Abstract
The respiration rates R (oxygen uptake per min) and body mass M (mg per individual) of sunflower (Helianthus annuus L.) seedlings were measured for populations raised in the dark (scotomorphogenesis) and for plants subsequently grown in white light (photomorphogenesis) to determine the allometric (scaling) relationship for R vs. M. Based on ordinary least squares and reduced major axis regression protocols, cellular respiration rates were found to increase non-linearly as a 'broken-stick' curve of increasing M. During germination, the scaling was ca. 7.5-fold higher than after the emergence of the cotyledons from the seed coat, which can be attributed to the hypoxic conditions of the enclosed embryo. During seedling development, R was found to scale roughly as the 3/7 power of body mass (i.e., R ~ M −3/7), regardless of whether plants were grown in the dark or subsequently in white light. The numerical value of 3/7 statistically significantly differs from that reported across field- or laboratory-grown plants (i.e., R ~ M −1.0). It also differs from the expectations of recent allometric theory (i.e., R ~ M −0.75 - 1.0). This difference is interpreted to be the result of species-specific tissue-compositions that affect the volume fractions of metabolically active and less activ cells. These findings, which are supported by cytological and ultrastructural observations (i.e., scanning- and transmission electron micrographs), draw attention to the need to measure R of developing plants in a tissue- or organ-specific context.
Introduction
The relationship between cellular respiration (i.e., metabolic rate, usually measured as oxygen consumption in darkness) and total body mass is one of the most widely discussed phenomena in biology. The majority of researchers agree that the whole-organism respiration rate (R) scales as some function of total body mass (M) according to the general formula R = βMα, where β is the normalization parameter and α is the scaling exponent.Citation1,Citation2 However, there is no agreement about the numerical value of α. Across the spectrum of animals, including ecto- and endotherms, there is evidence that the exponent is numerically close to either 2/3 or 3/4, although the exponent differs depending on which species-groupings are examined.Citation1,Citation3 The relationship between plant metabolism and body size is even more problematic, in part due to the fact that body size can change over many orders of magnitude as a consequence of an open and indeterminate development in the majority of embryophytes and because metabolically inert tissues, such as wood, can accumulate over the course of a plant's ontogeny.Citation3–Citation5
Another phenomenon that can obscure or confound the relationship between whole-plant respiration and body size is the extent to which oxygen consumption rates differ among metabolically active but different cells and tissues or the extent to which the metabolic activity of a particular organ increases or decreases during development. These possibilities have not been explored in sufficient detail, especially for small or very young plants, in large part because of the technological difficulties in measuring R for small tissue samples or juvenile organs.
Here, we present detailed measurements of the average respiration rates of populations of developing sunflower (Helianthus annuus L.) seedlings grown in darkness or grown in the dark followed by exposure to white light. In this context, we distinguish between two phases of development: germination and seedling growth.Citation6 We present correlation analyses of cellular respiration rate versus body size as gauged by fresh- and dry mass, supplemented by cytological investigations, in an effort to further inform the discussion and theory revolving around the scaling of metabolic rates with respect to differences in plant body size.
Results and Discussion
Germination and seedling development.
According to Bewley,Citation6 germination denotes those processes that commence with the uptake of water by the quiescent, de-hydrated seed and terminates with the elongation of the embryonic axis. This “visible germination” is shown in (days 0 to 2 after sowing). Upon release from the attached seed coat, plant development commences and terminates with the death of the etiolated organism when kept in the dark between days 6 and 8 after sowing (scotomorphogenesis). In seedlings irradiated with white light (WL, abbreviations see ), photomorphogenesis is induced (). This process is characterized by a reduction in the rate of hypocotyl elongation, the greening and expansion of the cotyledons, and changes in the metabolism of the cells.Citation7
Metabolic scaling and cytological investigations.
Our quantitative data show that the two developmental stages (i.e., seed germination and subsequent organ development; and B) differ markedly with respect to their metabolic scaling characteristics because respiration R increases non-linearly as a ‘broken-stick’ function of increasing fresh mass Mf (). Specifically, ordinary least square (OLS) regression of log-transformed R versus Mf yields a slope of 0.37 during the first two days (i.e., seed germination) and a slope of 0.05 subsequently (i.e., during organ development). This nearly 7.5-fold decrease in the scaling of R with respect to Mf is possibly due to the production of ethanol during germination as a result of an internal deficiency in oxygen.Citation6 A recent study by Borisjuk and RolletschekCitation8 has shown that, in sunflower embryos (i.e., day 0 in our ), the major barrier that inhibits diffusive O2 uptake of the cells is a thin, lipid-rich membrane covering the entire oil-storing cotyledons.Citation8 The much thicker multiple layers of the pericarp and the attached seed coat were found to be ineffective barriers of gas (O2 or CO2) exchange. We suggest that the membrane envelope of the embryo may be responsible for the hypoxic conditions of the cells of the imbibed storage cotyledons during the early phase of germination (i.e., days 0 to 1 after sowing).Citation6,Citation8 Scanning electron micrographs of the cotyledons of 3-day-old sunflower seedlings revealed that at this stage of organ development the embryonic lipid membrane described by Borisjuk and RolletschekCitation8 is no longer present (). Moreover, the stomatal pores on the outside of the cotyledons are open (). Hence, CO2 and O2 can readily diffuse out of and into the metabolically active cells of the growing sunflower seedling. Transmission electron micrographs document that the cells contain numerous mitochondria, lipid droplets (oleosomes) and starch-filled amyloplasts (). Within these heterotrophic cells, lipids and starch serve as the metabolites for O2-dependent mitochondrial respiration.Citation6
Turning attention again to the scaling of respiration during seedling development ( and ), regression of the log10-transformed data for plants grown in the dark and those subsequently grown in white light shows that, despite the small sample sizes (N = 5 and 3 for dark- and light-grown seedlings, respectively), the correlation between log R versus log Mf was statistically significant (0.978 ≤ r2 ≤ 0.987). Using these data, reduced major axis (RMA) regression analyses revealed no statistical difference in the slopes of log R versus log Mf for plants raised in the dark and for seedlings subsequently grown in white light (i.e., αRMA = 0.38 with 95% CIs = 0.35 and 0.40 and αRMA = 0.40 with 95% CIs = 0.33 and 0.48, respectively), nor in the Y-intercepts of log R versus log Mf (i.e., log βRMA = 0.76 with 95% CIs = 0.64 and 0.87, and log βRMA = 0.074 with 95% CIs = 0.164 and 1.32, respectively). The data-sets for plants raised in the dark and those subsequently grown in the light therefore were pooled to determine the functional (scaling) relationship for log R versus log Mf during seedling development. RMA regression of these pooled data (N = 8) gave αRMA = 0.440 (95% CIs = 0.386 and 0.493) and log βRMA = 0.595 (95% CIs = 0.321 and 0.869). Once again, the correlation between log R and log Mf was statistically significant (r2 = 0.932, F = 64.5, p > 0.0001).
Based on the 95% CIs of the RMA scaling exponent for log R versus log Mf, the data presented in indicate that the respiration of developing sunflower seedlings scales roughly as the 3/7 power of fresh body mass (i.e., R ∼ M−3/7; αRMA = 0.39 − 0.49). This exponent differs numerically from the isometric exponent (i.e., R ∼ M−1.0; αRMA ∼ 1.0) reported by Reich et al.Citation9 based on an interspecific analysis of data collected from ca. 500 laboratory and field-grown seedlings and tree saplings. It also differs from the 3/4 numerical value proposed by West et al.Citation10 (R ∼ M−3/4) based on their theoretical approach and from the isometric exponent predicted when the West et al.Citation10 theory is adjusted to deal with very small body sizes.Citation11 And, finally, it deviates significantly from a 2/3 scaling relationship. Importantly, these and other empirical and theoretical treatments of the scaling of respiration rates use dry rather than fresh mass as the measure of body size. However, this does not explain why our data indicate a much smaller scaling exponent for R versus body size because, for sunflower seedlings grown in WL or in the dark, dry mass scales as the -0.015 power of fresh mass (). Thus, regardless of whether dry or fresh mass is used as a measure of sunflower body size, the scaling exponent for R versus body size fails to confirm numerical values falling within the range of 0.66 to 1.0.
Materials and Methods
Plant material and growth conditions.
Sunflower (Helianthus annuus L. cv. Giganteus) achenes (containing seeds) were germinated in moist vermiculite in closed, transparent plastic boxes (25°C, darkness, 99% relative humidity) as described in references Citation13 and Citation14. Three days after sowing, half of the seedling trays were transferred into an irradiated growth chamber (continuous white light, WL; photosynthetically active radiation, PAR, ∼120 µmol m−2s−1 at plant level, see refs. Citation12 and Citation14). One to six days after sowing, imbibed embryos or germinated seedlings, respectively, were removed from the vermiculite, washed and blotted dry. After mechanical removal of the attached seed coat, representative seedlings (and dry embryos at day zero) were analyzed ( and B).
Determination of cellular respiration and plant mass.
Oxygen uptake (i.e., cell respiration of the whole organism in darkness, R) was measured polarographically using a Clarktype oxygen electrode as described by Kutschera.Citation15 The rate of O2-depletion of air-saturated water was recorded over a period of 20 min in darkness (25°C). Thereafter, the O2-uptake of killed (boiled-cooled down) samples was determined as a control (i.e., physical O2 absorption of the tissues unrelated to metabolic activity of the cells). These values were subtracted and the rates of oxygen uptake (i.e., cellular respiration) calculated.Citation15
The fresh and dry mass (Mf and Md, respectively) of embryos and entire seedlings, taken from the same batch of plants, were determined with a microbalance. All experiments were repeated six times using batches of fresh seeds (10 individuals each) and the average values of these independent replicates were calculated. The standard errors of the means (SEM) were calculated. The values were not larger than the size of the symbols and are therefore not shown. These data were used to statistically analyze the relationships between respiration (i.e., metabolic activity of the cells) and fresh and dry mass.
Statistical protocols.
Ordinary least squares (OLS) regression was used to determine the relationship between non-transformed data for respiration and mass. Reduced major axis (RMA, also called standard major axis) regression protocols were employed to calculate the slopes and Y-intercepts of log-log linear relationships (i.e., αRMA and log βRMA, respectively; see refs. Citation2 and Citation17). These parameters were computed using the formulas αRMA = αOLS/r and log βRMA = log R − αRMA log Mf, where αOLS is the OLS regression slope, r is the OLS correlation coefficient, and log denotes mean logCitation10-value. The protocols used in the software package, Standardized Major Axis Tests and Routines, were used to determine whether the numerical values of αRMA and log βRMA differed among the data collected from plants raised in the dark and those subsequently grown in white light.Citation18,Citation19 Heterogeneity in log αRMA and log βRMA was rejected in all cases if p > 0.05 ().
Scanning and transmission electron microscopy.
Scanning electron micrographs of cotyledons cut from 3-day-old etiolated sunflower seedlings were prepared and analyzed as described in reference Citation16. For transmission electron microscopy, sections, 2 mm in length, were cut in the region between the apical hook and the onset of the cotyledons, using 3-day-old dark-grown seedlings of average size (see ). The samples were fixed for 2 h in 4% glutaraldehyde, washed for 30 min in sodiumcacodylate buffer (0.1 M, pH 7.2) and fixed for 2 h in OsO4 (2%). After dehydration, the samples were embedded in Spurr's epoxyresin. Thereafter, ultrathin transverse sections were cut with a diamond knife and viewed in an electron microscope as described in references Citation12 and Citation20.
Conclusions and Outlook
The discrepancy between the numerical value of 3/7 reported here and that anticipated by theory or for plants grown in the field or in the laboratoryCitation4,Citation10 is likely attributable to anatomical differences among species and the very small sizes of the plants examined in our study. Because plant tissues differ in their volume fractions of water and carbon (e.g., storage parenchyma versus xylem), the metabolic rates of plant tissues undoubtedly differ or change as the volume fractions of individual cells and tissues change ontogenetically as a consequence of the accumulation of secondary cell wall layers and/or as woody plants increase in size.Citation2,Citation19 In this respect, the study of plant scaling relationships is far more complicated than the corresponding quantitative analyses of adult animals as model organisms.Citation1,Citation3 As a consequence, organ- and tissue-specific values of R should be measured in order to overcome these difficulties.
In keeping with these conclusions, our cytological studies ( and ) show that metabolically active cells contain numerous mitochondria and reserve materials (lipids, starch) that fuel cellular respiration. In eukaryotic cells, this series of biochemical processes occurs exclusively in mitochondria. However, about 20% of the sunflower hypocotyl is composed of vascular tissues and intracellular spaces.Citation13,Citation19 These parts of the developing plant axis are therefore metabolically less active (or inactive), which likely accounts for the lower metabolic rate of this organ.
Finally, it must be noted that Mori et al.Citation4 directly measured respiration for a total of 271 whole plants, spanning nine orders of magnitude in body mass across species native to tropical to boreal ecosystems. These authors report that the data conform to a biphasic, mixed-power function in which the allometric exponent varies continuously from 1.0 (for the smallest plants) to 3/4 (across the larger plants). In tandem with our data, which span a much smaller body size range than explored by Mori et al.Citation4, the relationships between plant respiration and body mass is likely tri-phasic, one in which the scaling exponent increases from <1.0, rises to ∼1.0, and then declines to ∼3/4. Regardless of the precise numerical range occupied by the scaling exponent, it is becoming increasingly clear that no single numerical value can be asserted as “canonical” or “invariant”. This general conclusion is in accordance with the hypothesis of White,Citation5 who argues that, in animals and plants, no universal scaling exponent adequately characterizes the effect of body mass on metabolism.
Figures and Tables
Figure 1 Germination (A) and seedling development (B) in sunflower (Helianthus annuus). Achenes were sown in moist vermiculite and raised in darkness (D, days 0 to 2) or grown for 3 days in the dark and subsequently irradiated for 1 to 3 days with continuous white light (WL). The seedlings were kept in 99% relative humidity at 25°C.
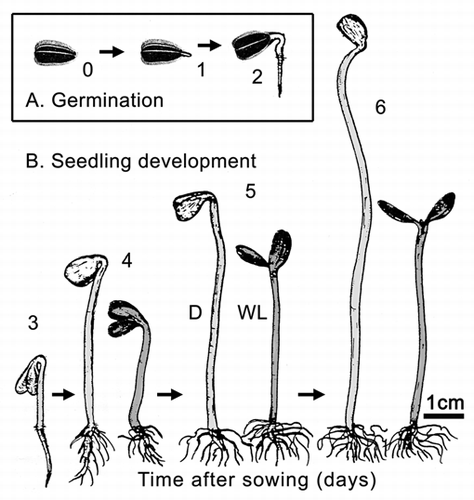
Figure 2 Bivariate plot of log10-transformed data for cellular respiration (R) versus fresh mass (Mf) during germination and seedling development. Sunflower seeds were raised in the dark or, after 3 days of plant growth, irradiated with continuous white light (WL). Lines are reduced major axis (RMA) regression curves (summary statistics provided in the text).
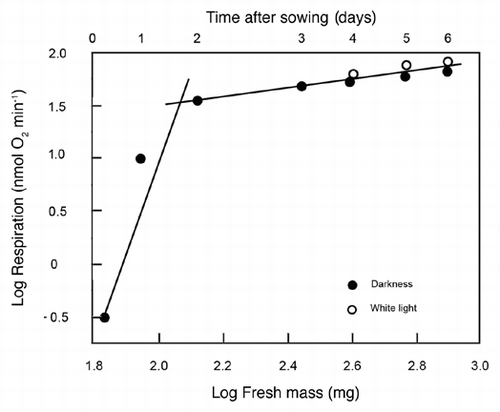
Figure 3 Representative scanning electron micrographs of the cotyledons (A) and epidermal cells (B) of 3-day-old sunflower seedlings that were grown in darkness. The peripheral cells (B) were photographed in the area indicated by the arrow (A). C, cotyledons; E, epidermal cells; S, open stomatum with open pore. Bars = 1 mm (A), 50 µm (B).
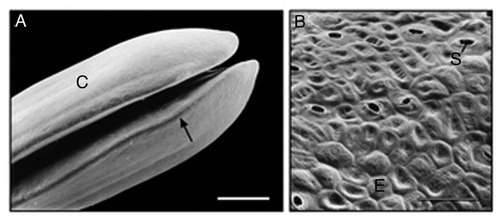
Figure 4 Ultrastructure of sub-epidermal cells in the region between the apical hook and the onset of the cotyledons in 3-day-old etiolated sunflower seedlings. The representative transmission electron micrograph shows the cytoplasmic region of three cells in the periphery of the organ that are separated by a thick wall. A, amyloplast; C, cytoplasm; L, lipid droplet (oleosome); M, mitochondrion; N, nucleus; S, starch; V, vacuole; W, cell wall. Bar, 2 µm.
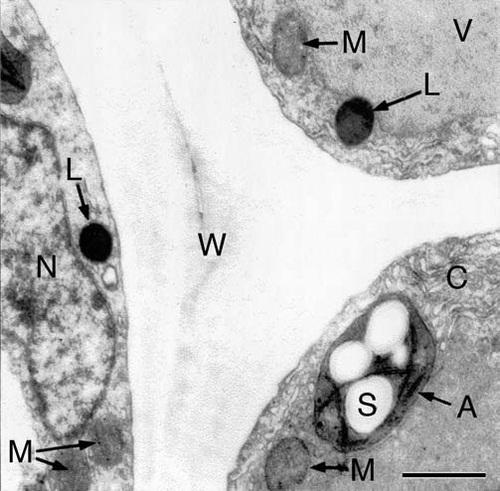
Figure 5 Bivariate plot of log10-transformed data for fresh mass (Mf) versus dry mass (Md) during germination and seedling development. Sunflower seeds were raised in the dark or, after 3 days of plant growth, irradiated with continuous white light (WL). The line is a reduced major axis (RMA) regression curve (summary statistics provided in the text).
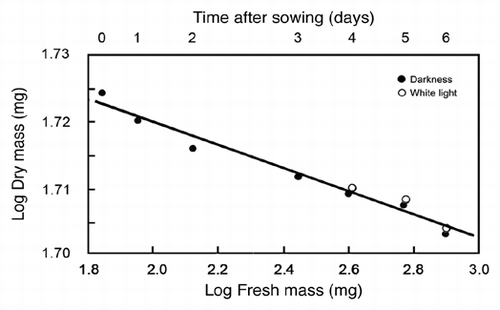
Table 1 List of abbreviations and definitions
Acknowledgements
We thank Prof. W.R. Briggs (Department of Plant Biology, Carnegie Institution for Science, Stanford, CA) for providing laboratory space and consultation.
This work was supported by the Alexander von Humboldt-Stiftung (AvH, Bonn, Germany, Fellowship Stanford/USA 2009–2010 to UK).
References
- Kleiber M. The Fire of Life: An Introduction to Animal Energetics 1961; New York John Wiley
- Niklas KJ. Plant Allometry. The Scaling of Form and Process 1994; Chicago and London The University of Chicago Press
- Kutschera U, Niklas KJ. Evolutionary plant physiology: Charles Darwin's forgotten synthesis. Naturwissenschaften 2009; 96:1339 - 1354
- Mori S, Yamaji K, Ishida A, Prokushkin G, Masyagina OV, Hagihara A, et al. Mixed-powered scaling of whole-plant respiration from seedlings to giant trees. Proc Natl Acad Sci USA 2010; 107:1447 - 1451
- White CR. Physiology: There is no single P. Nature 2010; 464:691 - 692
- Bewley JD. Seed germination and dormancy. Plant Cell 1997; 9:1055 - 1066
- Briggs WR, Olney MA. Photoreceptors in plant photomorphogenesis to date: Five phytochromes, two cryptochromes, one phototropin and one superchrome. Plant Physiol 2001; 125:85 - 88
- Borisjuk L, Rolletschek H. The oxygen status of the developing seed. New Phytol 2009; 182:17 - 30
- Reich PB, Tjoelker MG, Machado JL, Oleksyn J. Universal scaling of respiratory metabolism, size and nitrogen in plants. Nature 2006; 439:457 - 461
- West GB, Brown JH, Enquist BJ. A general model for the structure and allometry of plant vascular systems. Nature 1999; 400:664 - 667
- Enquist BJ, Allen AP, Brown JH, Gillooly JF, Kerkoff AJ, Niklas KJ, et al. Biological scaling: Does the exception prove the rule?. Nature 2007; 445:9 - 10
- Kutschera U. Cell-wall synthesis and elongation growth in hypocotyls of Helianthus annuus L. Planta 1990; 181:316 - 323
- Kutschera U, Niklas KJ. The epidermal-growth-control theory of stem elongation: An old and a new perspective. J Plant Physiol 2007; 164:1395 - 1409
- Kutschera U, Deng Z, Oses-Prieto JA, Burlingame AL, Wang ZY. Cessation of coleoptile elongation and loss of auxin sensivity in developing rye seedlings. A quantitative proteomic analysis. Plant Signal Behav 2010; 5:509 - 517
- Kutschera U. Fusicoccin-induced growth and dark respiration in rye coleoptiles. J Plant Physiol 1999; 154:554 - 556
- Kutschera U. Bacterial colonization of sunflower cotyledons during seed germination. J Appl Bot 2002; 76:96 - 98
- Sokal RR, Rohlf FJ. Biometry 1981; 2nd ed New York Freeman WH
- Warton DI, Weber NC. Common slope tests for bivariate errors-in-variables models. Biometrical J 2002; 44:161 - 174
- Niklas KJ. Plant Biomechanics. An Engineering Approach to Plant Form and Function 1992; Chicago and London The University of Chicago Press
- Hodick D, Kutschera U. Light-induced inhibition of elongation growth in sunflower hypocotyls. Biophysical and ultrastructural investigations. Protoplasma 1992; 168:7 - 13