Abstract
Ipecac alkaloids produced in the medicinal plant Psychotria ipecacuanha such as emetine and cephaeline possess a monoterpenoid-tetrahydroisoquinoline skeleton, which is formed by condensation of dopamine and secologanin. The condensation products are deglucosylated, and the resulting aglycon is further processed to protoemetine, which is condensed with the second molecule of dopamine, followed by conversion into cephaeline and emetine. Although four hydroxy groups derived from two molecules of dopamine need to be methylated to form emetine, the order of O-methylation reactions had been veiled. We recently identified three Ipecac alkaloid O-methyltransferases (IpeOMT1-IpeOMT3) that are sufficient for catalyzing O-methylations of all four hydroxy groups. Detailed characterization of their catalytic properties with integration of that of the previously identified Ipecac alkaloid β-glucosidase (IpeGlu1) revealed a large portion of the biosynthetic pathway of Ipecac alkaloids. The results provide proof-of-concept to the significance and the usefulness of the biosynthetic pathway strategy by EST analysis coupled with recombinant enzyme characterization. At the same time, however, the results raised an intriguing question about the subcellular network between the biosynthetic enzymes and intermediates. Here, we provide additional discussion about this point, and indicate what remains to be elucidated.
Plants produce a large variety of secondary metabolites. Due to their biological activities, many are considered to have roles in chemical defense against pathogens and herbivores. Some are also beneficial to humans because of their pharmacological activities. Alkaloids are one of the better utilized classes of plant natural products. The medicinal plant Psychotria ipecacuanha accumulates large amount of alkaloids known as ‘Ipecac alkaloids’; the roots have long been used as an emetic, expectorant and later as a medication for amebic dysentery.Citation1 Such medicinal effects of the root extracts derive from the principal alkaloid emetine. In addition, many kinds of emetine-related alkaloids such as cephaeline and ipecoside are also produced in P. ipecacuanha ().Citation1–Citation3 The biosynthetic pathway starts from the condensation of dopamine and secologanin. The condensation products are deglucosylated, and the resulting aglycon is further processed to protoemetine, which is condensed with the second molecule of dopamine, followed by conversion into cephaeline and emetine.
To identify the Ipecac alkaloid biosynthetic genes, a cDNA library was constructed from a P. ipecacuanha root culture that efficiently produced Ipecac alkaloids and was sequenced to collect 1,050 expressed sequence tags (ESTs).Citation4 Homology searches of those ESTs allowed us to identify candidates for Ipecac alkaloid biosynthetic genes. Full-length cDNAs were isolated and recombinant enzymes were assayed for substrate specificity and steady-state kinetic parameters. The strategy allowed us to rapidly elucidate a large portion of the biosynthetic pathway in two consecutive studies.Citation4,Citation5 We first reported the identification of Ipecac alkaloid β-glucosidase (IpeGlu1) that hydrolyzes glucosylated Ipecac alkaloids, including N-deacetylisoipecoside, an intermediate of emetine biosynthesis.Citation4 Then, recently we reported the identification of three O-methyltransferases (IpeOMT1-IpeOMT3) of which the enzymatic activities are sufficient to methylate all four hydroxy groups of the two isoquinoline moieties to form emetine, as well as to form all Ipecac alkaloid derivatives having distinct methylation patterns ().Citation5 The results indicated that the EST database of Psychotria root culture constructed is enriched in Ipecac alkaloid biosynthetic genes. A similar strategy has also achieved considerable success in isolation of alkaloid biosynthetic genes in opium poppy (Papaver somniferum) and coptis (Coptis japonica),Citation6–Citation11 showing the usefulness of an EST-sequencing approach coupled with the characterization of recombinant enzymes in terms of substrate specificity and kinetic properties. We have demonstrated that the transcript profiles of IpeOMTs and Ipeglu1 are well correlated,Citation5 suggesting that other unidentified genes of Ipecac alkaloid biosynthesis are also transcribed in a concerted manner. Deep transcriptome analysis with next-generation sequencing and subsequent data mining based on co-expression with IpeOMT and Ipeglu1 genes would enable the complete identification of the remaining Ipecac alkaloid biosynthetic genes.
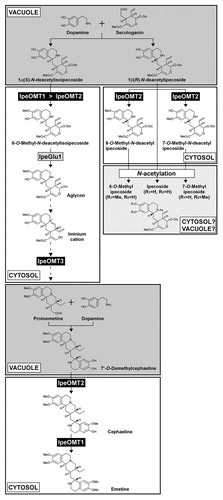
We have proposed a plausible emetine biosynthetic pathway, but the detailed reaction scheme from 6-O-methyl-N-deacetylisoipecoside aglycon to protoemetine has not yet been elucidated ().Citation4,Citation5 The aglycon is highly reactive, and thus the spontaneous intramolecular reactions proceed in vitro.Citation4 We hypothesized that the iminium cation, one of the spontaneous reaction products of 6-O-methyl-N-deacetylisoipecoside aglycon, would be an intermediate of emetine biosynthesis (). Formation of protoemetine from the iminium cation requires reductions of imine and olefin, methyl ester hydrolysis, decarboxylation and O-methylation. Although we have proposed the involvement of IpeOMT3 in the O-methylation of 7-hydroxy group with respect to isoquinoline skeleton by using the synthetic aglycon mimic (3-O-methylredipecamine) as substrate,Citation5 its natural substrate is still unknown. These facts raised a fundamental question about how such a highly labile aglycon can serve as intermediate for emetine biosynthesis. One possibility is the formation of a metabolon, complexes of enzymes that execute sequential metabolic transformations.Citation12,Citation13 The metabolon functions to effectively channel substrates between two successive enzymes, ensuring a high rate of metabolic transformation. It also prevents loss or dilution of substrate by diffusion and protects chemically labile intermediates. The metabolon formation of biosynthetic enzymes of secondary metabolites is becoming unveiled in plants.Citation14–Citation16 Such a mechanism may also function in Ipecac alkaloid biosynthesis. If so, what can be an alternative approach to prove the involvement of an enzyme in the biosynthetic reaction, when in vitro enzyme assay cannot be conducted due to a chemically labile intermediate? Generation of gene knockout/suppression lines would be one of the options. However, for example, suppression of salutaridinol 7-O-acetyltransferase, one of the morphine biosynthetic genes in opium poppy, did not lead to the accumulation of salutaridinol, but of salutaridine, the substrate for salutaridine reductase that functions prior to salutaridinol 7-O-acetyltransferase.Citation17 Therefore, it should be noted that the knockout or suppression of a biosynthetic gene does not always lead to the direct accumulation of its substrate. The other approach is to use a structural analog that is more stable than the natural substrate, as we used synthetic 3-O-methylredipecamine in the functional identification of IpeOMT3 enzyme.Citation5
Elucidation of the Ipecac alkaloid biosynthetic pathway disclosed discrepancies in subcellular localization between substrate and the corresponding enzyme (). We have demonstrated that the IpeOMT and IpeGlu1 enzymes are localized in cytosol.Citation5 On the other hand, 1α(S)-N-deacetylisoipecoside and its diastereomer 1β(R)-N-deacetylipecoside, which are the condensation products of dopamine and secologanin and are further processed by those enzymes, are thought to be localized in the vacuole (). As described in our recent paper,Citation5 those glucoalkaloids may be transported outside the vacuole presumably by a multidrug resistance-associated protein-type ATP-binding cassette transporter. However, according to the biosynthetic pathway revealed, N-deacetylisoipecoside first has to be captured by IpeOMT1, followed by deglucosylation by IpeGlu1 despite the cytosolic localization of both enzymes. Likewise, N-deacetylipecoside also needs to be methylated by IpeOMT2 without contacting IpeGlu1, because N-deacetylipecoside is one of the best substrates for IpeGlu1. We have suggested that these regulations would be possible by the cell-specific expression of the biosynthetic genes.Citation5 For example, the cells for the synthesis of R-configured Ipecac alkaloids might express IpeOMT2 but not IpeGlu1. Another possibility is the formation of metabolon. It is likely that a metabolon that does not include IpeGlu1 is formed to avoid deglucosylation of IpeOMT2 substrate and products for the synthesis of methylated glucoalkaloids having an R-configuration, whereas emetine biosynthetic enzymes may form complexes that include IpeOMT1 and IpeGlu1, as well as the unidentified biosynthetic enzymes responsible for the conversion of the labile 6-O-methyl-N-deacetylisoipecoside aglycon to protoemetine as mentioned above.
Figures and Tables
Figure 1 Schematic representation of the Ipecac alkaloid biosynthetic pathway. Major reactions catalyzed by IpeOMT1, IpeOMT2, IpeOMT3 and IpeGlu1, and the probable subcellular compartments where each reaction is performed are shown. Dopamine is synthesized in cytosol and accumulated in the vacuole.Citation18,Citation19 In comparison, strictosidine, an intermediate for terpenoid-indole alkaloid biosynthesis formed by condensation of tryptamine and secologanin, is synthesized and stored in the vacuole.Citation20,Citation21 Thus, the condensation reactions of dopamine and secologanin and of dopamine and protoemetine are likely to be performed in the vacuole. Subcellular compartments of the produced Ipecac alkaloids, such as emetine, cephaeline, ipecoside and its methyl derivatives, have not yet been elucidated. See refs. Citation4 and Citation5 for spontaneous reactions leading from 6-O-methyl-N-deacetylisoipecoside aglycon to the iminium cation and the branched Ipecac alkaloid biosynthetic pathway, respectively.
Addendum to:
References
- Janot M-M. Manske RHF, Holmes HL. The ipecac alkaloids. The Alkaloids 1953; 3:San Diego Academic Press 363 - 394
- Fujii T, Ohba M. Cordel GA. The Alkaloids 1998; 51:San Diego Academic Press 271 - 321
- Wiegrebe W, Kramer WJ, Shamma M. The emetine alkaloids. J Nat Prod 1984; 47:397 - 408
- Nomura T, Quesada AL, Kutchan TM. The new β-D-glucosidase in terpenoid-isoquinoline alkaloid biosynthesis in Psychotria ipecacuanha. J Biol Chem 2008; 283:34650 - 34659
- Nomura T, Kutchan TM. Three new O-methyltransferases are sufficient for all O-methylation reactions of ipecac alkaloid biosynthesis in root culture of Psychotria ipecacuanha. J Biol Chem 2010; 285:7722 - 7738
- Ziegler J, Diaz-Cháves ML, Kramell R, Ammer C, Kutchan TM. Comparative macroarray analysis of morphine containing Papaver somniferum and eight morphine free Papaver species identifies an O-methyltransferase involved in benzylisoquinoline biosynthesis. Planta 2005; 222:458 - 471
- Ziegler J, Voigtländer S, Schmidt J, Kramell R, Miersch O, Ammer C, et al. Comparative transcript and alkaloid profiling in Papaver species identifies a short chain dehydrogenase/reductase involved in morphine biosynthesis. Plant J 2006; 48:177 - 192
- Liscombe DK, Facchini PJ. Molecular cloning and characterization of tetrahydroprotoberberine cis-N-methyltransferase, an enzyme involved in alkaloid biosynthesis in opium poppy. J Biol Chem 2007; 282:14741 - 14751
- Pienkny S, Brandt W, Schmidt J, Kramell R, Ziegler J. Functional characterization of a novel benzylisoquinoline O-methyltransferase suggests its involvement in papaverine biosynthesis in opium poppy (Papaver somniferum L). Plant J 2009; 60:56 - 67
- Minami H, Dubouzet E, Iwasa K, Sato F. Functional analysis of norcoclaurine synthase in Coptis japonica. J Biol Chem 2007; 282:6274 - 6282
- Morishige T, Dubouzet E, Choi K-B, Yazaki K, Sato F. Molecular cloning of columbamine O-methyltransferase from cultured Coptis japonica cells. Eur J Biochem 2002; 269:5659 - 5667
- Srere PA. Complexes of sequential metabolic enzymes. Ann Rev Biochem 1987; 56:89 - 124
- Ovádi J, Srere PA. Macromolecular compartmentation and channeling. Int Rev Cytol 2000; 192:255 - 279
- Panicot M, Minguet EG, Ferrando A, Alcázar R, Blázquez MA, Carbonell J, et al. A polyamine metabolon involving aminopropyl transferase complexes in Arabidopsis. Plant Cell 2002; 14:2539 - 2551
- Jørgensen K, Rasmussen AV, Morant M, Nielsen AH, Bjarnholt N, Zagrobelny M, et al. Metabolon formation and metabolic channeling in the biosynthesis of plant natural products. Curr Opin Plant Biol 2005; 8:280 - 291
- Nielsen KA, Tattersall DB, Jones PR, Møller BL. Metabolon formation in dhurrin biosynthesis. Phytochemistry 2008; 69:88 - 98
- Kempe K, Higashi Y, Frick S, Sabarna K, Kutchan TM. RNAi suppression of the morphine biosynthetic gene salAT and evidence of association of pathway enzymes. Phytochemistry 2009; 70:579 - 589
- Roberts MF, McCarthy D, Kutchan TM, Coscia CJ. Localization of enzymes and alkaloidal metabolites in Papaver latex. Arch Biochem Biophys 1983; 222:599 - 609
- Kutchan TM, Rush M, Coscia CJ. Subcellular localization of alkaloids and dopamine in different vacuolar compartments of Papaver bracteatum. Plant Physiol 1986; 81:161 - 166
- McKnight TD, Bergey DR, Burnett RJ, Nessler CL. Expression and enzymatically active and correctly targeted strictosidine synthase in transgenic tobacco plants. Planta 1991; 185:148 - 152
- Stevens LH, Blom TJM, Verpoorte R. Subcellular localization of tryptophan decarboxylase, strictosidine synthase and strictosidine β-D-glucosidase in suspension cultured cells of Catharanthus roseus and Tabernaemontata divaricata. Plant Cell Rep 1993; 12:573 - 576