Abstract
Plant cells are encased within a complex polysaccharide wall that strengthens the cell and has key roles in all aspects of plant cell growth, differentiation, and interaction with the environment. This dynamic structure is under continual modification during plant development, and its synthesis and modification require the activity of a myriad of enzymes. The mucilage secretory cells (MSCs) of the Arabidopsis thaliana seed coat provide a model for the discovery of novel genes involved in the synthesis, secretion and modification of cell wall components, particularly pectin. These cells synthesize copious amounts of pectinaceous mucilage during development and, upon hydration of the desiccated seed, the mucilage rapidly swells, bursts from the MSCs and surrounds the seed in a gelatinous capsule. Several genes affecting MSC differentiation, pectin synthesis, and mucilage release have been identified and additional genes involved in these and related processes including pectin secretion and the mechanical alteration of cell walls await to be discovered.
Introduction
The plant cell wall is a complex, extracellular matrix mediating plant interaction with both the biotic and abiotic environments. It is composed of cellulose microfibrils cross-linked by various hemicelluloses and embedded in a malleable matrix of acidic polysaccharides known as pectins. These components undergo constant dynamic modifications in order to accommodate cell growth, elongation and division. Understanding this complex harmonization of elements remains a key goal for scientists interested in the manipulation of plant cell wall structure and function.
Pectins, the most complex and heterogeneous of the cell wall components, are characterized by the presence of galacturonic acid. The three main types of pectins are homogalacturonan (HG), rhamnogalacturonan I (RG I) and rhamnogalacturonan II (RG II).Citation1 HG is made up of straight chains of α-1-4 linked galacturonic acid residues, the carboxyl groups of which can be methyl esterified. Unesterified stretches of galacturonic acid on parallel HG chains can be cross-linked via Ca2+ bridges to form a gel. The porosity of the cell wall can, therefore, be regulated by the esterification of HG as well as by the presence of branched pectins like RG I and RG II.Citation2–Citation5 RG I is composed of a backbone of alternating α-1-2-linked rhamnose and α-1-4-linked galacturonic acid residues, which is frequently branched with side chains of arabinose, galactose and arabinogalactan molecules on its rhamnose residues.Citation1,Citation4,Citation5 RG II, like HG, has a backbone of α-1-4 linked galacturonic acid, with four complex, evolutionarily-conserved sidechains containing a variety of sugars.Citation1,Citation4,Citation5 The exact degree of association and interaction between these three pectins within the cell wall is unclear.Citation2
The complexity and heterogeneity of pectin polymers, their essential role in cell-cell adhesion and the redundancy of carbohydrate active enzymes has made the investigation of pectin biosynthesis and modification challenging. New approaches that will simplify the study of these important compounds are in demand. In this regard, the epidermal cells of the Arabidopsis thaliana seed coat are interesting because they produce copious amounts of easily-accessible, dispensable pectin that is extruded from mature dry seed upon exposure to water. In recent years, these seed coat mucilage secretory cells (MSCs) have been adopted as a model for the study of both the synthesis and secretion of pectin as well as aspects of the mechanical alteration of the primary cell wall. This review will highlight recent advances, as well as the power and limitations of this model system that is rapidly gaining recognition and significance.
Differentiation of the Arabidopsis Seed Coat Epidermis
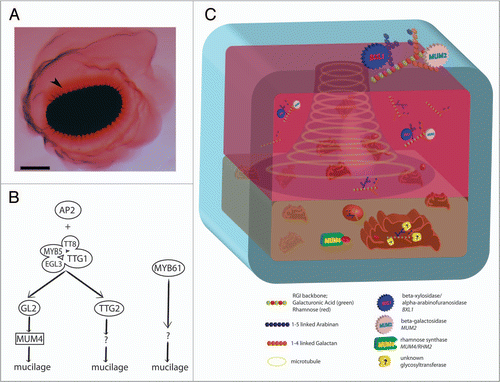
The MSCs of the Arabidopsis seed coat differentiate from the outermost layer of the ovule outer integument following fertilization.Citation6–Citation8 Initially, cells grow by vacuolar expansion. Growth is followed by the synthesis and polar secretion of large quantities of pectinaceous mucilage into the apoplast adjacent to the outer tangential primary cell wall. The secretion of the mucilage leads to cytoplasmic rearrangements that result in a volcano-shaped cytoplasmic column in the center of the cell. A volcano-shaped secondary cell wall known as the columellaCitation7 is then formed through the deposition of cell wall along the surface of the cytoplasmic column, ultimately displacing the cytoplasm toward the inner tangential side of the cell. Eventually, during the final stages of seed maturation, the cell undergoes apoptosis and the mucilage dries. Upon wetting, this hydrophilic mucilage rapidly expands, rupturing the primary cell wall and surrounding the seed.Citation7 Staining of Arabidopsis mucilage has revealed the presence of two distinct layers of mucilage, an inner, dense layer tightly associated with the seed, and an outer, water-soluble, diffuse layer (). Both layers have been shown to be comprised primarily of unbranched RG I, with lesser quantities of homogalacturonan, cellulose, xyloglucan, arabinans and galactans in the inner layer.Citation7,Citation9–Citation13 Thus Arabidopsis seed mucilage represents easily accessible and relatively homogeneous RG I.
Differentiated MSCs and mucilage have been shown to be dispensable for germination and development under laboratory conditions. The APETALA2 (AP2) gene encodes a transcription factor14 required for several developmental events including differentiation of the outer layers of the seed coat. Seed coats of plants homozygous for loss-of-function mutations in APETALA2 lack differentiated MSCs and underlying palisade cells. Consequently, ap2 seeds make little to no mucilage, but can still germinate and grow normally.Citation10,Citation15,Citation16 This dispensability of mucilage underscores the feasibility of identifying mutants with mucilage defects as an approach to identifying genes involved in pectin synthesis.
Lessons on Pectin Synthesis and its Regulation
A screen for mutants with abnormal mucilage capsules identified a set of genes, MUCILAGE-MODIFIED 1–5 (MUM1–5),Citation16 required for normal mucilage extrusion when the seed are exposed to water. One of these, the mum4 mutant, was found to have severely reduced mucilage and altered columnella shape. The cloning of MUM4 (aka RHM2) revealed that it encodes a UDP-L-rhamnose synthase that converts UDP-D-glucose to UDP-L-rhamnose, an important substrate for the synthesis of RG I, the main pectic component of mucilage ().Citation9,Citation17,Citation18 Though expressed throughout the plant, MUM4 was found to be specifically upregulated in developing seeds at the time of mucilage production, suggesting that RG I synthesis in MSCs is dependent on substrate availability.Citation9 These results demonstrate how a screen for mucilage mutants can be used for the identification of key pectin biosynthetic enzymes.
Loss-of-function mutations in a rhamnose synthase gene might be expected to result in lethality since rhamnose is such an important component of cell walls. However, even strong loss-of-function mutants of MUM4 are viable and make small amounts of mucilage RG I. This apparent paradox can be explained by redundancy and gene specialization. MUM4/RHM2 is one member of a family of three homologous genes with high sequence identity.Citation9 The other two rhamnose synthase genes (RHM1 and RHM3), like MUM4, are expressed in most tissues and presumably could provide redundant rhamnose synthase activity for most cell types in the absence of MUM4. This, however, is not the case in the MSCs where large amounts of rhamnose are required in a short time-frame and MUM4 is specifically upregulated to meet this demand. Thus, even essential genes can be identified using MSC mutant screens given redundancy and specialization. Whether mucilage mutant screens can be used to identify additional genes encoding essential enzymes remains to be determined.
The mum4 mutant has been useful as a tool for the isolation of additional mucilage mutants using a modifier screen. Mutagenesis of the mum4 mutant followed by a screen for second site mutations that enhance the phenotype identified six MUM ENHANCER mutants (men1-6), that are compromised in mucilage synthesis and release.Citation19
The ease of identifying mutations disrupting the copious mucilage produced by MSCs have made them a useful tool for reverse genetic analysis as well as forward genetics. Coincident with the cloning of the MUM4 locus using mum4 mutants by Western et al. (2004),Citation9 Usadel et al. (2004)Citation13 discovered the link between UDP-L-rhamnose and RG I synthesis in the seed coat by isolating knockout mutants for RHM2.Citation9,Citation17 More recently, the Arabidopsis GALACTURONOSYLTRANSFERASE (GAUT) gene family was investigated through the use of T-DNA insertional mutants. The appearance of reduced mucilage release and lower galacturonic acid levels in a gaut11 mutant revealed a role for GAUT11 in mucilage expansion and composition.Citation20 These mutants and others as yet to be characterized, promise to yield much more information about aspects of pectin synthesis.Citation16
The MSCs have also allowed for the elucidation of the regulation of specific aspects of mucilage synthesis. As described above, the AP2 transcription factor is required for the differentiation of the MSCs.Citation10,Citation15,Citation16,Citation21 Several other transcription factors, TRANSPARENT TESTA GLABRA1 (TTG1), TTG2, ENHANCER OF GLABRA3 (EGL3), TRANSPARENT TESTA8 (TT8), MYB5, TT2, GLABRA2 (GL2) and MYB61 have been shown to be required for mucilage synthesis since recessive mutations in any one of them result in MSCs with reduced mucilage similar to mum4 mutants.Citation22–Citation26 A considerable body of evidence suggests a regulatory pathway through which these transcription factors regulate mucilage biosynthesis (). Briefly, TTG1, a WD40 repeat protein, forms a complex with a bHLH protein (EGL3 and/or TT8) and a MYB protein (MYB5 and/or TT2) to activate the homeobox gene GL2, and the WRKY gene TTG2.Citation9,Citation20,Citation22 The R2R3 MYB gene MYB61 appears to define a separate pathway since it is not regulated by the TTG1 complex.Citation10 Expression analysis in mutant backgrounds have indicated that MUM4 is positively regulated by TTG1, GL2 and AP2, but not TTG2 and MYB61.Citation9 Thus, MUM4 defines one of the downstream targets of the TTG1/GL2 pathway in MSC cells. The targets of both TTG2 and MYB61 await discovery.
The MSC cells may also represent an avenue through which the regulatory role of hormones in pectin production can be elucidated. aba1 mutants have been shown to be affected in mucilage production and exogenous ABA treatment can be used to stimulate its synthesis.Citation27 Additionally, mutants in the GIBBERELLIN-3 OXIDASE4 (GA3OX4) gene appear to have uneven release of a reduced amount of mucilage.Citation28 The only observable defect in the development of the MSCs of ga3ox4 mutants is the delayed disappearance of starch granules, whose degradation has been implicated in the development of the central columnella.Citation7,Citation11,Citation28 The link between the delayed disappearance of starch granules in the ga3ox4 mutant and its mucilage phenotype, as well as the role of the normally antagonistic GA and ABA in pectin synthesis remains enigmatic. Future research using the MSC model will need to focus on the details of these pathways in order to determine their role in the development of MSCs, as well as pectin synthesis.
Lessons on Pectin Secretion and Targeting
Pectin biosynthesis is a Golgi-mediated process as initially shown by autoradiography.Citation29 More recent antibody work done primarily in root cap and tip-growing cells has expanded our understanding of the complexity of the Golgi apparatus and its role in pectin synthesis, though much remains unknown.Citation30–Citation32 However, unlike root cap and tip-growing cell types, the MSCs present a model system where pectin secretion occurs in copious amounts during a discrete developmental period and is targeted to a specific domain of the apoplast. As such, the MSCs represent an excellent platform from which to study pectin secretion as well as cell wall deposition. Indeed, using light and electron microscopy, a recent study of the changes in the endomembrane system during MSC differentiation underscores its potential.Citation13
Mucilage synthesis in MSCs begins at approximately 5 days post anthesis (dpa), and concludes by 9 dpa with secondary cell wall deposition having begun by that time.Citation7,Citation13 As one might expect for active secretory cells, significant changes in the number, density, morphology and cargo of Golgi can be detected in 7 dpa MSCs compared with the non-secreting cells of 4 dpa.Citation13 Golgi morphology changes dramatically in this period with 7 dpa Golgi having more electron-dense stacks with shorter diameter, swollen cis cisternae and a much more extensive TGN than 4 dpa MSCs.Citation13 Concomitant with the changes in morphology is the appearance of mucilage in most of the Golgi stacks throughout the MSCs. Indeed, the changes in Golgi morphology are probably a consequence of the increase in secretion of mucilage since they did not occur in the mum4 mutant defective in mucilage biosynthesis. In addition to the changes in Golgi appearance, there is a two-fold increase in Golgi number following 4 dpa, presumably to allow for the large volume of mucilage produced in a short time period. The increase in Golgi appears to be an integral part of the differentiation process since it occurs independently of the amount of mucilage made by the cell.
During mucilage secretion, the mucilage is targeted to a specific domain of the apoplast—the junction of the outer tangential and radial cell walls—to form the distinctive ring-shaped mucilage pocket.Citation7 Interestingly, unlike in root hairs and pollen tubes where Golgi cluster at the site of deposition, the mucilage- containing Golgi of the MSCs are distributed relatively evenly throughout the cytoplasm (). At present it is unclear how the mucilage is deposited at the site of the mucilage pocket but the answer to the question promises to be novel and interesting.
In a examination of MSC structure, microtubules were found to line the entire mucilage pocket ().Citation33 Tracking mucilage production revealed electron dense vesicles carrying the RG I antigen amongst the microtubules spanning the mucilage domain. By contrast, anti-actin immunofluorescence showed actin microfilaments throughout the cytoplasm with no apparent concentration around the mucilage pocket domain, or within the cell at all.Citation33 These results suggested that the microtubules might be involved in vesicle transport to the mucilage pocket. To test this hypothesis, MSCs were examined in the mor1-1 mutant, whose microtubules lose organization when grown at high temperatures.Citation34,Citation35 Although release of mucilage from seed coats was partially disrupted, the secretion of mucilage did not appear to be significantly affected in mor1-1 MSCs. Thus, the role of microtubules lining the mucilage pocket of MSCs remains enigmatic.
While the MSC system has proved useful in elucidating aspects of pectin secretion, there remain a number of difficulties in the study of the Golgi apparatus and cytoskeleton in these cells. First, the location of the seed within the silique makes it difficult to observe the in vivo development of the MSCs and accompanying changes in Golgi and cytoskeletal architecture. This difficulty is compounded by the presence of starch granules that hinder dissection with fluorescence microscopy. Finally, due to the technical challenges of delivering cytoskeleton disrupting drugs to the seed and the MSCs, further investigation is required to determine the role of these two cytoskeletal components in MSC development and mucilage production.
Lessons on Post-Synthesis Pectin Modification in Muro
The plant cell wall is a dynamic structure whose constant modification is necessary in order for plant cells to grow and divide. Several recent studies have demonstrated the utility of the MSCs as a model for identifying genes involved in cell wall modification. Four mutants with defects in mucilage release upon hydration have been identified: mum1, mum2, atbxl1 and atsbt1.7.Citation16,Citation36–Citation38 Their phenotypes demonstrate that mucilage synthesis and secretion to the apoplast is not sufficient to guarantee extrusion of mucilage when seeds are exposed to water. The characterization of these mutants and the cloning of three of the genes responsible (MUM2, AtBXL1 and AtSBT1.7) have provided evidence that the gene products are involved in structural modifications of either the primary cell wall and/or mucilage necessary for proper cell wall breakage and mucilage swelling.
mum2 mutant seeds are distinguished by the complete lack of extruded mucilage when hydrated. However, sectioning of developing seeds and monosaccharide analysis of whole seeds showed the mutant to be indistinguishable from wild type with respect to mucilage deposition and columella formation.Citation36,Citation38 When mature seeds were embedded and sectioned, effectively removing the primary cell wall as a barrier, mum2 mucilage failed to expand when exposed to water while that of wild type showed normal extrusion. Thus, mum2 mucilage appeared to be affected in its ability to hydrate and expand. The MUM2 gene encodes a β-galactosidase that is secreted to the apoplast.Citation36,Citation38 Chemical analysis of extracted mum2 and wild type mucilage showed changes consistent with the idea that mum2 mucilage has altered RG I that contains more arabinan side chains and terminal galactose residues than in wild type mucilage.Citation36,Citation38 Taken together the results suggest that removal of the RG I arabinogalactan side chains of mucilage following secretion to the apoplast is required for normal hydration and expansion properties of mucilage. Though this data presents a strong case for the necessity of mucilage modification for release, the possibility of concomitant cell wall modification could not be eliminated.
Similar to mum2 mutants, atbxl1 mutants showed a consistent decrease in released mucilage upon hydration, while sectioning of developing seeds showed seemingly wild type mucilage secretion and columella formation. AtBXL1 was found to encode a bi-functional β-xylosidase/α-arabinofuranosidase, which is expressed throughout the plant including the developing seed.Citation39–Citation41 Chemical analysis of extracted mucilage indicated increases in α-1-5 linked arabinans, a neutral side chain on the main pectic component RG I.Citation41 Immunofluorescence on sectioned MSCs also indicated an increase of α-1-5 linked arabinans in the primary cell walls.Citation41 Treating mutant seeds with an exogenous α-arabinofuranosidase was able to promote wild type mucilage release. While it is clear that the action of an α-arabinofuranosidase is required for proper mucilage release, it remains unclear whether the trimming of arabinan side chains is necessary for the weakening of the primary cell wall to facilitate breakage, the expansion properties of the mucilage itself needed for extrusion, or both.
In addition to leading to the identification and functional analysis of pectin modification enzymes, the MSC model has allowed for the identification of factors involved in the regulation of pectin modification. The recently identified atsbt1.7 mutant provides insight into the control of pectin modification required for mucilage release.Citation37 As with the mum2 and atbxl1 mutants, there appear to be no developmental differences between atsbt1.7 and wild type seeds that could account for the observed decrease in mucilage extrusion in the mutant. The atsbt1.7 mucilage did, however, show a decrease in mucilage methylation, a change that was correlated with increased pectin methyl esterase (PME) activity.Citation3,Citation11,Citation12,Citation37 Thus the subtilisin-like serine protease encoded by AtSBT1.7 may impact mucilage extrusion by directly degrading a PME, or through proteolytic cleavage to activate a PME inhibitor and thus ultimately preventing excessive de-methylesterification of the mucilage and/or the primary cell wall.
The discovery and characterization of MUM2, AtBXL1 and AtSBT1.7 has provided a glimpse into the active and complex modifications required for mucilage release. Although the exact targets of the enzymes encoded by these genes remains to be elucidated, it is not difficult to imagine them working in concert in both the cell wall and mucilage in order to bring about the optimal conditions for mucilage release. Both MUM2 and BXL1 modify RG I, the main component of mucilage, removing branches which may strengthen interactions with cellulose in the mucilage.Citation42 Such interactions with cellulose may be necessary during secretion and early deposition of the mucilage in the apoplast after which the side chains are removed by MUM2 and BXL1 to allow extrusion during hydration. Indeed, the increase of arabinans in the mum2 mucilage could be interpreted to mean that the removal of galactose by MUM2 is required in order for BXL1 to reach its target. The noted increase in anti-arabinan label at 7 dpa in the atbxl1 cell wall suggests that these modifications may indeed be occurring relatively quickly after mucilage deposition.Citation41 The action of SBT1.7 could affect the porosity of the mucilage allowing access of modification enzymes like MUM2 and BXL1. Similarly, these enzymes could also be affecting the strength of the MSCs' primary cell wall, weakening it sufficiently to allow the expanding mucilage to break through.
The MSC model has led to the identification of pectin modification enzymes and allowed researchers to observe their effects on properties of the cell wall, as well as demonstrated factors that are involved in the regulation of pectin modification. However, the variety of pectin synthesizing and modifying genes that can be studied may be limited by the forms of pectins present in the MSCs' walls and the types of modifications required for mucilage extrusion. Additionally, the structure of the seed coat is such that biophysical tests are less tenable compared to linear or planar plant structures such as stems or leaves. This makes it more difficult to directly assess the effects novel modification enzymes have on the mechanical properties of the cell wall.
Conclusions and Future Directions
The MSC model has provided new insight into the complexities of pectin production as well as cell wall modification. The dispensability of mucilage and MSCs under laboratory conditions allows a convenient and rapid means of identifying mutants affected in pectin synthesis, its regulation and release. As a result, genes encoding proteins involved in pectin synthesis, including biosynthetic enzymes and transcription factors that regulate them, have been identified. The MSC model also presents a unique cell type in which targeted secretion of vast amounts of pectin occurs in the absence of cell growth. This has allowed for the analysis of targeted pectin secretion with regard to the Golgi apparatus. Finally, the system has led to the identification of genes whose products act to modify pectin in the mucilage or cell wall, or both, in order for the mucilage to be extruded upon hydration of the seed. These findings have provided insight into the effects of pectin modification on cell wall and mucilage properties.
The future holds promise for more revelations from the MSC model concerning pectin biosynthesis and modification, secretion and secondary cell wall biosynthesis. There are novel mutants currently under investigation including mum1, mum3, mum5 and the MEN mutants (men1-6);Citation16,Citation19 and additional forward and reverse genetics screens are underway. Tools under development include a seed coat microarray analysis that will enable the identification of targets for reverse genetic analysis and MSC specific promoters that will allow manipulation of MSCs without detrimental effects to the rest of the plant. With each new piece of information, the value of the MSCs as a model grows. The full potential of MSCs as a model therefore has yet to be realized.
Abbreviations
| HG | = | homogalacturonan |
| RG I | = | rhamnogalacturonan I |
| RG II | = | rhamnogalacturonan II |
| MSCs | = | mucilage secretory cells |
| GA | = | gibberellic acid |
| ABA | = | abscisic acid |
| TGN | = | trans-golgi network |
| PME | = | pectin methylesterase |
Figures and Tables
Figure 1 (A) Arabidopsis seed with released mucilage. Upon hydration the hydrophilic mucilage breaks out of the MSCs and surrounds the seed. Staining with ruthenium red reveals a dense mucilage layer associated with the seed (arrowhead) surrounded by a more diffuse, less cohesive layer. Bar = 100 µm. (B) Proposed regulatory pathway of mucilage production. During MSC development, both AP2 and TTG1 regulate mucilage production through the upregulation of downstream transcription factors (GL2 and TTG2). TTG1 acts as a complex with the bHLH proteins EGL3 and TT8, and the R2R3 MYBs MYB5 and TT2. GL2 upregulates the rhamnose synthase MUM4, while the downstream targets of TTG2 remain unknown. A third, independent pathway of mucilage production exists under the control of MYB61. (C) The Arabidopsis mucilage secretory cell during mucilage production. At approximately 6–7 dpa, pectin synthesis begins in the MSC. At this stage, the Golgi apparatus and derivative secretory vesicles are distributed throughout the cytoplasm. nucleotide sugar interconversion enzymes embedded in the Golgi membrane or in the cytoplasm synthesize NDP-sugars, which are then transported into the Golgi lumen (sugar transporters not shown). A rhamnose synthase (MUM4/RHM2) is shown synthesizing UDP-L-rhamnose. As yet unidentified GTs in the Golgi lumen assemble pectins (RG I shown; GAUT11 is possible candidate). These polysaccharides are packaged into vesicles and secreted into the extracellular space as part of the mucilage. The apoplastic, mucilage-containing pocket forms a ring around cytoplasm in the apical portion of the cell, leading to a volcano-shaped cytoplasm constrained to a central column and basal portion of the cell. Hydrolytic enzymes (BXL1 and MUM2) modify RG I sidechains in the apoplast. it is possible that this modification occurs in the mucilage and the cell wall, facilitating mucilage release.
Acknowledgements
This work was supported by Discovery Grants from the Natural Science and Engineering Research Council of Canada to T.L.W. and G.W.H.
References
- Somerville CR, Bauer S, Brininstool G, Facette M, Hamann T, Milne J, et al. Toward a systems approach to understanding plant cell walls. Science 2004; 306:2206 - 2211
- Vincken J-P, Schols HA, Oomen RJFJ, McCann M, Ulvskov P, Voragen AGJ, et al. If homogalacturonan were a side chain of rhamnogalacturonan I. Implications for cell wall architecture. Plant Physiol 2003; 132:1781 - 1789
- Willats WGT, Orfila C, Limberg G, Buchholt HC, van Alebeek G-JWM, Voragen AGJ, et al. Modulation of the degree and pattern of methyl-esterification of pectic homogalacturonan in plant cell walls. J Biol Chem 2001; 276:19404 - 19413
- Mohnen D. Pectin structure and biosynthesis. Curr Opin Plant Biol 2008; 11:266 - 277
- Willats WGT, McCartney L, Mackie W, Knox JP. Pectin: cell biology and prospects for functional analysis. Plant Mol Biol 2001; 47:9 - 27
- Beeckman T, De Rycke R, Viane R, Inze D. Histological study of seed coat development in Arabidopsis thaliana. J Plant Res 2000; 113:139 - 148
- Western TL, Skinner DJ, Haughn GW. Differentiation of mucilage secretory cells of the Arabidopsis seed coat. Plant Physiol 2000; 122:345 - 355
- Windsor JB, Symonds VV, Mendenhall J, Lloyd AL. Arabidopsis seed coat development: morphological differentiation of the outer integument. Plant J 2000; 22:483 - 493
- Western TL, Young DS, Dean GH, Tan WL, Samuels AL, Haughn GW. MUCILAGE-MODIFIED4 encodes a putative pectin biosynthetic enzyme developmentally regulated by APETALA2, TRANSPARENT TESTA GLABRA1 and GLABRA2 in the Arabidopsis seed coat. Plant Physiol 2004; 134:296 - 306
- Penfield S, Meissner RC, Shoue DA, Carpita NC, Bevan MW. MYB61 is required for mucilage deposition and extrusion in the Arabidopsis seed coat. Plant Cell 2001; 13:2777 - 2791
- Willats WGT, McCartney L, Knox JP. In-situ analysis of pectic polysaccharides in seed mucilage and at the root surface of Arabidopsis thaliana. Planta 2001; 213:37 - 44
- Macquet A, Ralet M-C, Kronenberger J, Marion-Poll A, North HM. In situ, chemical and macromolecular study of the composition of Arabidopsis thaliana seed coat mucilage. Plant Cell Physiol 2007; 48:984 - 999
- Young RE, McFarlane HE, Hahn MG, Western TL, Haughn GW, Samuels AL. Analysis of the golgi apparatus in Arabidopsis seed coat cells during polarized secretion of pectin-rich mucilage. Plant Cell 2008; 20:1623 - 1638
- Kunst L, Klenz JE, Martinez-Zapater J, Haughn GW. AP2 gene determines the identity of perianth organs in flowers of Arabidopsis thaliana. Plant Cell 1989; 1:1195 - 1208
- Koornneef M. The complex syndrome of TTG mutants. Arabidopsis Inf Serv 1981; 18:45 - 51
- Western TL, Burn J, Tan WL, Skinner DJ, Martin-McCaffrey L, Moffatt BA, et al. Isolation and characterization of mutants defective in seed coat mucilage secretory cell development in Arabidopsis. Plant Physiol 2001; 127:998 - 1011
- Usadel B, Kuchinsky AM, Rosso MG, Eckermann N, Pauly M. RHM2 is involved in mucilage pectin synthesis and is required for the development of the seed coat in Arabidopsis. Plant Physiol 2004; 134:286 - 295
- Oka T, Nemoto T, Jigami Y. Functional analysis of Arabidopsis thaliana RHM2/MUM4, a multidomain protein involved in UDP-d-glucose to UDP-l-rhamnose conversion. J Biol Chem 2007; 282:5389 - 5403
- Arsovski AA, Villota MM, Rowland O, Subramaniam R, Western TL. MUM ENHANCERS are important for seed coat mucilage production and mucilage secretory cell differentiation in Arabidopsis thaliana. J Exp Bot 2009; 60:2601 - 2612
- Caffall KH, Pattathil S, Phillips SE, Hahn MG, Mohnen D. Arabidopsis thaliana T-DNA mutants implicate GAUT genes in the biosynthesis of pectin and xylan in cell walls and seed testa. Mol Plant 2009; 2:1000 - 1014
- Jofuku KD, den Boer BGW, Montagu MV, Okamuro JK. Control of arabidopsis flower and seed development by the homeotic gene APETALA2. Plant Cell 1994; 6:1211 - 1225
- Gonzalez A, Mendenhall J, Huo Y, Lloyd A. TTG1 complex MYBs, MYB5 and TT2, control outer seed coat differentiation. Dev Biol 2009; 325:412 - 421
- Li SF, Milliken ON, Pham H, Seyit R, Napoli R, Preston J, et al. The Arabidopsis MYB5 transcription factor regulates mucilage synthesis, seed coat development and trichome morphogenesis. Plant Cell 2009; 21:72 - 89
- Johnson CS, Kolevski B, Smyth DR. TRANSPARENT TESTA GLABRA2, a trichome and seed coat development gene of Arabidopsis, encodes a WRKY transcription factor. Plant Cell 2002; 14:1359 - 1375
- Zhang F, Gonzalez A, Zhao M, Payne CT, Lloyd A. A network of redundant bHLH proteins functions in all TTG1-dependent pathways of Arabidopsis. Development 2003; 130:4859 - 4869
- Rerie WG, Feldmann KA, Marks DM. The GLABRA2 gene encodes a homeodomain protein required for normal trichome development in Arabidopsis. Gen Dev 1994; 8:1388 - 1399
- Karssen CM, Brinkhorst-van der Swan DLC, Breekland AE, Koornneef M. Induction of dormancy during seed development by endogenous abscisic acid: studies on abscisic acid deficient genotypes of Arabidopsis thaliana (L.) Hehnh. Planta 1983; 157:158 - 165
- Kim Y-C, Nakajima M, Nakayama A, Yamaguchi I. Contribution of gibberellins to the formation of Arabidopsis seed coat through starch degradation. Plant Cell Physiology 2005; 46:1317 - 1325
- Northcote D, Pickett-Heaps J. A function of the Golgi apparatus in polysaccharide synthesis and transport in the root-cap cells of wheat. Biochem J 1966; 98:159 - 167
- Zhang GF, Staehelin LA. Functional compartmentation of the Golgi apparatus of plant cells. Immunocytochemical analysis of high-pressure frozen-and freeze-substituted Sycamore maple suspension culture cells. Plant Physiol 1992; 99:1070 - 1083
- Staehelin LA, Giddings TH, Kiss JZ, Sack FD. Macromolecular differentiation of Golgi stacks in root tips of Arabidopsis and Nicotiana seedlings as visualized in high pressure frozen and freeze-substituted samples. Protoplasma 1990; 157:75 - 91
- Staehelin LA, Moore I. The plant Golgi apparatus: structure, functional organization and trafficking mechanisms. Annu Rev Plant Physiol Plant Mol Biol 1995; 46:261 - 288
- McFarlane H, Young R, Wasteneys G, Samuels A. Cortical microtubules mark the mucilage secretion domain of the plasma membrane in Arabidopsis seed coat cells. Planta 2008; 227:1363 - 1375
- Whittington AT, Vugrek O, Wei KJ, Hasenbein NG, Sugimoto K, Rashbrooke MC, et al. MOR1 is essential for organizing cortical microtubules in plants. Nature 2001; 411:610 - 613
- Kawamura E, Himmelspach R, Rashbrooke MC, Whittington AT, Gale KR, Collings DA, et al. MICROTUBULE ORGANIZATION 1 regulates structure and function of microtubule arrays during mitosis and cytokinesis in the Arabidopsis root. Plant Physiol 2006; 140:102 - 114
- Dean GH, Zheng H, Tewari J, Huang J, Young DS, Hwang YT, et al. The Arabidopsis MUM2 gene encodes a beta-galactosidase required for the production of seed coat mucilage with correct hydration properties. Plant Cell 2007; 19:4007 - 4021
- Rautengarten C, Usadel B, Neumetzler L, Hartmann J, Büssis D, Altmann T. A subtilisin-like serine protease essential for mucilage release from Arabidopsis seed coats. Plant J 2008; 54:466 - 480
- Macquet A, Ralet MC, Oudet O, Kronenberger J, Mouille G, Marion-Poll A, et al. A naturally occurring mutation in an Arabidopsis accession affects a beta-d-galactosidase that increases the hydrophilic potential of rhamnogalacturonan I in seed mucilage. Plant Cell 2007; 19:3990 - 4006
- Goujon T, Minic Z, El Amrani A, Lerouxel O, Aletti E, Lapierre C, et al. AtBXL1, a novel higher plant (Arabidopsis thaliana) putative beta-xylosidase gene, is involved in secondary cell wall metabolism and plant development. Plant J 2003; 33:677 - 690
- Minic Z, Rihouey C, Do CT, Lerouge P, Jouanin L. Purification and characterization of enzymes exhibiting beta-d-xylosidase activities in stem tissues of Arabidopsis. Plant Physiol 2004; 135:867 - 878
- Arsovski AA, Popma TM, Haughn GW, Carpita NC, McCann MC, Western TL. AtBXL1 encodes a bifunctional beta-d-xylosidase/alpha-l-arabinofuranosidase required for pectic arabinan modification in Arabidopsis mucilage secretory cells. Plant Physiol 2009; 150:1219 - 1234
- Zykwinska AW, Ralet M-CJ, Garnier CD, Thibault J-FJ. Evidence for in vitro binding of pectin side chains to cellulose. Plant Physiol 2005; 139:397 - 407