Abstract
Accumulation of unfolded protein or misfolded protein causes endoplasmic reticulum (ER) stress. Increased salt concentration activates a stress response pathway in the ER in Arabidopsis thaliana to induce the expression of several salt stress response genes, leading to a more optimal protein folding environment in the ER. In addition, some salt stress-regulated proteins require zinc for their activity, including some zinc-dependent DNA binding proteins and zinc-finger proteins. In a recent study, we reported that ZTP29, a putative zinc transporter at the ER membrane, is involved in the response to salt stress through regulation of zinc level in the ER to induce the UPR pathway. In this addendum, we propose a testable hypothesis for the role of ZTP29 in the response to salt stress via the regulation of zinc levels in the ER.
High salinity is a common abiotic stress that adversely affects plant growth and crop production.Citation1 Plants must sense the stress and transduce stress signals to activate response pathways leading to adaptation to, or tolerance of, the abiotic stress in salt environment.Citation2 Salt stress activates a stress response pathway in the endoplasmic reticulum (ER) in Arabidopsis thaliana, indicating that the adaptation of plants to salt stress involves ER stress signal regulation.Citation3,Citation4 There is limited understanding of molecular mechanisms on ER stress in plants, as compared to yeast and mammalian cells. bZIP60, bZIP28, bZIP17 are three membrane-associated basic domain/leucine zipper (bZIP) factors, which have been reported as candidates for ER-folding proteins in plants.Citation5–Citation7 BiP acts as a general chaperone in the ER lumen, due to its ability to discriminate between properly folded and unfolded protein structures.Citation8 Unfolded or misfolded proteins are retained in the ER and form stable complexes with BiP and other ER resident chaperones.Citation9 Zinc deficiency induces unfolded protein response (UPR) in most eukaryotes.Citation10 Zinc is an important trace element, which participates in physiological and biochemical process in vivo. The requirement of zinc for proper ER function is evolutionarily conserved.
ZTP29 Functions Upstream of UPR Induced by Salt Stress
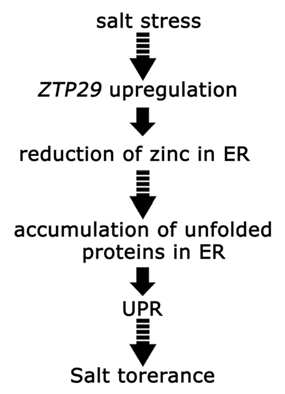
In a previous studyCitation11 we showed that ZTP29 is a putative zinc transporter at the ER membrane in Arabidopsis thaliana. ZTP29 expression is induced by salt stress and ztp29 mutant plants exhibit various phenotypes of NaCl hypersensitivity. In addition, salt stress-induced upregulation of the UPR pathway genes BiP2 and bZIP60 is inhibited in ztp29 mutant plants. These observations suggest that ZTP29 is involved in the response to salt stress through the decrease of zinc levels in the ER, which is required to induce the UPR pathway. However, the upregulation of BiP2 and bZIP60 still occurred in the ztp29 mutant plants, as well as wildtype plants, after treatment with turicamycin (TM) and dithiothreitol (DTT), two traditional UPR-induce reagents that can cause an ER stress response in plants, as well as in both yeast and mammalian cells.Citation12 These results indicate that loss-of-function mutation of ZTP29 does not cause protein unfolding or misfolding in the ER directly. Therefore, we suggest that ZTP29 might function upstream of proper folding of ER proteins in the ER (). In our model, salt stress induces upregulation of ZTP29 expression, which functions in the transportation of zinc from the ER to the cytoplasm. As a result, zinc levels in the ER are reduced, resulting in the induction of UPR. Activation of UPR leads tolerance to salt stress in plants. However, there are gaps in this suggested pathway. For instance, the mechanism by which the reduction of zinc in the ER results in protein unfolding or misfolding is unknown. Feasible techniques for the direct observation of the changes of zinc level in the ER under salt stress are needed for further studies.
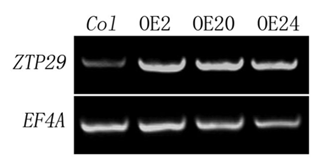
ZTP29 Maintains Zinc Homeostasis in Salt Stress
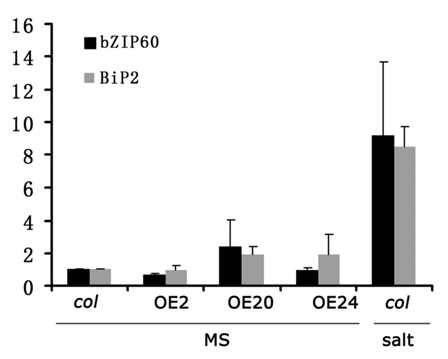
In the model we proposed, upregulation of ZTP29 expression may activate the UPR pathway. However, overexpression of ZTP29 in Arabidopsis plants did not result in obvious changes in BiP2 and bZIP60. Three ZTP29-overexpressing Arabidopsis lines, OE2, OE20 and OE24 were selected for evaluation (). No obvious upregulation of genes involved in UPR was observed in these overexpression transgenic plants under normal conditions, as compared to salt treated wildtype plants. Results from qRT-PCR showed that there were no significant changes of expression of BiP2 and bZIP60 in the Arabidopsis plants of ZTP29-overexpressing lines (OE2, OE20 and OE24), compared to that in salt-stress-induced wild-type plants (). These observations strengthen our hypothesis that expression of ZTP29 does not cause protein unfolding or misfolding in the ER directly. However, these observations suggest that in addition to ZTP29, other component(s) may be needed to activate the UPR pathway induced by salt stress. Upregulation of ZTP29 expression might play a key role in zinc transportation from the ER to cytoplasm, a necessary step for the activation of UPR pathway under salt stress. Nevertheless, other possibilities should be considered. Zinc in the ER may be one of the sources of cytoplasmic zinc under salt stress. Upregulation of ZTP29 expression in salt conditions not only induces UPR but also plays a role in keeping the cytoplasmic zinc level balanced, which might also contribute to plant adaptation to salt stress.
Conclusions and Perspectives
In salt stress conditions, ZTP29 plays a key role in induction of UPR. ZTP29 functions upstream of proper folding of ER proteins in the ER and might be also involved in maintaining zinc homeostasis, both of which contribute to plant adaptation to salt stress. Further studies are needed to elucidate the precise mechanisms involved in these processes. Methods are needed for the direct observation of changes in the zinc level in the ER under salt stress. Further studies will examine the mechanism by which reduction in the level of zinc in the ER results in protein unfolding or misfolding. In addition, investigation into the role of ZTP29 in the maintenance of zinc homeostasis will shed further light on the phenomena of plant adaptation to salt stress.
Figures and Tables
Figure 1 A shematic representation of the model of the involvement of ZTP29 in the induction of UPR by salt stress. This model propose that ZTP29 functions upstream of proper folding of ER proteins.
Acknowledgements
This work was supported by grants from the National Basic Research Program of China (2006CB100101), the 111 Project (B06003), and the National Natural Science Foundation of China (30830058 and 30721062) to M.Y.
Addendum to:
References
- Xiong LM, Schumaker KS, Zhu JK. Cell Signaling during Cold, Drought and Salt Stress. Plant Cell 2002; 14:165 - 183
- Serrano R, Rodriguez-Navarro A. Ion homeostasis during salt stress in plants. Curr Opin Cell Biol 2001; 13:399 - 404
- Liu JX, Srivastava R, Che P, et al. Salt stress responses in Arabidopsis utilize a signal transduction pathway related to endoplasmic reticulum stress signaling. Plant J 2007; 51:897 - 909
- Koiwa H, Li F, McCully MG, Mendoza I, et al. The STT3a subunit isoform of the Arabidopsis oligosaccharyl transferase controls adaptive responses to salt/osmotic stress. Plant Cell 2003; 15:2273 - 2284
- Iwata Y, Koizumi N. An Arabidopsis transcription factor, AtbZIP60, regulates the endoplasmic reticulum stress response in a manner unique to plants. Proc Natl Acad Sci USA 2005; 102:5280 - 5285
- Liu JX, Srivastava R, Che P, Howell SH. An endoplasmic reticulum stress response in Arabidopsis is mediated by proteolytic processing and nuclear relocation of a membrane-associated transcription factor, bZIP28. Plant Cell 2007; 19:4111 - 4119
- Liu JX, Srivastava R, Che P, et al. Salt stress responses in Arabidopsis utilize a signal transduction pathway related to endoplasmic reticulum stress signaling. Plant J 2007; 51:897 - 909
- Gulow K, Bienert D, Haas IG. BiP is feed-back regulated by control of protein translation efficiency. J Cell Sci 2002; 115:2443 - 2452
- Gething MJ, Sambrook JF. Protein folding in the cell. Nature 1992; 355:33 - 45
- Ellis CD, Wang F, MacDiarmid CW, et al. Zinc and the Msc2 zinc transporter protein are required for endoplasmic reticulum function. J Cell Biol 2004; 166:325 - 335
- Wang MY, Xu QY, Yu JJ, Yuan M. the putative Arabidopsis zinc transporter ZTP29 is involved in the response to salt stress. Plant Mol Biol 2010; 73:467 - 479
- Okushima Y, Koizumi N, Yamaguchi Y, et al. Isolation and characterization of a putative transducer of endoplasmic reticulum stress in Oryza sativa. Plant Cell Physiol 2002; 43:532 - 539