Abstract
Fluorescence resonance energy transfer (FRET)- sensitized emission imaging of Arabidopsis thaliana roots expressing the yellow cameleon 3.60 calcium (Ca2+) reporter showed that the concentration of calcium in the cytosol ([Ca2+]cyt) increased upon aluminum ion (Al3+) treatment in root cells from the transition zone within seconds. The Al3+-induced [Ca2+]cyt transients were biphasic and were modified by Ca2+ channel blockers and by an antagonist of neuronal glutamate receptors, 2-amino-5-phosphonopentanoate (AP-5), and by the anion channel blocker, 5-nitro-2-(3’-phenylpropyl-amino) benzoate (NPPB). The [Ca2+]cyt transients were not uniquely associated with Al3+ toxicity mechanisms since lanthanum (La3+) and gadolinium (Gd3+) also elicited [Ca2+]cyt transients that were similar to those induced by Al3+. Here a testable model that describes a possible mechanism and sequence of events that lead to the Al3+-induced [Ca2+]cyt transients and inhibition of root growth is proposed. This model can be applied to study also the signal-response coupling of the trivalent ions La3+ and Gd3+.
Aluminum (Al) is a naturally occurring component of soil particles and is the third most abundant element in the earth's crust.Citation1 In acidic soils, Al dissolves in the soil solution and different ionic Al species form.Citation2,Citation3 The most toxic Al species in acidic soils is ionic Al, Al3+.Citation4 Al3+ toxicity stems from its interference with a plethora of cellular processes that control plant growth and development.Citation3,Citation5–Citation7
The interactions between calcium (Ca2+) and Al3+ are well documented in the literature. One of the toxic effects of Al3+ on plant growth and development has been ascribed to the disruption of Ca2+ homeostasis by Al3+.Citation8,Citation9 The fact that Al3+ inhibits Ca2+ uptake by roots,Citation10 blocks voltage-regulated Ca2+ channels,Citation11,Citation12 and affects the concentration of Ca2+ in the cytosol ([Ca2+]cyt)Citation13–Citation18 support this view. Ca2+ alleviates Al3+ toxicityCitation19–Citation22 perhaps by inhibiting Al3+ accumulation in the roots and cells.Citation23,Citation24
Rincón-Zachary et al.Citation18 using fluorescence resonance energy transfer (FRET)-sensitized emission to image Arabidopsis thaliana roots expressing the yellow cameleon 3.60 Ca2+ reporter demonstrated increases in the concentration of free Ca2+ in the cytosol ([Ca2+]cyt) within seconds of Al3+ application. Al3+ induced distinct [Ca2+]cyt signatures in cells from the different developmental root regions-meristem, elongation and maturation zones. The [Ca2+]cyt signature in the transition zone, which is the most Al-sensitive root region,Citation25 was biphasic and was modified by treatments that chelate external Ca2+ (EGTA), block Ca2+ entry through the plasma membrane (verapamil), by an antagonist of neuronal glutamate receptors, 2-amino-5-phosphonopentanoate (AP-5), and by the anion channel blocker, 5-nitro-2-(3′-phenylpropyl-amino) benzoate (NPPB). All of these agents affected the first peak of the Al3+-induced [Ca2+]cyt signature by reducing its magnitude or abolishing it. These results support the notion that Al3+ interacts with different types of plasma membrane Ca2+ channels, causing them to open. Al3+-induced [Ca2+]cyt transients were also observed in the Arabidopsis Al-resistant and Al-sensitive mutants alr104 and als3, respectively. In addition, the trivalent ions lanthanum (La3+) and gadolinium (Gd3+) evoked [Ca2+]cyt signatures in the transition zone of the wild-type Arabidopsis and of the alr104 and als3 roots similar to those elicited by Al3+. Hence the authors concluded that the observed [Ca2+]cyt transients were not uniquely associated with Al3+ toxicity mechanisms. Al3+, La3+ and Gd3+ appear to elicit the same Ca2+ signaling pathway.
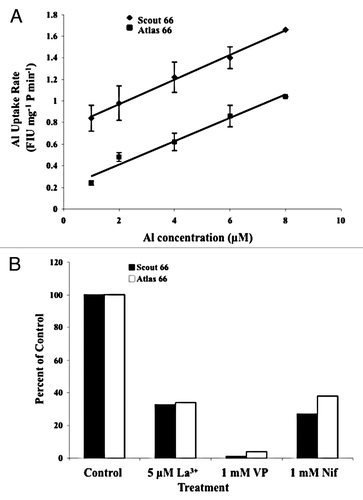
I would like to propose a testable model that describes the possible sequence of events during Ca2+ signaling triggered by trivalent ions using Al3+ as a prototype (). (1) Al3+ causes Ca2+ channels in cells of the root transition zone to open allowing Ca2+ influx into the cells. (2) [Ca2+]cyt rises producing the first peak of the biphasic [Ca2+]cyt signature. (3) Increased [Ca2+]cyt activates internal Ca2+ channels located in membranes of internal Ca2+ stores such as the vacuole, ER, mitochondria or plastids producing the second peak of the [Ca2+]cyt signature. Ca2+-induced Ca2+ release from internal stores has been described in plant cells.Citation26 (4) Al3+ may permeate plasma membrane Ca2+ and non-selective cation channels and interact with internal Ca2+ channels allowing Ca2+ to be released into the cytosol, contributing to the rise in [Ca2+]cyt. In this context, supporting data come from unpublished results (Leblanc J and Rincón-Zachary M) that show Al3+ transport across plasma membrane (PM) vesicles isolated from 5 mm wheat (Triticum aestivum) root tips by aqueous two-phase partitioningCitation27 (). In this experiment isolated PM vesicles were loaded with the fluorescent histochemical aluminum indicator morin (2′, 3′, 4′, 5, 7-pentahydroxyflavone) for 30 min at room temperature and then centrifuged at 100,000 xg for 15 min at 4°C and the pellet was washed twice to remove excess morin. The PM vesicles (25 µg protein mL−1) were then incubated in a 2 mL buffer (250 mM sucrose, 50 mM K2SO4, 1 mM DTT, 5 mM MES-Tris [pH 7.0]) containing different concentrations of Al3+ for 10 min at room temperature. Al3+uptake by the PM vesicles was monitored by fluorometry (excitation at 420 nm; emission at 475 nm). The results show that PM vesicles isolated from the Al-sensitive wheat cultivar Scout 66 root tips are more permeable to Al3+ than those isolated from the Al-tolerant cultivar Atlas 66 (). In this experiment, the relationship between the rate of Al3+ uptake and the Al3+ concentration in the solution was linear for both Scout 66 (Y = 0.114X + 0.741, R2 = 0.99) and Atlas 66 (Y = 0.108X + 0.193, R2 = 0.98) PM vesicles. In addition, LeblancCitation28 showed that compounds known to block Ca2+ channels inhibited Al3+ uptake by plasma membrane vesicles (; Leblanc J and Rincón-Zachary M, unpublished data). La3+, verapamil and nifedipine were very effective in inhibiting Al3+ uptake by plasma membrane vesicles: 5 µM La3+ and 1 mM nifedipine caused 67% and 73% inhibition, respectively, and 1 mM verapamil completely abolished the Al3+ uptake by the vesicles. Thus, it is feasible that Al3+ permeates non-selective cation channels or/and Ca2+ channels. (5) Lastly, the overall [Ca2+]cyt elevation could set off mechanisms that inhibit root growth (e.g., callose synthesis and its deposition in the cell wall, disruption of the cytoskeleton organization, formation of reactive oxygen species, etc.). Testing these hypotheses is underway.
Figures and Tables
Figure 1 A model that describes a possible mechanism and sequence of events that lead to the [Ca2+]cyt transients and inhibition of root growth. (1) Al3+ interacts with Ca2+ channels in the plasma membrane of root cells in the root transition zone. The Ca2+channels open and external Ca2+ enters the cytosol. (2) [Ca2+]cyt rises producing the first peak of the biphasic [Ca2+]cyt signature. (3) Increased [Ca2+]cyt activates internal Ca2+ channels located in membranes of internal Ca2+ stores (e.g., tonoplast, ER, mitochondria or plastids) producing the second peak of the [Ca2+]cyt signature. (4) Al3+ permeates the PM through Ca2+- and non-selective cation channels. (5) Al3+ opens internal Ca2+ channels in the tonoplast, ER, mitochondria or plastids and as a result more Ca2+ is released into the cytosol. (6) The overall [Ca2+]cyt elevation stimulates mechanisms that inhibit root growth.
![Figure 1 A model that describes a possible mechanism and sequence of events that lead to the [Ca2+]cyt transients and inhibition of root growth. (1) Al3+ interacts with Ca2+ channels in the plasma membrane of root cells in the root transition zone. The Ca2+channels open and external Ca2+ enters the cytosol. (2) [Ca2+]cyt rises producing the first peak of the biphasic [Ca2+]cyt signature. (3) Increased [Ca2+]cyt activates internal Ca2+ channels located in membranes of internal Ca2+ stores (e.g., tonoplast, ER, mitochondria or plastids) producing the second peak of the [Ca2+]cyt signature. (4) Al3+ permeates the PM through Ca2+- and non-selective cation channels. (5) Al3+ opens internal Ca2+ channels in the tonoplast, ER, mitochondria or plastids and as a result more Ca2+ is released into the cytosol. (6) The overall [Ca2+]cyt elevation stimulates mechanisms that inhibit root growth.](/cms/asset/56441425-cfa0-4b25-a46c-1f9554b6ba93/kpsb_a_10911973_f0001.gif)
Figure 2 Al3+ uptake by PM vesicles isolated from 5 mm root tips of both the Al-sensitive cultivar Scout 66 and the Al-tolerant cultivar Atlas 66. (A) Rate of Al3+ uptake by PM vesicles incubated in increasing concentrations of Al3+. The PM vesicles from the Al sensitive cultivar Scout 66 were more permeable to Al3+ than those of the Al-tolerant cultivar Atlas 66. The values are means ± SD. Rates of Al3+ uptake are expressed in Fluorescence Intensity Units (FIU) mg−1 protein min−1. (B) Effect of Ca2+ channel blockers on the rate of Al3+ uptake by PM vesicles s percent of the control. All Ca2+ channel blockers tested inhibited the rate Al3+ uptake by the PM vesicles in both cultivars. The accumulation of Al3+ in the PM vesicles was monitored by measuring the fluorescence emitted by the Al-morin complex as described in the text. Both experiments were repeated three times in triplicate (n = 9). The PM vesicles were pooled from multiple independent membrane isolations in order to obtain enough membrane protein for the assays.
Addendum to:
References
- Foy CD, Chaney RL, White MC. The physiology of metal toxicity in plants. Annu Rev Plant Physiol 1978; 29:511 - 566
- Martin RB. Sigel H, Sigel A. Bioinorganic chemistry of aluminum. Metal Ions in Biological Systems: Aluminum and Its Role in Biology 1988; New York Marcel Dekker 1 - 57
- Matsumoto H. Cell biology of aluminum toxicity and tolerance in higher plants. Int Rev Cytol 2000; 200:1 - 46
- Kinraide TB. Identity of rhizotoxic aluminum species. Plant Soil 1991; 134:167 - 178
- Taylor GJ. Sigel H, Sigel A. The physiology of aluminum phytotoxicity. Metal Ions in Biological Systems: Aluminum and Its Role in Biology 1988; New York Marcel Dekker 123 - 163
- Kochian LV. Cellular mechanisms of aluminum toxicity and resistance in plants. Annu Rev Plant Physiol Plant Mol Biol 1995; 46:237 - 260
- Ma JF. Syndrome of aluminum toxicity and diversityof aluminum resistance in higher plants. Int Rev Cytol 2007; 264:225 - 252
- Foy CD. Plant adaptation to acid, aluminum-toxic soils. Common Soil Sci Plant Anal 1988; 19:959 - 987
- Rengel Z, Zhang W-H. Role of dynamics of intracellular calcium in aluminium-toxicity syndrome. New Phytol 2003; 159:295 - 314
- Huang JW, Shaff JE, Grunes DL, Kochian LV. Aluminum effects on calcium fluxes at the root apex of aluminum-tolerant and aluminum-sensitive wheat cultivars. Plant Physiol 1992; 98:230 - 237
- Huang JW, Pellet DM, Parenik LA, Kochian LV. Aluminum interactions with voltage-dependent calcium transport in plasma membrane vesicles isolated from roots of aluminum-sensitive and aluminum-resistant wheat cultivars. Plant Physiol 1996; 110:561 - 569
- Piñeros M, Tester M. Calcium channels in higher plant cells: selectivity, regulation and pharmacology. J Exp Bot 1997; 48:551 - 577
- Jones DL, Gilroy S, Larsen PB, Howell SH, Kochian LV. Effect of aluminum on cytoplasmic Ca2+ homeostasis in root hairs of Arabidopsis thaliana (L.). Planta 1998; 206:378 - 387
- Jones DL, Kochian LV, Gilroy S. Aluminum induces a decrease in cytosolic calcium concentration in BY-2 tobacco cell cultures. Plant Physiol 1998; 116:81 - 89
- Zhang W-H, Rengel Z. Aluminium induces an increase in cytoplasmic calcium in intact wheat root apical cells. Aust J Plant Physiol 1999; 26:401 - 409
- Bhuja P, McLachlan K, Stephens J, Taylor G. Accumulation of 1,3-β-D-glucans in response to aluminum and cytosolic calcium in Triticum aestivum. Plant Cell Physiol 2004; 45:543 - 549
- Kawano T, Kadono T, Fumoto K, Lapeyrie F, Kuse M, Isobe M, et al. Aluminum as a specific inhibitor of plant TPC1 Ca2+ channels. Biochem Biophs Res Commun 2004; 324:40 - 45
- Rincón-Zachary M, Teaster ND, Sparks JA, Valster AH, Motes CM, Blancaflor EB. Fluorescence resonance energy transfer sensitized emission of yellow cameleon 3.60 reveals root-zone-specific calcium signatures in Arabidopsis in response to aluminum and other trivalent cations. Plant Physiol 2010; 152:1442 - 1458
- Aniol A. Aluminium uptake by roots of two winter wheat varieties of different tolerance to aluminium. Biochem Physiol Pflanzen 1983; 178:11 - 20
- Kinraide TB. Three mechanisms for the calcium alleviation of mineral toxicities. Plant Physiol 1998; 118:513 - 520
- Grauer UE, Horst WJ. Modeling cation amelioration of aluminum phytotoxicity. Soil Sci Soc Ame J 1992; 166 - 172
- Ryan PR, Kinraide TB, Kochian LV. Al3+-Ca2+ interactions in aluminum rhizotoxicity I. Inhibition of root growth is not caused by reduction of calcium uptake. Planta 1994; 192:98 - 103
- Teaster ND. The effect of calcium and magnesium on aluminum accumulation in wheat roots (Triticum aestivum L.) [master's thesis] 1999; Wichita Falls (TX) Midwestern State University
- Vitorello VA, Haug A. Short-term aluminum uptake by tobacco cells: growth dependence and evidence for internalization in a discrete peripheral region. Physiol Plant 1996; 97:536 - 544
- Sivaguru M, Horst WJ. The distal part of the transition zone is the most aluminum-sensitive apical root zone of maize. Plant Physiol 1998; 116:155 - 163
- Ng CKY, McAinsh MR. Encoding specificity in plant calcium signaling: Hot-spotting the ups and downs and waves. Ann Bot 2003; 92:477 - 485
- Palmgren MG, Askerlund P, Fredrikson K, Widell S, Sommarin M, Larsson C. Sealed inside-out and cytoplasmic-side-in plasma membrane vesicles. Plant Physiol 1990; 92:871 - 880
- Leblanc JA. Aluminum influx via a calcium channel in the plasma membrane of root tip cells of the winter wheat (Triticum aestivum L.) cultivars: Atlas 66 (Al-tolerant) and Scout 66 (Al-sensitive) [master's thesis] 2008; Wichita Falls (TX) Midwestern State University