?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Bioelectrochemical circuits operate in all plants including the sensitive plant Mimosa pudica Linn. The activation of biologically closed circuits with voltage gated ion channels can lead to various mechanical, hydrodynamical, physiological, biochemical, and biophysical responses. Here the biologically closed electrochemical circuit in pinnae of Mimosa pudica is analyzed using the charged capacitor method for electrostimulation at different voltages. Also the equivalent electrical scheme of electrical signal transduction inside the plant’s pinna is evaluated. These circuits remain linear at small potentials not exceeding 0.5 V. At higher potentials the circuits become strongly non-linear pointing to the opening of ion channels in plant tissues. Changing the polarity of electrodes leads to a strong rectification effect and to different kinetics of a capacitor. These effects can be caused by a redistribution of K+, Cl-, Ca2+, and H+ ions through voltage gated ion channels. The electrical properties of Mimosa pudica were investigated and equivalent electrical circuits within the pinnae were proposed to explain the experimental data.
Introduction
Signal transduction in Mimosa pudica Linn. has been attracting the attention of researchers since the XVI century.Citation1–Citation5 It is a sensitive plant in which the leaves and the petiole move in response to intensity of light, mechanical or electrical stimuli, drought and hot or cold stimuli.Citation1,Citation6–Citation8 It was found by HaberlandtCitation3 that long distance signal transduction in Mimosa pudica occurs through the phloem.
Mechanical movement of Mimosa pudica after electrical signal transduction is a defense mechanism6 and can be the simplest criterion of plant behavior and intelligence.Citation9 HookeCitation1 stated long ago, “And that this may be so, it seems with great probability to be argued from the strange phenomena of sensitive plants, wherein Nature seems to perform several animal actions with the same schematism or organization that is common to all vegetables, as may appear by some no less instructive then curious observations that were made by divers eminent members of the Royal Society on some of these kind of plants?…”
The Mimosa pudica plant contains long slender branches called petioles, which can fall due to mechanical, thermal or electrical stimuli. The petioles contain smaller pinnae arranged on the rachis of the pinna. The pinnules are the smallest leaflets while the entire leaf contains the petioles, pinnae and pinnules. Tertiary, secondary and primary pulvini are responsible for the movement of a pinna.
Thigmonastic movements, such as a response to mechanical stimuli, appear to be regulated by electrical and chemical signal transduction spreading the stimulus throughout the Mimosa pudica pinnae, petiole or stem.Citation6–Citation8 Electrical signals can induce chemical processes, which were described by RiccaCitation5 in the chemical transmission hypothesis. The action potentials that occur in plants have many of the same properties as the action potentials that occur in animals.Citation6–Citation8,Citation10–Citation15 These properties include the all-or-none law, threshold potentials and refractory periods.Citation15 The transmission of an action potential induced by a gentle touch is halted at the base of a single pinna and no further transmission occurs, leaving leaflets from neighboring pinna unfolded.Citation6,Citation16 Action potentials propagate in the phloem and protoxylem parenchyma of Mimosa pudica.Citation17 The cell's depolarization results from a Cl− efflux followed by a K+ efflux, which initiates the repolarization phase.Citation18–Citation20
There are different methods for the evaluation of electrical circuits in plants.Citation21,Citation22 The patch clamp method can be used to study electrical characteristics of a small part of a biological membrane in vitro. The electrochemical impedance method may be used for the investigation of electrical characteristics of membranes or cells. However different electrochemical circuits can have the same electrochemical impedance.Citation23–Citation31 The description of equivalent electrical circuits based on electrochemical impedance AC measurements is based on the research intuition and can lead to various mistakes.Citation26 The newly developed DC charged capacitor method permits direct in vivo evaluation of the simplest electrical circuits in a cluster of cells or in a single cell.Citation32–Citation36
In the study reported, the biologically closed electrical circuits in the pinnae of Mimosa pudica were analyzed through electrostimulation using the Charged Capacitor Method.Citation32–Citation36 An equivalent electrical scheme of the electrical signal transduction was then evaluated inside pinnae.
Results
47 µF capacitor discharge in a pinna.
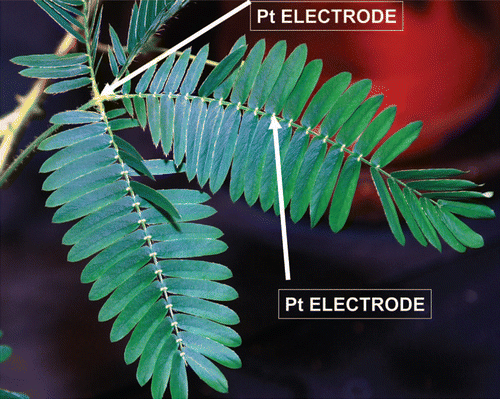
shows the location of Pt-electrodes in a pinna of Mimosa pudica. (+ in a secondary pulvinus and − in a rachis) and (− in a secondary pulvinus and + in a rachis) show the time dependencies of a 47 mkF charged capacitor discharging in the pinna of Mimosa pudica when the polarity of the same electrodes was reversed. and show the time dependencies of charged capacitors discharging in the Mimosa pudica in logarithmic coordinates.
If a capacitor of capacitance C is discharged during time t through a resistor R (), the logarithm of capacitor voltage U is 1
1 where U0 is the initial voltage of a capacitor and γ is the time constant. The circuit time constant γ = RC governs the discharging process. The voltage across the capacitor decreases exponentially from the initial value U0 to zero. As the capacitance or resistance increases the time of the capacitor discharge increases according to equation (1). and show that if the initial voltage on a charged capacitor is below 1 V, the dependence of voltage on a capacitor is close to linear in logarithmic coordinates and can be described by the simple equivalent electrical scheme shown on or B. If the initial voltage on a charged capacitor is higher than 1 V ( and ) there is strong deviation from the linear dependence predicted by equation 1.
Discussion
Electrical circuits in pinnae.
and show that electrical discharge of a capacitor through the plant can be described in the first approximation as a discharge through a constant resistance at small potentials (), though here, the better approximation can be achieved with the circuit in . At potentials higher than 0.5 V, the logarithmic plot in is definitely nonlinear meaning that the discharge can be described by the circuit in . The pattern of discharge strongly depends on polarity of the capacitor as can be seen in . Here the departure from linear logarithmic dependence can be noticed much earlier, even at U <0.5 V. Each curve can be satisfactorily modeled with the electrical circuit in . However, parameters of this circuit would be different for different cases.
To illustrate this fact, the input resistance of the circuits was calculated.Citation36 According to Ohm's law, this parameter is defined as voltage, U, divided by current, :
2
2
We found that in all cases presented in and the discharge process could be perfectly approximated with doubleexponential function corresponding to the scheme in : 3
3
Here a and c are the amplitudes of exponential functions and b and d are the time constants. Substituting eq. (3) in eq. (2) we can find the input resistance: 4
4
The characteristic time in the very beginning of discharge is 5
5
The characteristic time in the beginning of the capacitor discharge depends on initial voltage as presented in . shows constants a, b, c and d for experimental dependencies presented in and .
The curve in has a rough M-shape and is rather symmetrical with exception of the two middle points corresponding to the smallest voltages −0.1 V and +0.1 V. Here the dependence on polarity manifests itself in the strongest way. When the voltage is in the range from −1 V to +1 V, the characteristic time is between 120 and 150 seconds. At larger potentials the characteristic time sharply drops to 60 and even 30 seconds. That means that the plant's tissues are very electrically nonlinear which can be explained by the opening of various ion channels.
The non-linearity of electrical circuits in Mimosa pudica becomes especially obvious if data from and are presented in normalized form by dividing the experimental curves by initial voltage (). If the applied initial potential U0 is less or equal to 1 V, all curves fall in the narrow strip where they practically coincide. That means that at small potentials the discharge is governed by the same electrical circuit that can be considered as a linear one (). However at higher potentials the electrical circuit becomes strongly non-linear ( and D). The curves for U0 = ±1.5 V in strongly depart from the narrow strip corresponding to small voltages. Here the capacitor discharge is much faster, that can be explained by the opening of voltage gated channels and corresponding decrease of effective resistance.
There is a strong rectification effect inside a pinna, which is sensitive to the polarity of the capacitor. It is illustrated in , where the data from and are presented as the sum of positive and negative charges. One can see that at small potentials the curves cancel each other—the sum goes close to zero. At U0 = ±1.5 V there is a strong difference between the curves demonstrating the dependence of rectification on polarity. Once again this can be caused by a redistribution of K+, Cl− and Ca2+ ions through ion channels in the pinna.Citation19,Citation20
It is convenient to represent electrochemical properties of biologically closed electrical circuits with idealized equivalent electrical circuit models consisting of discrete electrical components.Citation37–Citation39 The voltage gated K+ and Cl− ion channels in Mimosa pudica can be simulated by diodes ( and D). The equivalent electrical circuit in is similar to the electrical circuit in the midrib of Venus flytrap,Citation24 which gives the same dependence of a capacitor discharge in a plant tissue.
Different polarity of the applied voltage can open different ion channels, such as K+ or Cl− ion channels and the equivalent electrical circuit can be simulated as shown in and D. These voltage gated ion channels were studied in detail a few years ago.Citation18–Citation20
The activation of biologically closed electrical circuits in Mimosa pudica can lead to different responses in plant tissue. A mechanical or electrical stimulus can induce electrical signals or action potentials, which propagate along the plasma membrane in phloem. Voltage-gated ionic channels control the plasma membrane potential and the movement of ions across membranes thereby regulating various biological functions.Citation40 These biological nanodevices play vital roles in signal transduction in higher plants.Citation15 Propagation of action potentials in Mimosa pudica along the pinna, petioles, pulvini and in the stem is documented in literature.Citation6,Citation16,Citation18,Citation36,Citation41,Citation42
Propagation of an action potential can induce a mechanical response by activating Cl− channels and voltage gated K+ ion channel in a pinna. Voltage gated K+ and Cl− ion channels can operate as an electrical starter of the osmotic motor in a pinna. Ion fluxes activate fast water transport through aquaporinsCitation43 between the lower half and upper half of the tertiary pulvinus or between outer and inner layers of pinnules following an electrical stimulus.
Processes associated with rapid movements of petioles and pinnae of sensitive plant Mimosa pudica have been attracting the attention of scientists since the Royal Society meetingCitation1 on July 17, 1661. There are few hypotheses about the mechanism of the mechanisms underlying the Mimosa movements such as osmotic motors,Citation8,Citation44 muscular movementsCitation45 and actin phosphorylation,Citation46 the chemical transmission hypothesis,Citation5 electrical signal transduction,Citation6 loss of turgor,Citation4 but exact mechanism of this widely investigated phenomenon remains unknown.
Closing of pinnules and a petiole bending can be triggered by electrical discharge between electrodes in a pulvinus.Citation35,Citation36 Both voltage and transmitted charge are responsible for the electrostimulated plant movement. Kinetics of a petiole bending or pinnules closing by electrical or mechanical stimulation is identical. Electrochemical properties of pinnae, a pulvinus, secondary pulvinus and a petiole are different.Citation6–Citation8 The equivalent electrical circuit of a petiole was evaluated and described by mathematical model recently.Citation36 In the present work we evaluated the simplest equivalent electrical circuit of a pinna by DC electrostimulation in vivo of Mimosa pudica's pinna using the new charged capacitor method.
Materials and Methods
Electrodes.
All measurements were conducted in the laboratory at 21°C inside a Faraday cage mounted on a vibration-stabilized table. Platinum electrodes were prepared from Teflon-coated platinum wires (A-M Systems, Inc., Sequim, WA). The response time of platinum electrodes was less than 0.1 µs, when we used square wave pulses from a function generator with the electrodes in the plant tissue. Insertion of the electrodes into Mimosa pudica caused the leaves to close, therefore each plant was allowed to rest for 3 hours until the leaves were completely relaxed and open. The time dependencies of the electrical discharge of the capacitor in Mimosa pudica were measured between 3 and 25 hours after insertion of the electrodes.
Data acquisition.
PCI eXtensions for Instrumentation (PXI) is a rugged PC-based platform that offers a high-performance solution for measurement and automation systems. PXI adds mechanical, electrical and software features that define complete systems for test, measurement and data acquisition. An NI-PXI-4071 digital multimeter (National Instruments, Austin, TX) connected to 0.1 mm thick Pt-electrodes was used to record the digital data.
Electrostimulation of cells clusters in vivo.
The Charge-Injection Method was used to precisely estimate the amount of electrical energy necessary to induce a response.Citation33–Citation36 A double pole-double throw (DPDT) switch was used to connect the 47 mkF capacitor to the PXI-4110 DC power supply (National Instruments) during charging and then to the plant during plant stimulation. By changing the switch position the charged capacitor can be instantaneously connected to the plant and induce a response. Since the charge of capacitor Q connected to the voltage source U is Q = CU, the amount of charge can be precisely regulated using different capacitors and applying various voltages.
Plants.
The seeds of Mimosa pudica L. were soaked in warm water (30°C) for 48 h. They were then grown in well drained peat moss at 21°C with a 12:12 hour light:dark photoperiod. After growing for two weeks the seedlings were transplanted into pots and placed in a plant growing chamber. Humidity averaged 45–50% and the plants were watered every day. Two to three month old plants were used for the experiments. Irradiance was 700–800 µE/m2s. All of the experiments were performed on healthy adult specimens.
Abbreviations
| a, b, c, d | = | constants of double-exponential function |
| C | = | capacitance |
| DPDT | = | double pole double throw switch |
| E | = | DC power supply |
| PXI | = | PCI extensions for instrumentation |
| R | = | resistance |
| R input | = | input resistance |
| τ | = | the circuit time constant |
| t | = | time |
| Q | = | charge of capacitor |
| U | = | voltage |
| U0 | = | is the initial voltage of a capacitor |
Figures and Tables
Figure 2 (A) Time dependence of electrical discharge in Mimosa pudica's pinna between electrodes connected to 47 µF charged capacitor (+ in a secondary pulvinus and − in a rachis) and an NI-PXI-4071 digital voltmeter. These results were reproduced 16 times. Location of Pt-electrodes is shown in . (B) Time dependence of electrical discharge in the Mimosa pudica's pinna between electrodes in logarithmic coordinates. U is the capacitor voltage in volts.
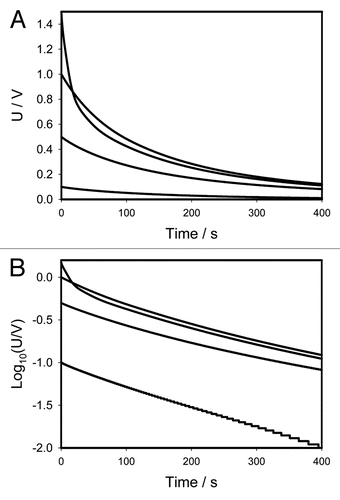
Figure 3 (A) Time dependence of electrical discharge in Mimosa pudica's pinna between electrodes connected to 47 µF charged capacitor (− in a secondary pulvinus and + in a rachis). Location of Pt-electrodes is shown in . These results were reproduced 16 times. (B) Time dependence of electrical discharge in the Mimosa pudica's pinna between electrodes connected to charged capacitors in logarithmic coordinates. U is the capacitor voltage in volts.
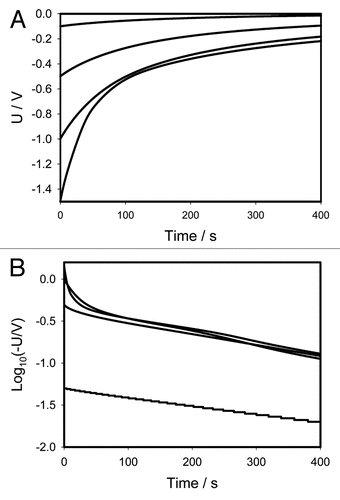
Figure 4 Electrical equivalent schemes of a capacitor discharge in a plant tissue. Abbreviations: C1, charged capacitor from voltage source U; C2, capacitance of plant tissue; R, resistance in the Mimosa pudica tissue; D, diode.
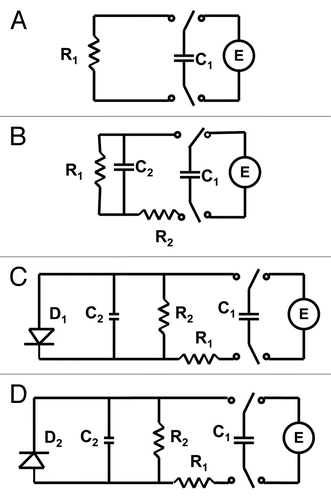
Figure 5 γ = RC dependence on the initial capacitor voltage during electrical discharge in Mimosa pudica's pinna between electrodes connected to charged capacitors. Location of Pt-electrodes is shown in .
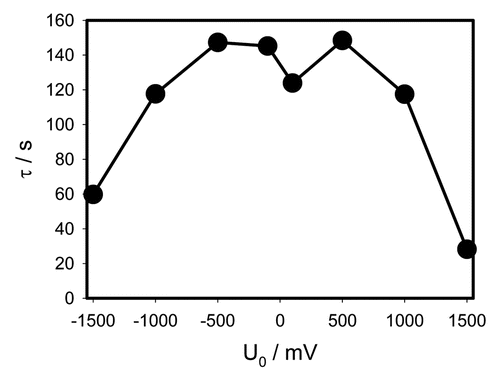
Figure 6 Normalized presentation of time dependence of electrical discharge in Mimosa pudica's pinna between electrodes connected to charged capacitor. U0 is the initial capacitor voltage in volts.
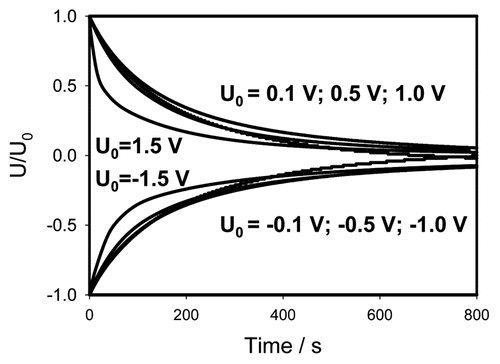
Figure 7 Time dependence of voltage differences ( and ) during electrical discharge in the Mimosa pudica's pinna between electrodes of different polarities connected to 47 mkF charged capacitor. Location of Pt-electrodes is shown in .
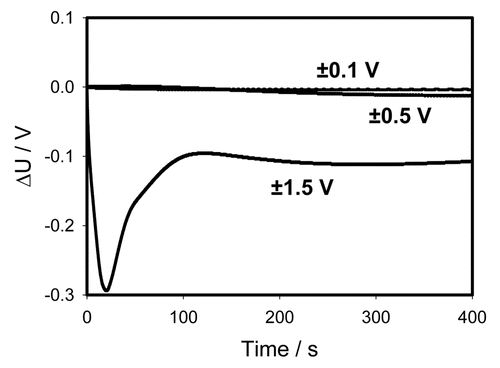
Table 1 Parameters of double-exponential function in equation 3 for experimental dependencies presented in and
Acknowledgements
This work was supported by the National Science Foundation (grant no. HRD0811507).
References
- Hooke R. Micrographia 1667; London The Royal Society
- Gardiner W. On the power of contractility exhibited by the protoplasm of certain plant cells. Annals Botany 1888; 1:362 - 367
- Haberlandt G. Das reisleitende Gewebesystem der Sinnpflanse 1890; Leipzig Engelmann
- Pfeffer W. The Physiology of Plants 1905; Oxford Clarendon Press
- Ricca U. Soluzione d'un problema di fisiologia. La propagazione di stimulo nella “Mimosa.”. Nuovo Giornale Botanico Italiano Nuovo Serie 1916; 23:51 - 170
- Bose JC. Movements in Plants 1918; Delhi B.R. Publishing Corp
- Bose JC. The Nervous Mechanism of Plants 1926; London Longmans Green
- Bose JC. The Motor Mechanism of Plants 1928; London Longmans Green
- Trewavas A. What is plant behavior?. Plant Cell Environment 2009; 32:606 - 616
- Bertholon M. De l'electricite des vegetaux: ouvrage dans lequel on traite de l'electricite de l'atmosphere sur les plantes, de ses effets sur leconomie des vegetaux, de leurs vertus medico 1783; Paris P.F. Didot Jeune
- Ksenzhek OS, Volkov AG. Plant Energetics 1998; San Diego Academic Press
- Volkov AG. Green plants: Electrochemical interfaces. J Electroanal Chem 2000; 483:150 - 156
- Volkov AG. Balushka F, Manusco S, Volkman D. Electrophysiology and Phototropism. Communication in Plants. Neuronal Aspects of Plant Life 2006; Berlin Springer 351 - 367
- Volkov AG. Plant Electrophysiology 2006; Berlin Springer
- Volkov AG, Brown CL. Kumar CSSR. Nanodevices in Nature. Nanodevices for Life Sciences 2006; Weinheim Wiley-VCH 440 - 463
- Fromm J, Lautner S. Electrical signals and their physiological significance in plants. Plant Cell Environm 2007; 30:249 - 257
- Sibaoka T. Excitable cells in Mimosa. Science 1962; 137:226
- Abe T. Chloride ion efflux during an action potential in the main pulvinus of Mimosa pudica. Bot Mag 1981; 94:379 - 383
- Stoeckel H, Takeda K. Plasmalemmal, voltage-dependent ionic currents from excitable pulvinar motor cells of Mimosa pudica. J Membr Biol 1993; 131:179 - 192
- Stoeckel H, Takeda K. Calcium-sensitivity of plasmalemmal delayed rectifier potassium current suggests that calcium influx in pulvinar protoplasts from Mimosa pudica L. can be revealed by hyperpolarizion. J Membr Biol 1995; 146:201 - 209
- Volkov AG, Carrell H, Markin VS. Biologically closed electrical circuits in Venus flytrap. Plant Physiol 2009; 149:1661 - 1667
- Volkov AG, Carrell H, Baldwin A, Markin VS. Electrical memory in Venus flytrap. Bioelectrochem 2009; 75:142 - 147
- Evert DR. Factors affecting electrical impedance of intermodal stem sections. Plant Physiol 1973; 51:478 - 480
- Harker FR, Maindonald JH. Ripening of nectarine fruit. Changes in the cell wall, vacuole and membranes detected using electrical impedance measurements. Plant Physiol 1994; 106:165 - 171
- Inaba A, Manabe T, Tsuji H, Iwamoto T. Electrical impedance analysis of tissue properties associated with ethylene induction by electric currents in cucumber (Cucumis sativus L.) fruit. Plant Physiol 1995; 107:199 - 205
- Laarabi S, Kinani KE, Ettouhami A, Limouri M. In vivo impedance of the aerial organs of some mono- and dicotyledonous plants. Comptes Rendus Biol 2005; 328:253 - 262
- McAdams ET, Jossinet J. Problems in equivalent circuit modeling of the electrical properties of biological tissues. Bioelectrochem Bioenerg 1996; 40:147 - 152
- Wang J, Zimmermann U, Benz R. Contribution of electrogenic ion transport to impedance of the algae Valonia utricularis and artificial membranes. Biophys J 1994; 67:1582 - 1593
- Zatsepina GN, Tsaplev YuB. Nature of electrical polarity of a higher plant. Biophysics 1980; 5:147 - 150
- Zhang MIN, Willison JHM. Electrical impedance analysis in plant tissues: A double shell model. J Exp Bot 1991; 42:1465 - 1475
- Zhang MIN, Willison JHM. Electrical impedance analysis in plant tissues: Impedance measurements in leaves. J Exp Bot 1993; 44:1369 - 1375
- Zhang MIN, Willison JHM. Electrical impedance analysis in plant tissues: in vivo detection of freezing injury. Canadian J Bot 1991; 42:1465 - 1475
- Volkov AG, Adesina T, Markin VS, Jovanov E. Closing of Venus flytrap by electrical stimulation of motor cells. Plant Signal Behav 2007; 2:139 - 144
- Volkov AG, Adesina T, Markin VS, Jovanov E. Kinetics and mechanism of Dionaea muscipula trap closing. Plant Physiol 2008; 146:694 - 702
- Volkov AG, Coopwood KJ, Markin VS. Inhibition of the Dionaea muscipula Ellis trap closure by ion and water channels blockers and uncouplers. Plant Sci 2008; 175:642 - 649
- Volkov AG, Foster JC, Ashby TA, Walker RK, Johnson JJ, Markin VS. Mimosa pudica: Electrical and mechanical stimulation of plant movements. Plant Cell Environm 2010; 33:163 - 173
- Volkov AG, Foster JC, Markin VS. Signal transduction in Mimosa pudica: Biologically closed electrical circuits. Plant Cell Environm 2010; 33:816 - 827
- Grattarola M, Massobrio G. Bioelectronics Handbook 1998; New York McGraw-Hill
- Keener J, Sneyd J. Mathematical Physiology 2001; New York Springer
- Nagumo J, Arimoto S, Yoshizawa S. An active pulse transmission line simulating nerve axon. Proc IRE 1962; 50:1061 - 2070
- Volkov AG, Deamer DW, Tanelian DL, Markin VS. Liquid Interfaces in Chemistry and Biology 1998; New York Wiley
- Roblin G. Movements, bioelectrical events and proton excretion induced in the pulvini of Mimosa pudica L. by a period of darkness. Z Pflanzenphysiol 1982; 108:295 - 304
- Umrath K. Der Erregungsvorgang bei höheren Pflancen. Erg Biol 1937; 14:1 - 42
- Tamiya T, Miyasaki T, Ishikawa H, Iruguchi N, Maki T, Matsumoto JJ, Tsuchiya T. Movement of water in conjunction with plant movement visualized by NMR imaging. J Biochem 1988; 104:5 - 8
- Moran N. Osmoregulation of leaf motor cells. FEBS Lett 2007; 581:2337 - 2347
- Balmer RT, Franks JG. Contractile characteristics of Mimosa pudica L. Plant Physiol 1975; 56:464 - 467
- Kameyama K, Kishi Y, Yoshomura M, Kanzawa N, Tsuchia T. Thyrosine phosphorylation in plant bending. Nature 2000; 407:37