7R), a regulatory role of epimerization in JA signaling was hypothesized.Citation13
COI1 is a Jasmonate Receptor
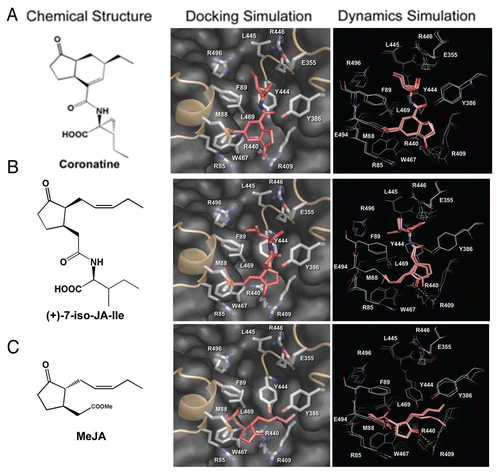
In the past few years much effort has been put into identification of the JA receptor. COI1 is an F-Box proteinCitation18 like TIR1, which was identified as an auxin receptor.Citation19,Citation20 Therefore, it was hypothesized that COI1 might be a receptor for JA.Citation21 The final proof, however, was missing. It becomes clear that COI1, which assembles SCF complexes with ASK1/2 and AtCullin1Citation22 interacts with the targets of SCFCOI1, the JAZ proteinsCitation15,Citation23,Citation24 in JA-Ile dependent manner.Citation15,Citation25 However, the receptor function of COI1 was shown only recently.Citation26 Direct binding of JA-Ile and coronatine with COI1 was shown with (1) the immobilized jasmonate approach showing that COI1 protein in crude extracts binds to the jasmonate moiety of JA-Ile; with (2) the surface plasmon resonance technology suggesting that the purified COI1 binds to JA-Ile/coronatine and subsequently interacts with JAZ1 protein; and finally by (3) photoaffinity labeling technology demonstrating that insect-expressed COI1 specifically binds to the photoaffinity-labeled coronatine. This experimental proof on binding of JA-Ile/coronatine to COI1 was nicely complemented by an extensive molecular modeling of interaction between COI1 and different jasmonate compounds such as JA, MeJA, OPDA, (+)-7-iso-JA and coronatine.Citation26 Only (+)-7-iso-JA and coronatine could fit to the COI1 surface pocket (), whereas MeJA and JA are too small to fit within the pocket and OPDA could not form a stable interaction with the surface pocket.Citation26 This is strongly supported by its above mentioned high bioactivity.Citation13 Although the crystal structure of the JA receptor is still missing, the nature of the receptor (COI1) and its ligand [(+)-7-iso-JA-Ile] is known. What does this mean for the measurements of jasmonates as indicator of JA-dependent processes?
(+)-7-iso-jasmonoyl-L-isoleucine can be Quantified as Stable compound
In the last two decades several methods have been developed for separation and quantitative analysis of oxylipins including jasmonates. Most of them are based on GC and GC/MS and were permanently improved in respect to the procedure and sensitivity as well as the instruments used. Several methods are based on the initial protocol of Schmelz et al.,Citation27 which was preferentially developed for volatiles, and that of Weber et al.,Citation28 convenient for new oxylipins. Improvement of these protocols led to large scale analysis of oxylipins including products of non-enzymatic oxidation such as phytoprostanes.Citation29 Recent refinement led to higher sensitivity in detection of free and esterified oxylipins including arabidopsides.Citation30,Citation31 Most of these methods are based on derivatization of a carboxylic acid group by esterification with pentafluorobenzyl bromide (PFBB) usually performed at 50 °C for 60 min. This derivatization is necessary for GC/MS but does not lead to the differentiation between the cis/trans isomers. The new aspects, however, in JA signaling including the high biological activity of a specific isomer, the (+)-7-iso-JA-Ile, require the quantitative analysis of the different stereoisomers. Regarding JA, the use of the PFBB ester method with freshly prepared extracts led to the detection of a preferential formation of 3R,7S-form, which is indicative for de novo JA biosynthesis (Miersch, pers. communication). A specific and quantitative analysis of the cis-form (3R,7S) could be achieved, however, only using PFBB oxime derivatives.Citation32
Recently quantitative analysis of (+)-7-iso-JA-Ile the terminal ligand of the JA receptor was shown.Citation11 Here the derivatization with PFBB performed in the known procedure was done only for 15 min. Consequently, epimerization of isomers could be strongly reduced, and there was no epimerization of (−)-JA-Ile to (+)-7-iso-JA-Ile and most of the JA-Ile of wounded tomato leaves could be stably detected as (+)-7-iso-JA-Ile for several hours. This indicates that in the wound response the most bioactive JA compound is formed steadily in a quite large time window. Therefore, epimerization of (+)-7-iso-JA-Ile seems not to be an important mechanism to switch off JA signaling.Citation11 The wound response in terms of JA-induced transcripts declined much earlier than the level of the most bioactive jasmonate conjugate.Citation33 As suggested recentlyCitation11, this putative discrepancy might be explained by stable accumulation of truncated JAZ proteins at elevated levels of JA-Ile thereby attenuating JA responsive gene expression.Citation16
Figures and Tables
Figure 1 Stuctures of (+)-7-iso-JA-L-ile [(3R,7S)-JA-L-ile], coronatine (COR), (−)-JA-L-Ile [(3R,7R)-JA-L-Ile] and (+)-JA-L-Ile [(3S,7S)-JA-L-Ile].
![Figure 1 Stuctures of (+)-7-iso-JA-L-ile [(3R,7S)-JA-L-ile], coronatine (COR), (−)-JA-L-Ile [(3R,7R)-JA-L-Ile] and (+)-JA-L-Ile [(3S,7S)-JA-L-Ile].](/cms/asset/cea72482-bc07-4ee8-aafc-1f2708403002/kpsb_a_10911574_f0001.gif)
Figure 2 Coronatine and JA-Ile could fit within the surface pocket of COI1. Molecular modeling of the interaction between COI1 and Coronatine (A), (+)-7-iso-JA-Ile (B) or MeJA (C). Left panel: chemical structures of the jasmonates. Middle panel: the pose with the highest GoldScore fitness value in the molecular docking simulation. The jasmonates are shown as red sticks. The surface pocket of COI1 is shown in grey. Right panel: superposition of representative frames of the restricted molecular dynamics simulation. Frames at the early, intermediate and late stages were extracted and superimposed. The jasmonates are shown as pink sticks, and their interacting residues are shown as white lines. Polar contacts are shown as yellow dotted lines (modified from Yan et al., 2009Citation26).
Acknowledgements
The authors' laboratory work was financially supported by a grant of the graduate program of the excellence cluster initiative of Sachsen-Anhalt, Germany (C.W.) and by the National Science Foundation of China, The National Basic Research 973 Program of China, and the Tsinghua-Yue-Yuen Medical Sciences Fund and grant from Chinese Academy of Sciences (D.X.). We thank B. Hause for critical reading of the manuscript.
References
- Schaller A, Stintzi A. Enzymes in jasmonate biosynthesis - Structure, function, regulation. Phytochemistry 2009; 70:1532 - 1538
- Wasternack C, Kombrink E. Jasmonates: structural requirements for lipid-derived signals active in plant stress responses and development. ACS Chem Biol 2010; 5:63 - 77
- Holbrook L, Tung P, Ward K, Reid DM, Abrams S, Lamb N, et al. Importance of the chiral centers of jasmonic acid in the responses of plants - activities and antagonism between natural and synthetic analogs. Plant Physiol 1997; 114:419 - 428
- Wasternack C. Jasmonates: An update on biosynthesis, signal transduction and action in plant stress response, growth and development. Ann Bot 2007; 100:681 - 697
- Miersch O, Neumerkel J, Dippe M, Stenzel I, Wasternack C. Hydroxylated jasmonates are commonly occurring metabolites of jasmonic acid and contribute to a partial switch-off in jasmonate signaling. New Phytol 2008; 177:114 - 127
- Glauser G, Grata E, Dubugnon L, Rudaz S, Farmer EE, Wolfender J-L. Spatial and temporal dynamics of jasmonate synthesis and accumulation in Arabidopsis in response to wounding. J Biol Chem 2008; 283:16400 - 16407
- Staswick PE, Su W, Howell SH. Methyl jasmonate inhibition of root growth and induction of a leaf protein are decreased in an Arabidopsis thaliana mutant. Proc Natl Acad Sci USA 1992; 89:6837 - 6840
- Staswick PE, Tiryaki I. The oxylipin signal jasmonic acid is activated by an enzyme that conjugates it to isoleucine in Arabidopsis. Plant Cell 2004; 16:2117 - 2127
- Suza WP, Staswick PE. The role of JAR1 in jasmonoyl-L-isoleucine production during Arabidopsis wound response. Planta 2008; 227:1221 - 1232
- Wang L, Halitschke R, Kang JH, Berg A, Harnisch F, Baldwin IT. Independently silencing two JAR family members impairs levels of trypsin proteinase inhibitors but not nicotine. Planta 2007; 226:159 - 167
- Suza W, Rowe ML, Hamberg M, Staswick PE. A tomato enzyme synthesizes (+)-7-iso-jasmonoyl-L- isoleucine in wounded leaves. Planta 2010; 231:717 - 728
- Feys BJF, Benedetti JE, Penfold CN, Turner JG. Arabidopsis mutants selected for resistance to the phytotoxin coronatine are male sterile, insensitive to methyl jasmonate, and resistant to a bacterial pathogen. Plant Cell 1994; 6:751 - 759
- Fonseca S, Chini A, Hamberg M, Adie B, Porzel A, Kramell R, et al. (+)-7-iso-Jasmonoyl-L-isoleucine is the endogenous bioactive jasmonate. Nat Chem Biol 2009; 5:344 - 350
- Browse J. Jasmonate passes muster: A receptor and targets for the defense hormone. Ann Rev Plant Biol 2009; 60:183 - 205
- Thines B, Katsir L, Melotto M, Niu Y, Mandaokar A, Liu G, et al. JAZ repressor proteins are targets of the SCFCOI1 complex during jasmonate signalling. Nature 2007; 448:655 - 661
- Chung HS, Niu Y, Browse J, Howe GA. Top hits in contemporary JAZ: An update on jasmonate signaling. Phytochemistry 2009; 70:1547 - 1559
- Yi H, Preuss ML, Jez JM. The devil (and an active jasmonate hormone) is in the details. Nat Chem Biol 2009; 5:273 - 274
- Xie DX, Feys BF, James S, Nieto-Rostro M, Turner JG. COI1: an Arabidopsis gene required for jasmonate-regulated defense and fertility. Science 1998; 280:1091 - 1094
- Kepinski S, Leyser O. The Arabidopsis F-box protein TIR1 is an auxin receptor. Nature 2005; 26:446 - 451
- Dharmasiri N, Dharmasiri S, Estelle M. The F-box protein TIR1 is an auxin receptor. Nature 2005; 435:441 - 445
- Woodward AW, Bartel B. A receptor for auxin. Plant Cell 2005; 17:2425 - 2429
- Xu L, Liu F, Lechner E, Genschik P, Crosby WL, Ma H, et al. The SCFCOI1 ubiquitin-ligase complexes are required for jasmonate response in Arabidopsis. Plant Cell 2002; 14:1919 - 1935
- Chini A, Fonseca S, Fernandez G, Adie B, Chico JM, Lorenzo O, et al. The JAZ family of repressors is the missing link in jasmonate signalling. Nature 2007; 448:666 - 671
- Yan YX, Stolz S, Chetelat A, Reymond P, Pagni M, Dubugnon L, Farmer EE. A downstream mediator in the growth repression limb of the jasmonate pathway. Plant Cell 2007; 19:2470 - 2483
- Katsir L, Schilmiller AL, Staswick PE, He SY, Howe GA. COI1 is a critical component of a receptor for jasmonate and the bacterial virulence factor coronatine. Proc Natl Acad Sci USA 2008; 105:7100 - 7105
- Yan J, Zhang C, Gu M, Bai Z, Zhang W, Qi T, et al. The Arabidopsis CORONATINE INSENSITIVE1 protein is a jasmonate receptor. Plant Cell 2009; 21:2220 - 2236
- Schmelz EA, Engelberth J, Alborn HAT, O'Donnell P, Sammons M, Toshima H, et al. Simultanous analysis of phytohormones, phytotoxins and volatile organic compounds in plants. Proc Natl Acad Sci USA 2003; 100:10552 - 10557
- Weber H, Vick BA, Farmer EE. Dinor-oxophytodienoic acid: a new hexadecanoid signal in the jasmonate family. Proc Natl Acad Sci USA 1997; 94:10473 - 10478
- Mueller MJ, Mene-Saffrane L, Grun C, Karg K, Farmer EE. Oxylipin analysis methods. Plant J 2006; 45:472 - 489
- Göbel C, Feussner I. Methods for the analysis of oxylipins in plants. Phytochemistry 2009; 70:1485 - 1503
- Thiocone A, Farmer EE, Wolfender JL. Screening for wound-induced oxylipins in Arabidopsis thaliana by differential HPLC-APCI/MS profiling of crude leaf extracts and subsequent characterisation by capillaryscale NMR. Phytochem Anal 2008; 19:198 - 205
- Schulze B, Lauchli R, Sonwa M M, Schmidt A, Boland W. Profiling of structurally labile oxylipins in plants by in situ derivatization with pentafluorobenzyl hydroxylamine. Anal Biochem 2006; 348:269 - 283
- Koo AJ, Gao X, Daniel Jones A, Howe GA. A rapid wound signaling activates the systemic synthesis of bioactive jasmonates in Arabidopsis. Plant J 2009; 59:974 - 986