Abstract
The effects of root hypoxia on ethylene biosynthesis and perception have been documented in many vegetative organs, but not extensively in fruit. Therefore, in the present study, the effects of root hypoxia on ethylene biosynthesis and perception were investigated in tomato (Solanum lycopersicum L.) fruit at five stages of the maturation phase. Our results showed that root hypoxia do not affect ethylene biosynthesis in fruit, but stimulates its reception from other plant part, as indicated by the increase in the expression of ethylene receptors ETR1 and 3.
Introduction
Ethylene is a gaseous plant hormone involved in specific developmental processes, as well as in response to many externa1 stresses.Citation1 Ethylene biosynthesis is increased in response to stimuli such as wounding, pathogen attack and drought.Citation2 During normal development, ethylene promotes a number of events, including senescence, seed germination, abscission and fruit ripening.Citation2
The pathway of ethylene synthesis is well established in higher plants.Citation3 Ethylene biosynthesis begins with the conversion of methionine to S-adenosyl-methionine (SAM), catalyzed by SAM synthase, followed by the formation of 1-aminocyclopropane-1-carboxylic acid (ACC) by ACC synthase (ACS). Finally, ACC is converted by ACC oxidase (ACO) to ethylene.Citation4 Genes encoding enzymes involved in ethylene biosynthesis have been cloned from a number of species.Citation5 In tomato, nine ACS genes have been identified,Citation6 but only two of these genes, ACS2 and ACS4, are expressed at a high level in fruit.Citation7,Citation8 ACO is encoded by a four gene family, but only ACO4 is expressed in fruit.Citation9
Our understanding of the role of ethylene in the regulation and coordination of fruit ripening has been enhanced by studies using tomato (Solanum lycopersicum L.) as a model system. Typically, ripening of climacteric fruits is characterized by increased respiration and ethylene biosynthesis.Citation10 Genetic studies have revealed the presence of several putative ethylene biosynthesis and receptor proteins in tomato that appear to initiate a signal transduction pathway that culminates in expression of ripening-related enzymes.Citation11
Enhanced ethylene biosynthesis is a normal part of ripening in climacteric fruits but is induced by various stresses.Citation4,Citation12 Indeed, numerous studies showed that ethylene biosynthesis in vegetative organs increased under hypoxic conditionsCitation4 and that the molecular basis for this increase is the induction of the ACS.Citation13 However, in none of these studies was investigated the ethylene biosynthesis and perception in fruits when hypoxia is applied in roots.
The aim of the present study was to investigate the effect of root hypoxia on ethylene biosynthesis and perception in tomato fruit, during the maturation phase.
Results
Effect of root hypoxia on fruit ethylene content.
The effects of prolonged root hypoxia were evaluated on ethylene content in fruits at the Mature Green (MG, 28–31 DPA), Breaker (B, 31–33 DPA), Turning (T, 34–36 DPA), Orange (O, 36–40 DPA) and Red Ripe (RR, 40–45 DPA) stages of ripening. As shown in , fruit ethylene content increased irrespective to the growing conditions to reach its maximal value at the O stage and then decreased. No differences were seen in ethylene content between fruits of control and hypoxically treated plants ().
Effects of root hypoxia on the expression of genes encoding ethylene biosynthesis and perception.
Ethylene is also known to be a major regulator of fruit ripening. To explain the absence of effect of root hypoxia on ethylene biosynthesis in fruit, we measured the expression level of genes encoding 1-aminocyclopropane-1-carboxylic acid synthase (ACS) and 1-aminocyclopropane-1-carboxylic acid oxidase (ACO), two enzymes that catalyse respectively the first and the second step of ethylene biosynthesis. Among the tomato ACS and ACO genes, ACS2, ACS4 and ACO4 are induced at the onset of fruit ripening, whereas ACS6 is expressed in green fruit before ripening and is repressed by ripening ethylene.Citation19 Semi-quantitative RT-PCR analysis demonstrated that these 4 genes displayed the expected expression pattern during the ripening process of both control and hypoxically treated plants without any significant effect of hypoxia ().
In order to determine whether root hypoxia affects the synthesis of ethylene receptors, the expression level of the ETR1, 3, 4 and 5 genes was also analysed. ETR 1 and ETR3 mRNA levels were significantly increased in B, T, O and RR fruits of hypoxically treated as compared to control plants, whereas ETR 4 and ETR5 mRNA levels were not affected by root hypoxia ().
Discussion
Micro-Tom is a miniature tomato (Solanum lycopersicum L.) cultivar with determinate growth, which has been proposed to be a suitable model for molecular and metabolic studies in tomato species due to its relatively small genome, ease of genetic manipulation and relatively short life cycle.Citation20 As previous observations showed that hypoxic conditions impacted Micro-Tom growth in a way similar to other tomato cultivars,Citation15,Citation21 we used this cultivar to perform the present investigation.
Generally, the ripening of fleshy fruits corresponds to a series of biochemical, physiological and structural changes that make the fruit attractive to the consumer. Although these processes vary from one type of fruit to another, fruits can be divided into two broad groups, known as climacteric and non-climacteric.Citation22 Categorisation into one group or the other depends on whether or not a fruit exhibits a peak in respiration and ethylene production during ripening.Citation10 In climacteric fruits, the sharp increase in ethylene production at the onset of ripening is considered as controlling the initiation of changes in colour, aromas, texture, flavour and other biochemical and physiological attributes.Citation11
Prolonged root hypoxia is known to increase ethylene biosynthesis in plant vegetative organs.Citation13 Consistently, the present work clearly shows that prolonged root hypoxia had no effect on ethylene content in tomato fruit. As ethylene is known to regulate fruit ripening by coordinating the expression of genes involved in this process,Citation23 we have analyzed the regulation of genes involved in ethylene synthesis and perception in hypoxic conditions. At that time, analysis of the expression of ACS and ACO genes is not consistent with a modulation of ethylene synthesis in fruits in response to root hypoxia. Thus, it is unlikely that ethylene production is directly involved in the limitation of lycopene and ascorbate accumulation in fruits of hypoxically treated tomato plants.Citation14 It is however noteworthy, that both ETR1 and ETR3 gene expression was enhanced at late stages of fruit ripening grown on hypoxically treated plants, as compared to the control.
Globally, this study demonstrates that prolonged root hypoxia do not affect ethylene biosynthesis in tomato fruit, but stimulates its reception from other plant parts.
Material and Methods
Plant material and fruit harvest.
Tomato (Solanum lycopersicum L. cv. Micro-Tom) plants were grown hydroponically in a growth chamber with a 16 h day (23°C)/9 h night (18°C) cycle, an irradiance of 350 µmolm−2s−1 and a 75–80% relative humidity. Root hypoxia was applied at first flower anthesis by stopping air bubbling as previously described in reference Citation14. Flowers were tagged at anthesis and the fruit number per plant was limited to six with 3 fruits per truss. Fruits were harvested at the mature green (MG), breaker (B), turning (T), orange (O) and red ripe (RR) stages, according to the L*a*b* uniform color space (CIELAB) as previously described in reference Citation15, using a CHROMA-METER (CR-200, Minolta France). The equatorial pericarp of fruits was hand dissected and immediately frozen in liquid nitrogen, ground to a fine powder and stored at −80°C until use.
Ethylene measurements.
Ethylene was measured from fruits of different developmental stages by sealing whole fruits in airtight jars for 2 h at 22°C after which a 1 ml sample of the headspace was taken and injected on to a Hewlett-Packard 5890 series II gas chromatograph equipped with a flame ionization detector.Citation16 Samples were compared to a standard of known concentration and normalized for fruit mass.
RNA extraction and semi-quantitative RT-PCR analysis.
Total RNA was isolated from approximately 10 mg of dried powder using the NucleoSpin RNA plant extraction kit (Promega). Reverse transcription was carried out using 1 µg of total RNA as described in Mortain-Bertrand et al.Citation17 Semi-quantitative RT-PCR was performed essentially as described in Télef et al.Citation18 Primers used for semi-quantitative RT-PCR are shown in .
Figures and Tables
Figure 1 Ethylene content in fruits of control (▴) and hypoxically treated (■) plants during the maturation phase. Values represent the mean ± SD of at least three measurements. MG, mature green; B, breaker; T, turning; O, orange; RR , red ripe.
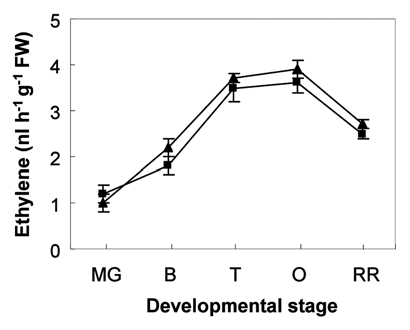
Figure 2 Semi-quantitative RT-PCR analysis of genes implicated in ethylene biosynthesis in fruits of control and hypoxically treated plants during the maturation phase. Signals were acquired using the Biorad Gel doc system and quantified with the quantity one software (Biorad). Values represent the mean ± SD (from two independent experiments) of the relative intensity of each signal normalized to MG control and EF1α signals. ACS, 1-aminocyclopropane-1-carboxylic acid synthase; ACO, 1-aminocyclopropane-1-carboxylic acid oxidase.
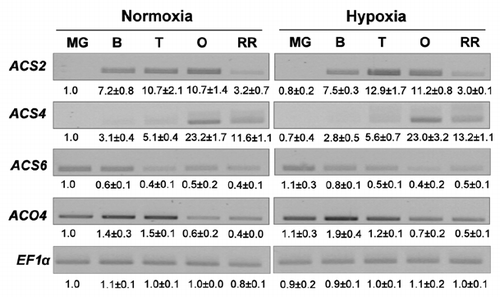
Figure 3 Semi-quantitative RT-PCR analysis of genes implicated in ethylene perception in fruits of control and hypoxically treated plants during the maturation phase. Signals were acquired using the Biorad Gel doc system and quantified with the quantity one software (Biorad). Values represent the mean ± SD (from two independent experiments) of the relative intensity of each signal normalized to MG control and EF1α signals. ETR: ethylene receptor.
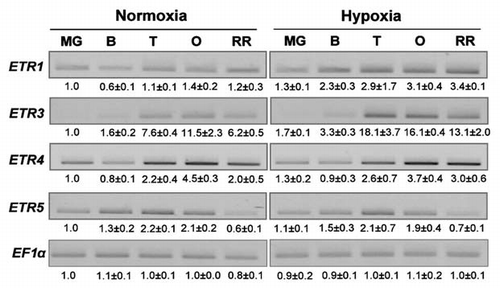
Table 1 Primers used for semi quantitative RT-PCR analysis
References
- Dat JF, Capelli N, Folzer H, Bourgeade P, Badot PM. Sensing and signalling during plant flooding. Plant Physiol Biochem 2004; 42:273 - 282
- Abeles FB, Morgan PW, Salveit ME. Ethylene in plant biology 1992; 2nd ed. San Diego CA Academic Press Inc.
- Bleecker AB, Kende H. Ethylene: a gaseous signal molecule in plants. Annu Rev Cell Develop Biol 2000; 16:1 - 40
- Yang SF, Hoffman NE. Ethylene biosynthesis and its regulation in higher plants. Annu Rev Plant Physiol 1984; 35:155 - 189
- Kende H. Ethylene biosynthesis. Annu Rev Plant Physiol Plant Mol Biol 1993; 44:283 - 307
- Zarembinski TI, Theologis A. Ethylene biosynthesis and action: a case of conservation. Plant Mol Biol 1994; 26:1579 - 1597
- Rottmann WE, Peter GF, Oeller PW, Keller JA, Shen NF, Nagy BP, et al. 1-Aminocyclopropane-1-carboxylate synthase in tomato is encoded by a multigene family whose transcription is induced during fruit and floral senescence. J Mol Biol 1991; 222:937 - 961
- Yip WK, Moore T, Yang SF. Differential accumulation of transcripts for 4 tomato 1-aminocyclopropane-1-carboxylate synthase homologs under various conditions. Proceed Nat Acad Sci USA 1992; 89:2475 - 2479
- Holdsworth MJ, Schuch W, Grierson D. Organization and expression for a wound/ripening-related small multigene family from tomato. Plant Mol Biol 1988; 11:81 - 88
- Rhodes M°C. KV Thimann. The maturation and ripening of fruits. Senescence in Plants 1980; Boca Raton FL CRC Press
- Giovannoni JJ. Molecular biology of fruit maturation and ripening. Annu Rev Plant Physiol Plant Mol Biol 2001; 52:725 - 749
- Yu YB, Yang SF. Auxin-induced ethylene production and its inhibition by amino-ethoxyvinylglycine and cobalt ion. Plant Physiol 1979; 64:1074 - 1077
- Grichko VP, Glick BR. Flooding tolerance of transgenic tomato plants expressing the bacterial enzyme ACC deaminase controlled by the 35S, rolD or PRB-1b promoter. Plant Physiol Biochem 2001; 39:19 - 25
- Horchani F, Gallusci P, Baldet P, Cabasson C, Maucourt M, Rolin D, et al. Prolonged root hypoxia induces ammonium accumulation and decreases the nutritional quality of tomato fruits. J Plant Physiol 2008; 165:1352 - 1359
- Horchani F, Khayati H, Raymond P, Brouquisse R, Aschi-Smiti S. Contrasted effects of prolonged root hypoxia on tomato (Solanum lycopersicum) roots and fruits metabolism. J Agron Crop Sci 2009; 195:313 - 318
- Barry CS, McQuinn RP, Thompson AJ, Seymour GB, Grierson D, Giovannoni JJ. Ethylene insensitivity conferred by the green-ripe and never-ripe ripening mutants of tomato. Plant Physiol 2005; 138:267 - 275
- Mortain-Bertrand A, Stammitti L, Télef N, Colardelle P, Brouquisse R, Rolin D, Gallusci P. Effects of exogenous glucose on carotenoid accumulation in tomato leaves. Physiol Plant 2008; 134:246 - 256
- Télef N, Stammitti-Bert L, Mortain-Bertrand A, Maucourt M, Carde JP, Rolin D, Gallusci P. Sucrose deficiency delays lycopene accumulation in tomato fruit pericarp discs. Plant Mol Biol 2006; 62:453 - 469
- Alexander L, Grierson D. Ethylene biosynthesis and action in tomato: a model for climacteric fruit ripening. J Exp Bot 2002; 53:2039 - 2055
- Meissner R, Jacobson Y, Melamed S, Levyatuv S, Shalev G, Ashri A, et al. A new model system for tomato genetics. Plant J 1997; 12:1465 - 1472
- Horchani F, Aloui A, Brouquisse R, Aschi-Smiti S. Physiological responses of tomato plants (Solanum lycopersicum L.) as affected by root hypoxia. J Agron Crop Sci 2008; 194:297 - 303
- Biale JB. Growth, maturation and senescence in fruits. Science 1964; 146:180 - 188
- Giovannoni JJ. Genetic regulation of fruit development and ripening. Plant Cell 2004; 16:170 - 180