Abstract
Lateral root (LR) stimulation during early signal exchange between plant roots and ectomycorrhizal (ECM) fungi has recently been shown to be achieved by modulation of auxin gradients. We suggested that this modulation could occur through altered polar auxin transport (PAT) and through activation of auxin signalling pathways in the root. However, it remains unclear, which fungal molecules alter auxin pathways inside the plant partner. It has been suggested in previous studies that auxin released by the fungus could trigger observed plant responses during early signal exchange and later on during root colonization. Here we focus on the early interaction and we provide evidence for an alternative mechanism. Indeed, LR stimulation by the fungus in A. thaliana followed a totally different timing than with exogenously applied auxin. Furthermore, experimental conditions that excluded the exchange of soluble molecules while allowing exchange of volatile(s) between the plant and the fungus were sufficient for LR induction, therefore questioning the role of secreted fungal auxin. These data suggest that volatiles released by the fungus and sensed by the plant may act upstream of altered auxin signalling in the plant.
Interactions of plant roots with symbiotic, ectomycorrhizal soil fungi lead to lateral root (LR) stimulation during the very early interaction phase.Citation1 This LR stimulation has recently been shown to be independent of root colonization and to occur as well in non-mycorrhizal plants, such as Arabidopsis suggesting that fungal signals have a broad perception spectrum.Citation1,Citation2 However, little is known about the type of signals exchanged between fungi and their plant partners during this early interaction phase. Several studies have proposed a role for the phytohormone auxin produced and secreted by ECM fungi as the signalling molecule during ECM fungus/plant signaling.Citation2–Citation7 Recently we studied changes in auxin response and auxin transport in poplar and Arabidopsis thaliana roots during contact with the ECM fungus Laccaria bicolor.Citation1 We demonstrated that the presence of the fungus enhances the auxin response and distribution at the root apex and that this, as well as LR stimulation, is reliant on polar auxin transport through AtPIN2 and probably through PtPIN9 in poplar. Here, using Arabidopsis thaliana, whose LR stimulation by Laccaria bicolor has been demonstrated, we propose that not yet identified fungal volatiles may regulate auxin homeostasis in the plant, questioning the contribution of the auxin released by the fungus on the induction of LR.
Exogenous Auxin and the Presence of Laccaria bicolor Differently Affect Lateral Root Formation in Arabidopsis thaliana
Laccaria is known to secrete indole-3-acetic acid (IAA), but the amount is rather low (about 10 nM in four week old liquid Laccania cultures).Citation8 In order to investigate whether an exogenous auxin treatment could quantitatively and qualitatively mimic LR stimulation observed during Arabidopsis/Laccaria interaction, we transferred Arabidopsis seedlings at 5 days after germination (DAG) to Petri dishes containing different concentrations of IAA and/or covered the seedlings' roots with Laccaria mycelium grown on cellophane membranes (reviewed in ref. Citation1). Applying low amounts of auxin (such as those released by the fungusCitation8) to Arabidopsis seedlings did not alter LR formation (data not shown). However it is possible that fungal auxin is heterogeneously distributed in the culture medium, which might result in an underestimation of fungal auxin in the direct vicinity of the root when auxin is quantified from the entire medium.Citation8 This is why higher concentrations, known to influence LR formation (1 and 10 µM), were also exogenously applied. Both concentrations led to rapid induction of LRs already detectable after one day () and this stimulation lasted up to two days before reaching a plateau. Unlike IAA treatments, LR induction by Laccaria increased more slowly but continuously, with the first significant difference being observable after two days. After six days, the LR number on plants in contact with the fungus reached similar values as for plants treated with 1 µM IAA and after eight days the number of LRs in the fungal treatment exceeded LR stimulation with 10 µM IAA. Furthermore, exogenous IAA treatments caused rapid (after one day) and enduring arrest of primary root growth in Arabidopsis, whereas the sole application of the fungus did not interfere with root elongation for up to eight days (data not shown).
We next analyzed the outcome of a combined treatment of Arabidopsis seedlings with Laccaria and a high exogenous auxin concentration (10 µM). Interestingly, the effect of both exogenous IAA and fungus on LR development appeared additive. After eight days significantly more LRs had developed in combined IAA plus fungus treatments compared to IAA treatment alone (). LRs developing in the combined treatment emerged as secondary order LRs (arrowheads in ).
As exogenous IAA did not mimic LR stimulation by the fungus and as high IAA concentrations applied in parallel to the fungus had a synergistic outcome, we questioned whether the LR stimulating signalling molecule released by the fungus could be different from auxin.
Volatiles Released by Laccaria bicolor are Sufficient to Stimulate LR Formation in Arabidopsis thaliana
To evaluate whether Laccaria could also stimulate LR formation in Arabidopsis when exchange of soluble molecules (such as fungal auxin) was prevented, we exposed Arabidopsis seedlings at 5 DAG in a bi-compartmented plate to volatiles released by Laccaria (). These conditions resulted in a significant LR increase compared to plants growing in control plates without fungus (). The pattern and quantity of LR stimulation was similar to LR stimulation observed during indirect contact (). This result demonstrates that fungal volatiles alone are sufficient to induce LR stimulation in the plant partner and may therefore be part of the early signalling molecules released by the fungus. Thus, fungal auxin is unlikely the (unique) trigger inducing LR formation.
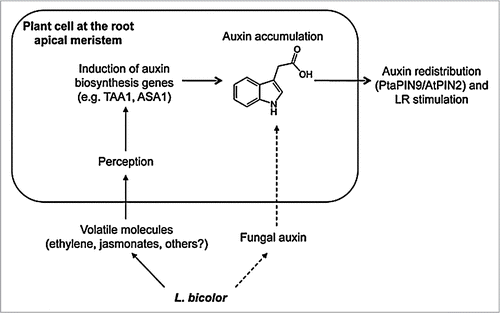
Which volatile fungal molecules could we consider as LR inducers? First, the gas ethylene released by Laccariaand other ECM fungi has already been proposed to play a role in fungal signalling.Citation2,Citation9 Furthermore, it has been demonstrated that fungi can produce jasmonate derivatives,Citation10 which may be involved in volatile signalling. Interestingly, exogenously applied ethylene and jasmonates interact with auxin pathways by activating auxin biosynthetic enzymes (Anthranilate Synthase 1—ASA1) and (Tryptophane Amino Transferase—TAA1) in roots and hence stimulate LR formation.Citation11–Citation13 Thus it may be taken into consideration that the increased auxin response in roots observed during contact with LaccariaCitation1 may be a result of an enhanced auxin biosynthesis in planta in response to fungal volatiles rather than only due to an uptake of fungal auxin itself (). Further analysis on the different compounds released by the fungus that could play a role in primary responses as well as auxin biosynthesis activity inside roots will certainly help to confirm this hypothesis.
Figures and Tables
Figure 1 Comparison of LR stimulation in Arabidopsis thaliana in contact with Laccaria bicolor and during exogenous IAA treatment. (A) LR development on 5 DAG Arabidopsis seedlings covered by a cellophane membrane with or without fungal mycelium or in the presence of 1 or 10 µM IAA. IAA rapidly stimulated LR development but led after two days to a plateau whereas LR stimulation by Laccaria is slower but persists over the first ten days. (B) LR development after 8 days of 10 µM IAA treatment. (C) LR development after 8 days indirect contact in the presence of 10 µM IAA. Note the high number of second degree LRs in (C) (arrows) that are absent in (B). LR stimulation in Arabidopsis by volatile molecules released by Laccaria mycelia. (D) Two-compartmented plate with Arabidopsis seedlings (left) and Laccaria (right). (E) LR development in the presence of volatiles released by Laccaria. Compared to controls, LR development was stimulated from three days of co-culturing with mycelium. Per treatment 15 to 25 (A–C) and 50 seedlings (E) were analyzed respectively. Different letters indicate significant difference between the respective conditions at each time-point (A and E) (Student's t-test, p < 0.05).
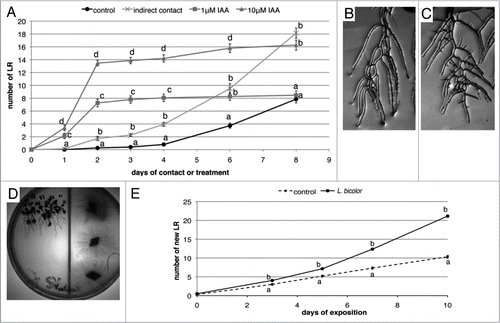
Figure 2 Hypothetical model of early L. bicolor/root signaling. The fungus releases some volatiles in addition to small amounts of auxin. Volatiles such as ethylene and jasmonate may be perceived by plant cells therefore activating the transcription of auxin biosynthesis genes, for instance Anthranilate synthase ASA1 or Tryptophane Amino Transferase TAA1. The activity of these genes could increase endogenous auxin levels in root apex. If present, fungal auxin may enrich this pool additionally. Increasing auxin levels may activate polar auxin transport and control redistribution of auxin, which will lead ultimately to LR stimulation.
Acknowledgements
We are grateful to Krista Plett for proofreading the final version of the manuscript. We thank Zachary Gaudin for help with experiments and Francis Martin for critical reading. This work has been supported by the European Commission within the Seventh Framework Program for Research, Project Energypoplar (FP7-211917), the French Space Agency (CNES) and the EVOLTREE network of excellence.
Addendum to:
References
- Felten J, Kohler A, Morin E, Bhalerao RP, Palme K, Martin F, et al. The ectomycorrhizal fungus Laccaria bicolor stimulates lateral root formation in poplar and Arabidopsis through auxin transport and signaling. Plant Physiol 2009; 151:1991 - 2005
- Splivallo R, Fischer U, Gobel C, Feussner I, Karlovsky P. Truffles regulate plant root morphogenesis via the production of auxin and ethylene. Plant Physiol 2009; 150:2018 - 2029
- Ek M, Ljungquist PO, Stenström E. Indole-3-acetic acid production by mycorrhizal fungi determined by gas chromatography-mass spectrometry. New Phytol 1983; 94:401 - 407
- Gay G, Normand L, Marmeisse R, Sotta B, Debaud JC. Auxin overproducer mutants of Hebeloma cylindrosporum Romagnesi have increased mycorrhizal activity. New Phytol 1994; 128:645 - 657
- Gea L, Normand L, Vian B, Gay G. Structural aspects of ectomycorrhiza of Pinus pinaster (Ait.) Sol. formed by an IAA-overproducer mutant of Hebeloma cylindrosporum Romagnesi. New Phytol 1994; 128:659 - 670
- Niemi K, Vuorinen T, Ernstsen A, Haggman H. Ectomycorrhizal fungi and exogenous auxins influence root and mycorrhiza formation of Scots pine hypocotyl cuttings in vitro. Tree Physiol 2002; 22:1231 - 1239
- Tranvan H, Habricot Y, Jeannette E, Gay G, Sotta B. Dynamics of symbiotic establishment between an IAA-overproducing mutant of the ectomycorrhizal fungus Hebeloma cylindrosporum and Pinus pinaster. Tree Physiol 2000; 20:123 - 129
- Karabaghli-Degron C, Sotta B, Bonnet M, Gay G, Le Tacon F. The auxin transport inhibitor 2,3,5-tri-iodobenzoic acid (TIBA) inhibits the stimulation of in vitro lateral root formation and the colonization of the tap-root cortex of Norway spruce (Picea abies) seedlings by the ectomycorrhizal fungus Laccaria bicolor. New Phytol 1998; 140:723 - 733
- Rupp LA, DeVries HE, Mudge KW. Effect of aminocyclopropane carboxylic acid and aminoethoxyvinylglycine on ethylene production by ectomycorrhizal fungi. Can J Bot 1989; 67:483 - 485
- Miersch O, Porzel A, Wasternack C. Microbial conversion of jasmonates-hydroxylations by Aspergillus niger. Phytochemistry 1999; 50:1147 - 1152
- Ivanchenko MG, Muday GK, Dubrovsky JG. Ethylene-auxin interactions regulate lateral root initiation and emergence in Arabidopsis thaliana. Plant J 2008; 55:335 - 347
- Stepanova AN, Robertson-Hoyt J, Yun J, Benavente LM, Xie DY, Dolezal K, et al. TAA1-mediated auxin biosynthesis is essential for hormone crosstalk and plant development. Cell 2008; 133:177 - 191
- Sun J, Xu Y, Ye S, Jiang H, Chen Q, Liu F, et al. Arabidopsis ASA1 is important for jasmonate-mediated regulation of auxin biosynthesis and transport during lateral root formation. Plant Cell 2009; 21:1495 - 1511