Abstract
Humic substances (HS) represent the organic material mainly widespread in nature. HS have positive effects on plant physiology by improving soil structure and fertility and by influencing nutrient uptake and root architecture. The biochemical and molecular mechanisms underlying these events are only partially known. HS have been shown to contain auxin and an “auxin-like” activity of humic substances has been proposed, but support to this hypothesis is fragmentary. In this review article, we are giving an overview of available data concerning molecular structures and biological activities of humic substances, with special emphasis on their hormone-like activities.
Introduction
Humic substances (HS) are the largest constituent of soil organic matter (∼60%) and are considered as a key component of the terrestrial ecosystem, being responsible for many complex chemical reactions in soil.Citation1 They can not be decomposed readily because of their intimate interactions with soil mineral phases and are chemically too complex to be used by micro organisms. As far as soil is concerned, one of the most striking characteristics of HS is their ability to interact with metal ions, oxides, hydroxides, mineral and organic compounds,Citation2 including toxic pollutants,Citation3–Citation5 to form water-soluble and water-insoluble complexes. Through the formation of these complexes, can dissolve, mobilize and transport metals and organics in soils and waters, or accumulate in soil horizons. Accumulation of such complexes can contribute to a reduction of toxicity, e.g., of aluminium (Al),Citation6,Citation7 or to remove Cr(VI) from aqueous solutions.Citation8 Recently Wang and Mulligan (2009) have used HS in the arsenic and heavy metal remediation, indicating the HS as a possible remedial to reduce and avoid further contamination. Moreover HS can interact with xenobiotic organic molecules such as pesticidesCitation2,Citation9–Citation11 and influence nutrient availability (N, S, P), especially of those nutrients present at a very low concentration.Citation12 Moreover, these are able to produce various morphological, physiological and biochemical effects on higher plants.Citation13–Citation15
Numerous studies have shown that HS enhance root, leaf and shoot growth but also stimulate the germination of various crop species.Citation16 These positive effects are explained as an interaction between HS and physiological and metabolic processes.Citation15,Citation17 The addition of HS stimulate nutrient uptake,Citation16,Citation20 cell permeabilityCitation21 and seems to regulate mechanisms involved in plant growth stimulation.Citation22–Citation26
It is not easy to distinguish between the direct and indirect effects of these substances. In fact, some of their positive effects may be ascribed to a general improvement of soil fertility, leading to a higher nutrient availability for plants. Whilst, in other cases, HS seem to positively influence metabolic and signalling pathways involved in the plant development, by acting directly on specific physiological targets.Citation27,Citation28 For this reason, understanding HS biological activity and the molecular mechanisms through which they exert their functions is becoming an important ecological task and a valid tool in facing environmental problems.
This review is aimed at clarifying the main signalling events governing the physiological effects of HS on plant metabolism, in order to shed more light on nature, properties, dynamics and functions of HS as part of soil and agricultural ecosystems.
Molecular Structure of Humic Substances
A focal point of this topic is to verify the existence of a relationship between structure and biological properties of HS.Citation29 So far the structure of HS is under debated. The main obstacle is the lack of a repetitive sequences and the variety of chemical and biological reactions involved in their genesis,Citation30 that make HS very complex and multifaceted molecules, able to exert important signalling and nutritional functions in soil-plant system. The points of view more debated concern the polymeric nature and the supramolecular association of HS. Different authors have considered the HS a polymeric material with high molecular mass (100–300 kDa),Citation1,Citation29,Citation31,Citation32 originated from the lignin decompositionCitation33 and from abiotic catalysts such as primary minerals and layer silicates.Citation34
Alvarez-Puebla et al.Citation35 proposed a macromolecular model of HS in which simple (though heterogeneous) monomeric units progressively build up into high molecular weight polymers by random condensation and oxidation processes ().Citation35 In this model is demonstrated that linear or branched polymeric chain, assuming several conformational folding that would give more resistant to microbial degradation and lengthen their turnover in the soil.Citation36
A supramolecular association of heterogeneous molecules held together by hydrophobic interactions (van der Waals, π-π, ion-dipole) and hydrogen bonds has been proposed by Piccolo,Citation37,Citation38 Simpson et al.Citation39 and Schaumann.Citation40 The whole of these forces stabilizes the structure of molecular aggregates. Moreover, the stability of HS aggregates in solution seems to be of a dynamic nature and influenced by the solution ionic strength and pH.Citation41,Citation42 At the alkaline pH the HS are in disperse form because intramolecular hydrogen bondings are completely disrupted.Citation43 Acidification of HS with weak acids (i.e., acetic acid) solutions produces a significant decrease of molecular sizes or disaggregation for disruption of weak non covalent interactions, such as van der Waals, π-π, and CH-π.Citation43,Citation44 At lowest pH values (<3) the HS collapse into smaller aggregated. This phenomenon is mainly due to the protonation of carboxylate groups that favor an increase in the number of intra- and intermolecular hydrogen bonds inside the humic macromolecules.Citation38,Citation45
From a chemical point of view the HS are molecular aggregates consisting of sugar, fatty acids, polypeptides, aliphatic chains and aromatic rings.Citation39 HS are operationally classified into humin and humic and fulvic acids in relation to different solubility at acid and alkaline pH.Citation1 This classification was based only on superficial criteria and no indication on chemical behavior or structures was deduced.Citation1
Biological Activities
Different authors hypothesized that HS may be adsorbed by plant roots, even if high molecular weight (HMW) and low molecular weight (LMW) fractions seem to behave differently.Citation15,Citation18,Citation21,Citation46,Citation71 Up to today it has not been yet completely clarified the mechanisms through which HS interact with the root cells and may subsequently influence plant physiology and growth. Among the modifications induced by HS on treated plants, changes in size and development were the first to be studied analytically. Under particular conditions, HS can stimulate plant growth in terms of increase in plant length and dry or fresh weight.Citation47,Citation48 These effects seem to depend on the concentrationCitation49 and source of the substance,Citation50 on the plant speciesCitation47,Citation48 and age, as well as on the culture conditions of the trial. Recently, many studies have confirmed the hypothesis of a direct effect of HS on plant physiology, in particular concerning root hair formationCitation51 and lateral root development ().Citation23,Citation24,Citation52
The effects of HS on plant metabolic processes have been extensively reviewed.Citation15,Citation44 For instance, many reports showed that HS influence respiration,Citation53 protein synthesisCitation54 and enzyme activity in higher plants.Citation53,Citation55–Citation57 As far as the photosynthesis process is concerned, few reports, focusing on the chlorophyll content and electrons transport, are available.Citation58–Citation61
In light of the importance of mineral nutrition for overall plant productivity, the effects of HS on ion uptake represent one of the topics which received more attention by scientists. They appear to be variable and selective, depending on the HS typology and concentration, on plant species, and on composition and pH of the medium.Citation14,Citation15,Citation25,Citation62–Citation65 A positive effect of HS on nutrient uptake has been reported for the major inorganic elements, such as nitrogen, phosphorus, potassiumCitation66 and sulphur,Citation48 but different HS fractions seem to differently affect their uptake.Citation67,Citation68 A recent study focused on iron uptake and assimilation in cucumber plants treated with purified leonardite humic acid (PHA), evidenced an induction of the expression of CsFRO1 and CsIRT1, encoding a Fe(III) chelate-reductase and a Fe(II) root transporter respectively.Citation69 These results strongly support the hypothesis that beneficial effects of HS on plant development may, at least in part, depend on their capacity to improve Fe availability for plant uptake under Fe-deficient conditions.Citation65 Pinton et al.Citation70 evidenced the capacity of a specific fraction of HS (water extracted fraction WEHS) to stimulate some of the typical plant responses to Fe deficiency, such as the induction of Fe(III) chelate-reductase activity. This action of WEHS was also associated to significant increase in rhizosphere acidification.
Furthermore, as a consequence of the environmental impact that the nitrogen fertilization has assumed during the last century, several studies were conduced to figure out how the presence of HS may interfere with the nitrate uptake and assimilation by plants.Citation14,Citation25,Citation27,Citation63,Citation70–Citation72 Results obtained demonstrated a strong positive effect of the LMW fractions on nitrate uptake and assimilation, whereas HMW fractions induced only weakly the same pathways,Citation15 in agreement with previous data.Citation71
More recently, three independent studies aimed to better clarify the mechanisms through which HS stimulate the nitrate uptake in maize,Citation23,Citation27,Citation70 demonstrated that the increment of nitrate influx measured in response to HS depends at least partially on a transcriptional activation of a gene encoding a major H+-ATPase of maize (Mha2), likely leading to the generation of a more favorable electrochemical gradient. In fact, nitrate influx across the plasmalemma of root cells is coupled to the favorable H+ electrochemical gradient created by the plasma membrane H+-ATPases.Citation73–Citation76
A recent study used a wide transcriptomic approach to study the biological processes involved in the Arabidopsis thaliana response to HS.Citation77 This study represents the first report concerning the global molecular mechanisms governing the HS-plant interaction. From our preliminary data it may be hypothesized that HS influence plant development by interfering with the transcription of genes involved in meristem formation and organization, cell cycle, microtubule organization and cytokinesis. Moreover, a great amount of the transcripts isolated has been shown to belong to several classes of transcription factors and DNA binding proteins, confirming. Even if the exact mechanisms through which HS exert their effects on plant physiology are still partially unclear, many evidences suggest that they may involve at least in part a hormone-like activity.
Hormone-Like Activities
A putative HS hormone-like activity is not surprising as it is known that soils vary in their native auxin contentCitation78 and fertile soils contain greater amounts of auxin then less fertile ones.Citation79,Citation80 Auxin and gibberellin levels are usually higher in the rhizosphere than in the bulk soil, probably as a consequence of increased microbial populations or of an accelerated metabolism owing to the presence of root exudates. Although numerous soil and rhizosphere microorganisms, as well as the root systems of higher plants, have been reported as producing auxinCitation81 and gibberellins,Citation82 there is little information about their stability and only indirect conclusions have been made about their presence in amounts high enough to be biologically active.Citation83
At the beginning of the 20th century, BottomleyCitation84 hypothesized that the growth promoting activity of the HS could be due to a hormone-like activity. Such argument was then deeply faced by a number of studies and later definitely corroborated by results demonstrating the immunological or spectrometric identification of indol acetic acid (IAA) inside several HS.Citation17,Citation23,Citation27,Citation85 In addition, the hypothesis of a HS auxin-like activity was also supported by reports showing a positive effect of such substances on specific targets of auxin action. Mha2, a major maize isoform of H+-ATPase that is preferentially expressed in guard cells, phloem and root epidermal cells and that appears to be strongly stimulated at the transcriptional level in response to auxin,Citation86 evidenced a significant upregulation of its mRNA abundance in roots of maize seedlings treated for 48 hours with earthworm low molecular size HS.Citation27 Furthermore, Russel and collaborators,Citation87 by studying the effects of two different molecular weight fractions of HS on pea, evidenced an auxin-like effect of both fractions on stomatal opening as influenced by phospholipase A2, that is considered to be involved in auxin signalling.Citation88,Citation89
In former experiments, Muscolo et al.Citation90,Citation91 showed a morphogenetic effect of HS on Nicotiana plumbaginifolia leaf explants, probably triggered by a modification of peroxidase and esterase activities. These effects, peculiar to humic fraction with a low relative molecular mass (<3,500 Da), were similar to those produced by IAA. A subsequent study on homogeneous carrot (Daucus carota) cell cultures compared the effects of the low relative molecular mass humic fraction to different auxins.Citation17 This humic fraction caused an increase in carrot cell growth similar to that induced by 2,4-dichlorophenoxyacetic acid (2,4-D) and promoted morphological changes similar to those induced by IAA. In addition, Muscolo et al.Citation17 demonstrated that IAA and LMW fractions richer in carboxylic groups bind in the same way with carrot cell membranes. Zandonadi et al.Citation52 comparatively evaluate the effects of indole-3-acetic acid and humic acids (HA) isolated from different soils substances on maize root development and on activities of plasmalemma and tonoplast H+ pumps. They observed that HA as well as low IAA concentrations (10−10 and 10−15 M) stimulated root growth by inducing the proliferation of lateral roots () along with a differential activation not only of the plasma membrane, but also of vacuolar H+-ATPases and H+-pyro-phosphatase.
A different theory about the auxin-like activity of the HS has been assumed by Schmidt et al.Citation92 (2007). To further investigate a possible hormone-like effect of water-soluble humic molecules (WEHS), they grown Arabidopsis plants in sterile medium containing WEHS in concentrations ranging from 1–20 mg C org. Application of WEHS were found to significantly increase the number and length of root hairs. Further experiments reported that the phenotypes of Arabidopsis auxin-related mutants, all exhibiting a reduced number of root hairs, were not rescued by the application of WEHS. In addition, mutants defective in root hair initiation such as rhd6, known to develop normal hairs in the presence of ethylene or auxin, were not affected by a wide range of applied concentration of WEHS. The authors concluded that HS cannot substitute for these hormones in promoting root hair growth, and suggested that HS can alter root development without significantly affecting the plant's auxin homeostasis. This assumption was also supported by the lack of responsiveness of the auxin-responsive GH3 gene. Transcripts of genes from the GH3 family accumulate following auxin exposure, probably to dampen auxin signaling by inactivating IAA via conjugation to amino acids.Citation93 Application of high concentration of HS (more than 5 mg C L−1) for two hours evidenced only a slight increase in transcript abundance in roots and caused no significant change in mRNA accumulation in leaves, further confirming the hypothesis that the changes in root morphology are not mediated via an auxin-signalling pathway.
It is important to note that this theory is not complemented with a detailed characterization of the HS analyzed. Because of the different features and the complex chemical composition of HS, an efficient and complete characterization, from both a chemical and a spectroscopic point of view, is an essential requirement to match data obtained from different studies. In this case the extraction procedure and the lacking in characterization of the humic substances analyzed make the results not completely suitable for further comparison with any other information present in literature. Moreover, the authors expressed the theory that WEHS do not exert their effects in an auxin-like manner without investigate its inner content of indole-3 acetic acid. According to all these lacking in description, the conclusion of the authors could be speculative rather than theoretical and it would need a more detailed investigation to be considered.
On the contrary, recently Dobbss and collaborators,Citation26 using Arabidopsis and tomato seedlings, demonstrated that various characterized humic acids need the auxin transduction pathway to be active. The increase in number of lateral root exhibited in Arabidopsis and tomato wild-type seedlings treated with different HS led authors to hypothesize the presence of auxin-like compounds in these organic matter. Nevertheless the same substances did not induce lateral root formation in a tomato mutant (dgt) characterized by a defective gene for auxin response (). They concluded that probably HS may act as a “buffer”, either absorbing or releasing signalling molecules, according to modifications in the rizosphere, such as the acidification brought about by the activity of plasma membrane H+-ATPaseCitation23,Citation52 or exudation of organic acids,Citation24,Citation94 thus behaving as a regulator of hormonal balance with respect to lateral root emergence.
More recently, the auxinic activity of HS in the initiation of lateral roots has been deeply studied in the model plant Arabidopsis thaliana,Citation28 by utilizing a combination of genetic and molecular approaches ( and ). The widely used auxin reporter DR5::GUSCitation95 was employed to visualize auxin responses in rootsCitation96,Citation97 and to characterize the distribution of LRP stages in both wild type (Col-0) and aux1 mutant background. In addition, the transcription of the known early auxin responsive genes IAA5 and IAA19,Citation98–Citation101 in parallel treatments with HS and comparable IAA concentrations was evaluated.
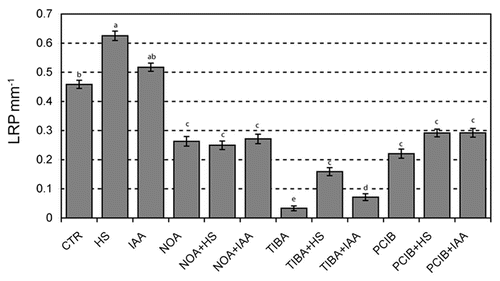
The authors concluded that HS exert their action on lateral root development mostly through their auxinic activity and clearly demonstrated the presence of a small amount of IAA in the fraction analyzed, corresponding to a concentration of 34 nM. At the same time, the presence of additional factors independent from auxin was evident based on IAA5 and IAA19 expression suggesting that more systematic approaches are needed to unravel the molecular mode of action of HS. These aspects are currently under investigation in our laboratory.
Because of their complicated and changeable nature, a debate on HS auxin-like activity is still open. However, the observed hormonal effects did not always correlate to the amount of IAA detected in the humic acids. For this reason, the presence of different compounds of the auxin family or of molecules that might either mimic the action or stimulate the plant endogenous metabolism of auxin cannot be ruled out. Functional genomics, transcriptomics or proteomics may represent a good strategy in shedding light on HS biological activity.
Conclusions and Perspectives
HS, as the major component of soil organic matter, have been widely studied in various areas of agriculture, such as soil chemistry, fertility and plant physiology. HS plays an important role in controlling the behavior and mobility of polluters in the environment and contribute substantially in improving the global soil fertility status. These features together with a major demand of safe food and sustainable agricultural have contributed to enlarge the environmental significance of HS, which have been recently recognized as a possible tool in facing environmental problems.
Many of their positive effects on soil and plant growth have been demonstrated to rely on their chemical composition, but progress in research on HS has been considerably hampered by the lack of characterization of the humic fractions being used.
The auxinic activity of HS, demonstrated in recent studies, is probably the main biological factor responsible for the positive effects exerted by HS on plant physiology. The stimulatory effect on Arabidopsis lateral root development observed in response to HS, has been found mainly in the first stages, when cells start to divide, suggesting that HS response may involve mechanisms as the stimulation of cell division and differentiation, which it is know to be under the control of auxin. Moreover, physiological and molecular data suggest brassinosteroids as a putative additional factor throught which HS could exert their effects on plant development. This finding has been further supported by recent transcriptomic results. A great amount of the genes isolated by means of a cDNA-AFLP approach have been demonstrated to be auxin regulated and related to developmental process, as differentiation and organization of meristems, embriogenesys, citokynesis and microtubules organization (Trevisian, personal communication).
All together, these results provide evidence that HS need auxin transduction pathway to establish their action on plant physiology, but evidenced also the existence of different signalling cascades involved in the global physiological response of plants to these substances (). This could be considered a starting point in elucidating mechanisms that occur in plant at molecular level in response to HS. Further studies are needed to assess the molecular targets and signalling pathways involved in the cross-talk between HS and plant cells. These features together with a major demand of safe food and sustainable agricultural have contributed to enlarge the environmental significance of HS.
Fertilizer factories are now redirecting their production to biostimulants, based on humic substances and other organic compounds and recently, in Italy, biostimulants were inserted in the Legislative Decree n. 217/2006 (“New regulation about fertilizer” MiPAF).
This is an important result which supports the fundamental project of recycling partially humified organic wastes, derived from plant, wood, food and other human activities, as beneficial soil amendments. For this reason, understanding HS biological activity and the molecular mechanisms through which they exert their functions is becoming an important ecological task and a valid tool in facing environmental problems.
Figures and Tables
Figure 1 Two perspectives (van der Waals surfaces) of two aggregated monomer HS. (A) Circles indicate the pores and their size. (B) Results obtained for two polymers of 23 units under the same conditions.Citation35
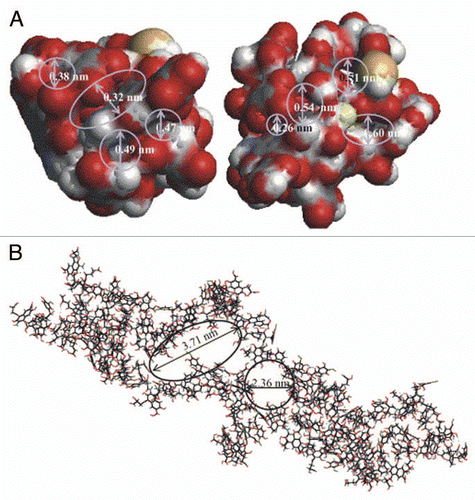
Figure 2 (A) Maize seedlings grown for 72 h in nutrient solution alone (control) or with addition of humic acids (HAs) at 50 mg C L−1 in the presence of increasing concentrations of citric acid (0, 0.0005, 0.005 and 0.05 mM). Bar = 4 mm. (B) Three maize seedlings grown for 168 h in nutrient solution alone (control) and three grown with the addition of HA at a concentration of 50 mg C L−1 (0). The arrows indicate the additional regions of primary roots with lateral roots. Bar = 20 mm.Citation24
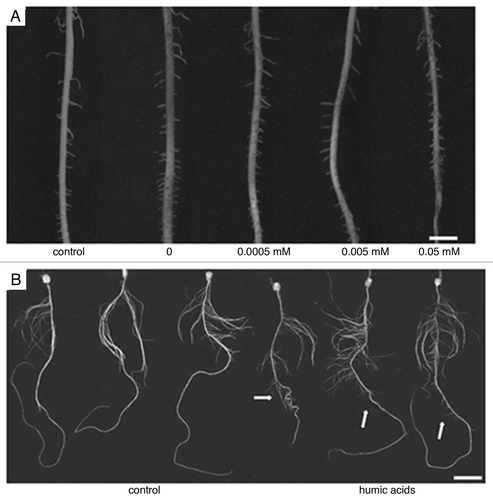
Figure 3 (A–C) Effect of HA and IAA on maize root development evaluated by the induction of mitotic sites (MS) of lateral root emergence (LRE) and lateral root density (LRE per millimetre primary root length—LRE/mm). (A) Representative images of mitotic sites (arrow) of a control root, bar 0.8 mm; hyperinduction of mitotic sites in a root treated with HAU (20 mg C l−1), or in a root treated with IAA (10−10 M), bars 1.2 mm. (B) Representative roots treated with IAA (0, 10−5, 10−10 M) or HAU (20 mg C l−1), bar 10 mm. (C) Quantification of early mitotic sites (clear columns), lateral root emergence (dashed columns) and lateral root density (grey columns). The data represent means from four independent experiments with ten plants analyzed per treatment (§SD, n = 40).Citation52
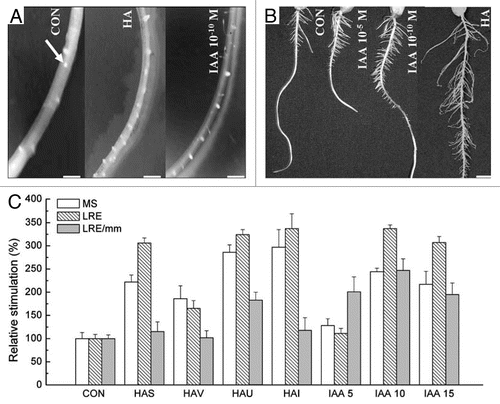
Figure 4 Effect of humic acids (HA; 40 mg C L−1) isolated from Xanthic Hapludox (P1), Sombrihumox (P4) and Rhodic Hapludox (P7) on the induction of lateral roots in micro-tom (MT; control) and dgt (an auxin-insensitive mutant) plants. Plants were cultivated in vitro for 30 days in Murashige-Skoog medium. No lateral root growth is noticed in dgt plants, suggesting that the various HA need the auxin-signalling transduction pathway to be active.Citation26
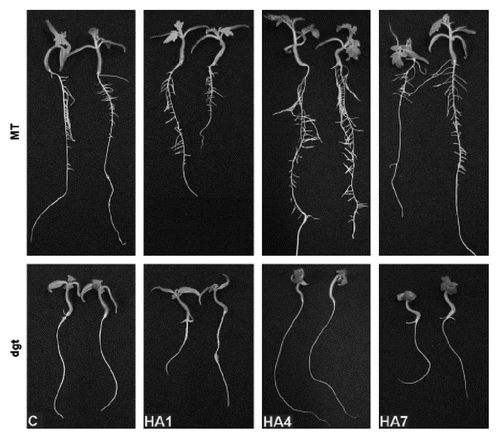
Figure 5 Visualization of Gus activity in root of DR5::GUS transgenic plants treated with different auxin inhibitors. Plants were grown for 4 days in MS medium plates and then transferred for 24 hours in: water, CTR (A), 50 µM NOA (B), 50 µM TIBA (C), 50 µM PCIB (D), 1 mgC L−1 HS (E), 50 µM NOA + 1 mgC L−1 HS (F), 50 µM TIBA + 1 mgC L−1 HS (G), 50 µM PCIB + 1 mgC L−1 HS (H), 34 nM IAA (I), 50 µM NOA + IAA 34 nM (J), 50 µM TIBA + IAA 34 nM (K), 50 µM PCIB + 34 nM IAA (L). Histochemical GUS staining was performed as described by Jefferson (1987). Lateral root primordia are represented at different developmental stages: I (B, G, H, K and L), II (A, F and J) and III (E and I). Scale bar is 50 µm.Citation28
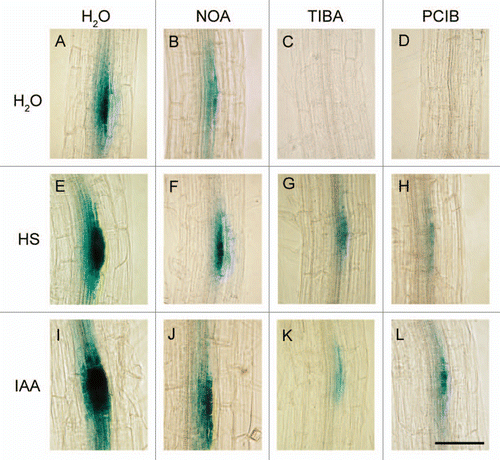
Figure 6 Changes in the density of LRP mm−1 in four-day-old DR5::GUS Arabidopsis seedlings after 24 hours of treatments with IAA (34 nM) or HS (1 mgC L−1) and in response to different auxin inhibitors.Citation28
References
- Stevenson FJ. Wiley John. Organic forms of soil nitrogen. Humic Chemistry: Genesis, Composition, Reaction 1994; New York 59 - 95
- Albers CN, Banta GT, Hansen PE, Jacobsen OS. Effect of different humic substances on the fate of diuron and its main metabolite 3,4-dichloroaniline in soil. Environ Sci Technol 2008; 1:8687 - 8691
- Cattani I, Zhang H, Beone GM, Del Re AA, Boccelli R, Trevisan M. The role of natural purified humic acids in modifying mercury accessibility in water and soil. J Environ Qual 2009; 6:493 - 501
- Luo W, Gu B. Dissolution and mobilization of uranium in a reduced sediment by natural humic substances under anaerobic conditions. Environ Sci Technol 2009; 43:152 - 156
- Wang S, Mulligan CN. Enhanced mobilization of arsenic and heavy metals from mine tailings by humic acid. Chemosphere 2009; 74:274 - 279
- Tan KH, Binger A. Effect of humic acid on aluminium toxicity in corn plants. Soil Sci 1986; 14:20 - 25
- Elkins KM, Nelson DJ. Spectroscopic approaches to the study of the interaction of aluminum with humic substances. Coord Chem Rev 2002; 228:205 - 225
- Janos P, Hula V, Bradnová P, Pilarová V, Sedlbauer J. Reduction and immobilization of hexavalent chromium with coal- and humate-based sorbent. Chemosphere 2009; 75:732 - 738
- Vermeer AWP. Interactions between humic acid and hematite and their effects on metal ion speciation 1996; The Netherlands Wageningen University (PhD thesis)
- Martin-Neto L, Traghetta DG, Vaz CM, Crestana S, Sposito G. On the interaction mechanisms of atrazine and hydroxyatrazine with humic substances. J Environ Qual 2001; 30:520 - 525
- Celano G, Smejkalová D, Spaccini R, Piccolo A. Interactions of three s-triazines with humic acids of different structure. J Agric Food Chem 2008; 27:7360 - 7366
- Arslan G, Pehlivan E. Uptake of Cr3+ from aqueous solution by lignite-based humic acids. Bioresour Technol 2008; 99:7597 - 7605
- Vaughan D, Malcolm RE. Vaughan D, Malcolm RE. Influence of humic substances on growth and physiological processes. Soil Organic Matter and Biological Activity 1985; Dordrecht Martinus Nijhoff/Junk W, The Netherlands 37 - 76
- Chen Y, Aviad T. MacCarthy P, Malcolm RL, Clapp CE, Bloom PR. Effects of humic substances on plant growth. Humic Substances in Soil and Crop Science: Selected Readings 1990; Madison American Society of Agronomy and Soil Science Society of America 161 - 187
- Nardi S, Pizzeghello D, Muscolo A, Vianello A. Physiological effects of humic substances on higher plants. Soil Biol Biochem 2002; 34:1527 - 1536
- Piccolo A, Celano G, Pietramellara G. Effects of fractions of coal-derived humic substances on seed germination and growth of seedlings (Lactuga sativa and Lycopersicum esculentum). Biol Fertil Soil 1993; 16:11 - 15
- Muscolo A, Bovalo F, Gionfriddo F, Nardi S. Earthworm humic matter produces auxin-like effects on Daucus carota cell growth and nitrate metabolism. Soil Biol Biochem 1999; 3:1303 - 1311
- Muscolo A, Sidari M, Francioso O, Tugnoli V, Nardi S. The auxin-like activity of humic substances is related to membrane interactions in carrot cell cultures. J Chem Ecol 2007; 33:115 - 129
- Linehan DJ. Humic acid and iron uptake by plants. Plant and Soil 1978; 50:663 - 670
- Dell'Agnola G, Nardi S. Hormone-like effect and enhanced nitrate uptake induced by depolycondensed humic fractions obtained from Allobophora rosea and A. caliginosa faeces. Biol Fertil Soils 1987; 4:115 - 118
- Vaughan D, Ord BG. Uptake and incorporation of 14C-labelled soil organic matter by roots of Pisum sativum L. J Exp Bot 1981; 32:679 - 687
- Lee YS, Bartlett RJ. Stimulation of plant growth by humic substances. Soil Sci Soc Am J 1976; 40:876 - 879
- Canellas LP, Olivares FL, Okorokova-Façanha AL, Façanha AR. Humic acids isolated from earthworm compost enhance root elongation, lateral root emergence and plasma membrane H+-ATPase activity in maize roots. Plant Physiol 2002; 130:1951 - 1957
- Canellas LP, Teixeira Junior LRL, Dobbss LB, Silva CA, Medici LO, Zandonadi DB, Façanha AR. Humic acids crossinteractions with root and orgnic acids. Ann Appl Biol 2008; 153:157 - 166
- Clapp CE, Chen Y, Hayes MHB, Cheng HH. Swift RS, Sparks KM. Plant growth promoting activity of humic substances. Understanding and Managing Organic Matter in Soils, Sediments and Waters 2001; St. Paul International Humic Science Society 243 - 255
- Dobbss LB, Medici LO, Peres LEP, Pino-Nunes LE, Rumjianek VM, Façanha AR, Canellas LP. Changes in root development of Arabidopsis promoted by organic matter from oxisols. Ann Appl Biol 2007; 151:199 - 211
- Quaggiotti S, Ruperti B, Pizzeghello D, Francioso O, Tugnoli V, Nardi S. Effect of low molecular size humic substances on the expression of genes involved in nitrate transport and reduction in maize (Zea mays L.). J Exp Bot 2004; 55:803 - 813
- Trevisan S, Pizzeghello D, Ruperti B, Francioso O, Sassi A, Palme K, et al. Humic substances induce lateral root formation and expression of the early auxinresponsive IAA19 gene and DR5 synthetic element in Arabidopsis. Plant Biol 2009; http://dx.doi.org/10.1111/j.1438-8677.2009.00248.x
- Schulten HR, Schnitzer M. Chemical model structures for soil organic matter and soils. Soil Sci 1997; 162:115 - 130
- Ziechmann W. Humic Substances 1994; Mannheim Wissenschaftsverlag
- Kononova MM. Soil organic matter. Its nature, its role in soil formation and in soil fertility 1966; Oxford Pergamon 544
- Schulten HR, Leinweber P. New insights into organicmineral particles: composition, properties, and models of molecular structure. Biol Fertil Soils 2000; 30:399 - 432
- Flaig W, Beutelsacher H, Rietz E. Gieseking JE. Chemical composition and physical properties of humic substances. Soil components: Organic components 1975; 1:New York Springer-Verlag 1 - 211
- Bollag JM, Myers C, Pal S, Huang PM. Huang PM, Berthelin J, Bollag JM, McGill WB, Page AL. The role of abiotic and biotic catalysts in the transformation of phenolic compounds. Environmental impacts of soil component interactions 1995; Chelsea Lewis Publisher 297 - 308
- Alvarez-Puebla RA, Goulet PJG, Garrido JJ. Characterization of the porous structure of different humic. Colloids Surf A: Physicochem Eng Asp 2005; 256:129 - 135
- Insam H, Rangger A, Henrich M, Hitzl W. The effect of grazing on soil microbial biomass and community on alpine pastures. Phyton 1996; 36:205 - 216
- Piccolo A. The supramolecular structure of humic substances. Soil Sci 2001; 166:810 - 833
- Piccolo A. The Supramolecular structure of humic substances. A novel understanding of humus chemistry and implications in soil science. Advances in Agronomy 2002; 75:57 - 134
- Simpson AJ, Kingery WL, Hayes MH, Spraul M, Humpfer E, Dvortsak P, et al. Molecular structures and associations of humic substances in the terrestrial environment. Naturwissenschaften 2002; 89:84 - 88
- Schaumann GE. Soil organic matter beyond molecular structure 1. Macromolecular and supramolecular characteristics. J Plant Nutr Soil Sci 2006; 169:145 - 156
- Baalousha M, Motelica-Heino M, Le Coustumer P. Conformation and size of humic substances: Effects of major cation concentration and type, pH, salinity and residence time. Colloids Surf A Physicochem Eng Asp 2006; 272:48 - 55
- Kucerik J, Smejkalova D, Cechlovska H, Pekar M. New insights into aggregation and conformational behavior of humic substances: Application of high resolution ultrasonic spectroscopy. Org Geochem 2007; 38:2098 - 2110
- Smejkalova D, Piccolo A. Aggregation and disaggregation of humic supramolecular assemblies by NMR diffusion ordered spectroscopy (DOSY-NMR). Environ Sci Technol 2008; 42:699 - 706
- Nardi S, Concheri G, Dell'Agnola G. Piccolo A. Biological activity of humus. Humic Substances in Terrestrial Ecosystems 1996; The Netherlands Elsevier 361 - 406
- Corrado G, Sanchez-Cortes S, Francioso O, Garcia-Ramos JV. Surface-enhanced Raman and flourescence joint analysis of soil humics. Anal Chim Acta 2008; 616:69 - 77
- Vaughan D, Chesire MV, Mundie CM. Uptake by the beetroot tissue and biological activity of 14C-labelled fractions of soil organic matter. Biochem Soc Trans 1974; 2:126 - 129
- Blanchet RM. The direct and indirect effect of humified, organic matter on the nutrition of vascular plants. Annales agronomiques 1958; 9:499 - 532
- Guminski S. Present-day views on physiological effects induced in plant organism by humic compounds. Sov Soil Sci 1968; 1250 - 1256
- Elgala AM, Metwally AJ, Khalil RA. The effect of humic acid and Na2 EDDHA on the uptake of Cu, Fe and Zn by barley in sand culture. Plant Soil 1978; 49:41 - 48
- Hernando V, Ortega BC, Fortun C. Study of the action of two types of humic acid on the maize plant. Soil Organic Matter Studies Vol 2, Report of IAEA Meeting Vienna 1977; Oxford Pergamon Press
- Schmidt W, Cesco S, Santi S, Pinton R, Varanini Z. Hartmann A, Schmid M, Wenzel W, Hinnsinger P. Water-extractable humic substances as nutrient acquisition signals for root hairs development in Arabidopsis. Rizosphere 2004—Perspectives and Challenges 2005; Neuherberg GSF-Berich 71
- Zandonadi DB, Canellas LP, Façanha AR. Indolacetic and humic acids induce lateral root development through a concerted plasmalemma and tonoplast H+ pumps activation. Planta 2007; 225:1583 - 1595
- Nardi S, Muscolo A, Vaccaro S, Baiano S, Spaccini R, Piccolo A. Relationship between molecular characteristics of soil humic fractions and glycolytic pathway and krebs cycle in maize seedlings. Soil Biol Biochem 2007; 39:3138 - 3146
- Carletti P, Masi A, Spolaore B, Polverino De Laureto P, De Zorzi M, et al. Protein Expression Changes in Maize Roots in Response to Humic Substances. J Chem Ecol 2008; 34:804 - 818
- Sladký Z. The effect of extracted humus substances on growth of tomato plants. Biol Plant 1959; 1:142 - 150
- Vaughan D. The stimulation of invertase development in aseptic storage tissue slices by humic acids. Soil Biol and Biochem 1967; 1:15 - 28
- Nardi S, Pizzeghello D, Remiero F, Rascio N. Chemical and biochemical properties of humic substances isolated from forest soils and plant growth. Soil Sci Soc Am J 2000; 64:639 - 645
- Thomas SM, Thorne GN, Pearman I. Effect of nitrogen on growth, yield and photorespiratory activity in spring wheat. Ann Bot 1978; 42:827 - 837
- Oettmeier W, Masson K, Donner A. Anthraquinone inhibitors of photosystem II electron transport. FEBS Letts 1988; 231:259 - 262
- Jezierski A, Czechowski H, Jerzykiewicz M, Drozd J. EPR investigations of structure of humic acids from compost, soil, peat and soft brown coal upon oxidation and metal uptake. Appl Magn Reson 2000; 18:127 - 136
- Pflugmacher S, Pietsch C, Rieger W, Steinberg CEW. Dissolved natural organic matter (NOM) impacts photosynthetic oxygen production and electron transport in coontail Ceratophyllum demersum. Sci Total Environ 2006; 357:169 - 175
- Varanini Z, Pinton R. Lüttge. Humic substances and plant nutrition. Progress in Botany 1995; Berlin Springer 97 - 117
- Varanini Z, Pinton R. Pinton R, Varanini Z, Nannipieri P. Direct versus indirect effects of soil humic substances on plant growth and nutrition. The Rizosphere 2001; Basel Marcel Dekker 141 - 158
- Tan KH. Humic Matter in Soil and the Environment 2003; New York Marcel Dekker
- Chen Y, De Nobili M, Aviad T. Magdoff FR, Weil RR. Stimulatory effects of humic substances on plant growth. Soil Organic Matter in Sustainable Agriculture 2004; Boca Raton CRC Press 103 - 129
- Mylonas VA, McCants CB. Effects of humic and fulvic acids on growth of tobacco 2. Tobacco growth and ion uptake. J Plant Nutr 1980; 2:377 - 393
- Vaughan D, Linehan DJ. The growth of wheat plants in humic acid solutions under axenic conditions. Plant Soil 1976; 44:445 - 449
- Marino G, Francioso O, Carletti P, Nardi S, Gessa C. Mineral content and root respiration of in vitro grown kiwifruit plantlets treated with two humic fractions. J Plant Nutr 2008; 31:1074 - 1090
- Elena A, Leménager D, Bacaicoa E, Fuentes M, Baigorri R, Zamarreño AMA, García-Mina JMA. The root application of a purified leonardite humic acid modifies the transcriptional regulation of the main physiological root responses to Fe deficiency in Fe-sufficient cucumber plants. Plant Physiol Biochem 2008; 47:215 - 223
- Pinton R, Cesco S, Iacoletti G, Astolfi S, Varanini Z. Modulation of NO3− uptake by water-extractable humic substances: involvement of root plasma membrane H+ATPase. Plant Soil 1999; 215:155 - 161
- Vaughan D. Burns RG, Dell'Agnola G, Miele S, Nardi S, Savoini G, Schnitzer M, et al. Effetto delle sostanze umiche sui processi metabolici delle piante. Sostanze Umiche effetti sul terreno e sulle piante 1986; Roma Ramo Editoriale degli Agricoltori 59 - 81
- Sessi E, Nardi S, Gessa C. Effects of low and high molecular weight humic substances from two differ ent soils on nitrogen assimilation pathway in maize seedlings. Humic Substances in the Environment 2000; 2:39 - 46 (ISSN:1506-7696)
- Thibaud JB, Grignon C. Mechanism of nitrate uptake in corn roots. Plant Sci Let 1981; 22:279 - 289
- Ruiz-Cristin J, Briskin DP. Characterization of a H+/NO3− symport associated with plasma membrane vescicles of maize roots using 36ClO3- as a radiotracer analog. Arch Biochem Biophys 1991; 285:74 - 82
- Meharg AA, Blatt MR. NO3− transport across the plasma membrane of Arabidopsis thaliana root hairs: kinetic control by pH and membrane voltage. J Membr Biol 1995; 145:49 - 66
- Miller AJ, Smith SJ. Nitrate transport and compartmentation in cereal root cells. J Exp Bot 1996; 300:843 - 854
- Trevisan S. A genomic approach for studying the biological activity of humic substances 2009; Padua University of Padua (PhD thesis)
- Hamence JH. The determination of auxins in soils, including a note on synthetic growth substances. Analyst 1946; 71:111 - 116
- Stewart WS, Anderson MS. Auxins in some american soils. Bot Gaz 1942; 103:570 - 575
- Dahm H, Sitek JM, Strzelczyk E. Synthesis of auxins by bacterial isolated from the roots of pine seedlings inoculated with rusty forest soil. Pol J Soil Sci 1977; 10:131 - 137
- Lebuhn M, Hartmann A. Method for determination of indole-3-acetic acid and related compounds of L-tryptophan catabolism in soils. J Chromatogr 1993; 629:255 - 266
- Rademacher W. Occurrence of gibberellins in different species of the fungal genera Sphaceloma and Elsinoe. Phytochemistry 1992; 31:4155 - 4157
- Rankenberger WT, Arshad M. Phytormones in Soils 1995; New York Marcel Dekker Inc
- Bottomley WB. Some effects of organic growth-promotion substances (auximones) on the growth of Lemma minor in mineral cultural solutions. Proc R Soc Lond B Biol Sci 1917; 89:481 - 505
- Muscolo A, Cutrupi S, Nardi S. IAA detection in humic substances. Soil Biol Biochem 1998; 30:1199 - 1201
- Frias I, Caldeira MT, Perez-Castineira JR, Navarro-Avino JP, Culianez-Macia FA, Kuppinger O, et al. A major isoform of the maize plasma membrane H+-ATPase: characterization and induction by auxin in coleoptiles. Plant Cell 1996; 8:1533 - 1544
- Russell L, Stokes AR, Macdonald H, Muscolo A, Nardi S. Stomatal responses to humic substances and auxin are sensitive to inhibitors of phospholipase A2. Plant Soil 2006; 283:175 - 185
- Macdonald H. Auxin perception and signal transduction. Physiol plantarum 1997; 100:423 - 430
- Scherer GFE. Second messengers and phosholipase A2 in auxin transduction. Plant Mol Biol 2002; 49:357 - 372
- Muscolo A, Felici M, Concheri G, Nardi S. Effect of humic substances on peroxidase and esterase patterns during growth of leaf explants of Nicotiana plumbaginifolia. Biol Fertil Soils 1993; 15:127 - 131
- Muscolo A, Panuccio MR, Abenavoli MR, Concheri G, Nardi S. Effect of molecular complexity and acidity of earthworm faeces humic fractions on glutamate dehydrogenase, glutamine synthetase and phosphoenolpyruvate carboxylase in Daucus carota II cells. Biol Fertil Soils 1996; 22:83 - 88
- Schmidt W, Santi S, Pinton R, Varanini Z. Water-extractable humic substances alter root development and epidermal cell pattern in Arabidopsis. Plant Soil 2007; 300:259 - 267
- Hagen G, Kleinschmidt A, Guilfoyle T. Auxin-regulated gene expression in intact soybean hypocotyl and excised hypocotyl sections. Planta 1984; 162:147 - 153
- Façanha AR, Façanha ALO, Olivares FL, Guridi F, Santos GA, Velloso ACX, et al. Bioatividade de àcidos hùmicos: efeito sobre o desenvolvimento redicular e sobre a bomba de pròtons da membrana plasmàtica. Pesqui Agropecu Bras 2002; 37:1301 - 1310
- Ulmasov T, Murfett J, Hagen G, Guilfoyle TJ. Aux/IAA proteins repress expression of reporter genes containing natural and highly active synthetic auxin response elements. Plant Cell 1997; 9:1963 - 1971
- Sabatini S, Beis D, Wolkenfelt H, Murfett J, Guilfoyle T, Malamy J, et al. An auxin-dependent distal organizer of pattern and polarity in the Arabidopsis root. Cell 1999; 99:463 - 472
- Benkova E, Michniewicz M, Sauer M, Teichmann T, Seifertova D, Jurgens G, Friml J. Local, Efflux-Dependent Auxin Gradients as a Common Module for Plant Organ Formation. Cell 2003; 115:591 - 602
- Goda H, Shimada Y, Aasmi T, Fujioka S, Yoshida S. Microarray analysis of brassinosteroid-regulated genes in Arabidopsis. Plant Physiol 2002; 130:1319 - 1334
- Nakamura A, Higuchi K, Goda H, Fujiwara MT, Sawa S, Koshiba T, et al. Brassinolide Induces IAA5, IAA19 and DR5, a Synthetic Auxin Response Element in Arabidopsis, Implying a Cross Talk Point of Brassinosteroid and Auxin Signalling. Plant Physiol 2003; 133:1843 - 1853
- Oono Y, Oura C, Rahman A, Aspuria ET, Hayashi K, Tanaka A, Uchimiya H. p-Chlorophenoxyisobutyric acid impairs auxin response in Arabidopsis root. Plant Physiol 2003; 111:1135 - 1147
- Yamazoe A, Hayashi K, Kepinski S, Leyser O, Nozaki H. Characterization of terfestatin A, a new specific inhibitor for auxin signaling. Plant Physiol 2005; 139:779 - 789