Abstract
Light is the ultimate energy source for photo-autotrophs on earth. For green plants, however, it can also be toxic under certain stressful environmental conditions and at critical developmental stages. Anthocyanins, a class of flavonoids, act as an effective screening mechanism that allows plant survival and proliferation under occasional periods of harmful irradiation through modulation of light absorption. Apart from light-sensing through photoreceptors such as phytochrome and cryptochrome, plants use the photosynthetic electron transfer (PET) chain to integrate light information. The redox status of the plastoquinone (PQ) pool of the PET chain regulates anthocyanin biosynthesis genes, together with the plant hormone ethylene and plant hormone-like sugars. A complex signaling apparatus in acyanic cells appears to transduce information to cyanic cells to regulate anthocyanin production through an intercellular signaling pathway that remains largely uncharacterized. This review will highlight recent advances in this field and their implications for the regulation of anthocyanin pigmentation.
Light is the key stimulus for anthocyanin biosynthesis among numerous other environmental cues such as temperature, nutrient deficiency, water status, wounding and pathogen attack.Citation1 The production of anthocyanin in young seedlings requires prolonged exposure to visible and near-visible wavelengths of light at a relatively high photon flux, and the extent of the plant response to light is a function of light quality and quantity.Citation2 High-light conditions trigger the accumulation of anthocyanin in vegetative tissues, which serves as a means to safeguard against the detrimental effects of excess light on the photosynthetic apparatus, which can lead to photo-inhibition. Sugar is a common regulator of a number of genes involved in photosynthesis, carbohydrate metabolism and pathogenesis. It also induces anthocyanin biosynthesis in Arabidopsis seedlings in the form of disaccharide sugars such as sucrose (Suc) and maltose.Citation3–Citation5 Plant hormones such as abscisic acid, jasmonic acid, cytokinin and gibberellic acid act in concert with sugar in the presence of light to regulate anthocyanin accumulation in either a positive or negative manner.Citation6 Thus, light, sugar and hormone signals interact in an intricate signaling network that simultaneously coordinates plant homeostasis and regulates anthocyanin pigmentation. Here, we review recent advances in our understanding of these interactions between light, sugar and ethylene and how they regulate anthocyanin pigmentation in Arabidopsis.
Sucrose, Ethylene and Light Coordinately Regulate Anthocyanin Accumulation in the Leaf
Carbohydrates are potent inducers of anthocyanin pigmentation in different organs in several plant species through their ability to transcriptionally activate anthocyanin biosynthesis genes. For instance, Suc promotes anthocyanin accumulation in the developing corollas of Petunia hybrida, Vitis vinifera cell suspensions and radish (Raphanus sativus) hypocotyls.Citation7–Citation9 In Arabidopsis, anthocyanins are produced in cotyledons or leaves when plants are grown on a sugar-containing medium, as evidenced by purple coloration of the organs.Citation10 More recently, genetic elements through which Sucspecific induction of anthocyanin biosynthesis is mediated have been identified in Arabidopsis seedlings.Citation4,Citation5 Interactions or crosstalk, among hormones and sugars form a complex network of overlapping signals that coordinate overall plant growth and development.Citation11 Ethylene markedly suppresses anthocyanin accumulation. Several studies have shown that inhibition of ethylene biosynthesis by Co2+ and prevention of ethylene action by silver increases anthocyanin content in maize (Zea may) seedlings.Citation12–Citation14 Similarly, in transgenic tobacco (Nicoatiana tabacum) plants expressing a mutated melon (Cucumis melo) ethylene receptor gene, ethylene response 1 (ETR1H69A), higher levels of anthocyanin accumulate in flower petals as compared to control plants.Citation15 In contrast, the concentration of anthocyanin in constitutive triple response 1 (ctr1) mutants, which exhibit a constitutive response to ethylene, is similar to wild-type (WT) plants in the presence of a high concentration of Suc.Citation16,Citation17 Subsequent finding in Arabidopsis confirmed that ethylene is a negative regulator of anthocyanin biosynthesis, and ethylene signaling is mitigated through a triple response pathway.Citation18
In Arabidopsis, light signaling is perceived and transduced by photoreceptors, including UV-B photoreceptor, cryptochrome 1 (CRY1) and phytochrome (PHY) A and B.Citation19–Citation21 The plant transcription factors HY5 and PIF3 positively regulate anthocyanin biosynthesis through direct binding to the promoters of anthocyanin structural genes, including CHS, CHI, F3H, F3'H, DFR and LDOX.Citation21 However, negative regulation of pigmentation by ethylene was shown to be independent of HY5.Citation18 In addition to photoreceptors, photosynthesis also contributes to the formation of anthocyanin. In turnip seedlings and non-chlorophyllous corn leaf, light-dependent anthocyanin accumulation was significantly inhibited by treatment with diuron, 3-(3,4-dicrhlorophenyl)-1, 1-dimethylurea (DCMU), a photosynthetic inhibitor.Citation21,Citation22 Consistent with this, in Arabidopsis, DCMU treatment of seedlings suppressed the accumulation of anthocyanin pigmentation in photosynthetically active leaf tissues in wild-type Columbia seedlings (shown in ), as well as in the ethylene-insensitive mutant etr1-1.Citation18 This suppression of anthocyanin accumulation was shown to be mediated through regulation of the transcription factors of the MYB-bHLH-WD40 (MBW) complex such as PAP1, Gl3 and EGL3. Additionally, there was an inverse relationship between the activity of the MBW complex and the level of MYBL2, which inhibits the formation of active MYB complexes. Ethylene maintains anthocyanin pigmentation in Arabidopsis leaves through the regulation of MYBL2 at the transcriptional level.Citation18
Physiological Basis of the Interaction between Light, Sugar and Ethylene
A feature of anthocyanin pigmentation during development is the transient nature of accumulation when either environmental or developmental changes render the plant more sensitive to environmental conditions. Prolonged accumulation of anthocyanin occurs only in tissues that do not have a photosynthetic carbon assimilation function and is favorable only under conditions of high light or in an arid habitat. The effects of developmental and environmental factors on anthocyanin pigmentation have been extensively reviewed in reference Citation1. Plants have evolved such temporal and spatial regulation systems in part because the accumulation and maintenance of anthocyanin involves an investment of energy that may reduce light capture and eventually carbon assimilation.Citation23,Citation24 Hence, the regulation of anthocyanin pigmentation seems to be intimately connected to the universal phenomenon of overall homeostasis, wherein negative reciprocity between pathways ensures that anthocyanin is synthesized and greening is suppressed during early stages of seeding growth, when the seedlings are most susceptible to light stress. Anthocyanin suppression appears to be mediated in part through the desensitization of certain structural genes and/or negative regulation of other interrelated pathways.Citation25,Citation26 Thus, negative regulation of anthocyanin accumulation by ethylene might be a mechanism whereby the proper balance between carbon assimilation and anthocyanin accumulation is maintained in target tissues, via the suppression of light- and sugar-induced anthocyanin pigmentation. Such a view is supported by the observation that an increase in the level of ethylene in vivo is accompanied by an increase in sugar and light dosage.Citation18
Mesophyll Cell-Derived Factors Signal Anthocyanin Biosynthesis in the Epidermal Cells
The accumulation of anthocyanin is a cell-autonomous response, meaning that color development is controlled at the level of the individual cell.Citation25,Citation27 Cotyledons and leaves are comprised of epidermis, mesophyll and vasculature. Light signals for anthocyanin synthesis seem to originate in photosynthetic cells in response to exogenously supplied sugars and then act at a distance on subepidermal and some vasculature cells, suggesting the presence of intercellular signaling molecules (see for details). These mesophyll-derived signals are as-yet unidentified, but signaling molecules could be generated in response to the plastoquinone (PQ) pool redox state and then transferred to the target cells. A similar phenomenon has been observed in petunia seedlings wherein corolla pigmentation is subject to regulation through light-derived signals originating from the calyx and leaves.Citation28 However, a role for photosynthetic electron transport in the regulation of corolla pigmentation was called into question by a later finding that DCMU treatment resulted in an insignificant change in corolla pigmentation, despite the fact that the cell layer underneath the epidermis contained green chloroplast.Citation28–Citation30 Such a discrepancy could arise from differences in the ability and efficiency of the photosynthetic apparatus of the underlying tissues, as well as the involvement of other organ-specific factors in generating the requisite signal.Citation30,Citation31 Photosynthesis signals could be induced via the thylakoid kinase STN7 or hydrogen peroxide and then transmitted to epidermal cells after being converted into an intercellular signal.Citation32 Alternatively, hormones that are known to either positively or negatively regulate anthocyanin biosynthesis, such as the gibberellins, jasmonic acid and abscisic acid, could be involved.Citation6 In fact, any intercellular signaling protein that is expressed in the inner mesophyll tissue and secreted to act on acyanic epidermal and vasculate cells could be involved in the induction of anthocyanin accumulation. The mesophyll-derived intercellular signaling protein stomagen has been shown to induce stomata development in Arabidopsis.Citation33 Regardless of which molecules participate in intercellular signaling, the regulation of anthocyanin content in the epidermis would protect inner photosynthetic cells from photoinhibition.
Conclusions and Future Perspectives
The positive and negative regulation of anthocyanin pigmentation by light, sugar and ethylene has important physiological implications. A role for tissue-specific mesophyll-derived redox signals in the intercellular signaling between cyanic and acyanic cells is emerging, and is likely to be significant in light of recent advances in our understanding of mesophyll-derived intercellular signaling mechanisms. This is a relatively new area of research that opens new avenues and presents new challenges for future investigations into the genetic components responsible for signaling between cyanic and acyanic cells in plants.
Figures and Tables
Figure 1 GUS expression in transgenic Arabidopsis seedlings harboring a GUS reporter gene driven by the PAP1 promoter (PAP1pro:GUS rep). Nine day-old seedlings were transferred to 0 (A) and 60 mM (B and C) Suc in the presence of 10 µM DCMU (C) and then exposed to white light (140 µmolm−2s−1) for 48 hours.
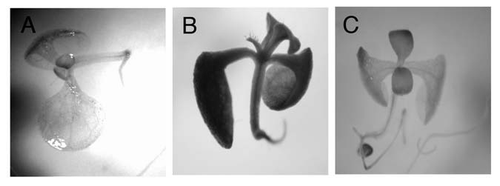
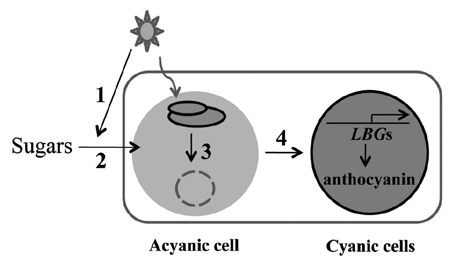
Figure 2 Proposed model for redox mediated signaling between cyanic and acyanic cells. (1) Light signals induce exogenous Suc uptake. (2) Apoplastic Suc in cotyledons and leaves is sensed by photosynthetic electron transport in mesophyll cells and generates a plastid signal. (3) The plastid signal is transformed into a mesophyll signal and then (4) transmitted to epidermal or vasculate cells, activating the MBW (Myb-bHLH-WD40) regulatory complex and downregulating MybL2 expression. This in turn leads to the specific upregulation of several late anthocyanin biosynthesis genes (LBGs), resulting in the accumulation of anthocyanin. The signaling molecule that mediates this intercellular response remains elusive.
Acknowledgements
This work was supported by the Crop Functional Genomics Center (grant no. CG2151) and the NRF (grant no. 2009-008146) funded by the Ministry of Education, Science, and Technology, Korea, and by a Korea Research Foundation Fellowship (grant no. KRF-2006-C00083 to P.K.D.).
References
- Steyn WJ, Wand SJE, Holcroft DM, Jacobs G. Anthocyanins in vegetative tissues: a proposed unified function in photoprotection. New Phytol 2002; 155:349 - 361
- Mancinelli AL. Light-dependent anthocyanin synthesis: a model system for the study of plant photomorphogenesis. Bot Rev 1985; 51:107 - 157
- Rolland F, Baena-González E, Sheen J. Sugar sensing and signaling in plants: conserved and novel mechanisms. Annu Rev Plant Biol 2006; 57:676 - 709
- Teng S, Keurentjes J, Bentsink L, Koornneef M, Smeekens S. Sucrose-specific induction of anthocyanin biosynthesis in Arabidopsis requires the MYB75/PAP1 gene. Plant Physiol 2005; 139:1840 - 1852
- Solfanelli C, Poggi A, Loreti E, Alpi A, Perata P. Sucrose-specific induction of the anthocyanin biosynthetic pathway in Arabidopsis. Plant Physiol 2006; 140:637 - 646
- Loreti E, Povero G, Novi G, Solfanelli C, Alpi A, Perata P. Gibberellins, jasmonate and abscisic acid modulate the sucrose-induced expression of anthocyanin biosynthetic genes in Arabidopsis. New Phytol 2008; 179:1004 - 1016
- Weiss D. Regulation of flower pigmentation and growth: multiple signaling pathways control anthocyanin synthesis in expanding petals. Physiol Plant 2000; 110:152 - 157
- Larronde F, Krisa S, Decendit A, Cheze C, Merillon JM. Regulation of polyphenol production in Vitis vinifera cell suspension cultures by sugars. Plant Cell Rep 1998; 17:946 - 950
- Hara M, Oki K, Hoshino K, Kuboi T. Enhancement of anthocyanin biosynthesis by sugar in radish (Raphanus sativus) hypocotyl. Plant Sci 2003; 164:259 - 265
- Ohto M, Onai K, Furukawa Y, Aoki E, Araki T, Nakamura K. Effects of sugar on vegetative development and floral transition in Arabidopsis. Plant Physiol 2001; 127:252 - 261
- Leon P, Sheen J. Sugar and hormone connections. Trends in Plant Sci 2003; 8:110 - 116
- Craker LE, Wetherbee PJ. Ethylene, light and anthocyanin synthesis. Plant Physiol 1973; 51:436 - 438
- Kang BG, Burg SP. Role of ethylene in phytochrome induced anthocyanin biosynthesis. Planta 1973; 110:227 - 235
- Rengel Z, Kordan HA. Effects of growth regulators on light-dependent anthocyanin production in Zea mays seedlings. Physiol Plant 1987; 69:511 - 519
- Takada K, Ishimaru K, Minamisawa K, Kamada H, Ezura H. Expression of a mutated melon ethylene receptor gene Cm-ETR1/H69A affects stamen development in Nicotiana tabacum. Plant Sci 2005; 169:935 - 942
- Kieber JJ, Rothenberg M, Roman G, Feldmann KA, Ecker JR. CTR1, a negative regulator of the ethylene response pathway in Arabidopsis, encodes a member of the Raf family of protein kinases. Cell 1993; 72:427 - 441
- Gibson SI, Laby RJ, Kim D. The sugar-insensitive1 (sis1) mutant of Arabidopsis is allelic to ctr1. Biochem Biophys Res Commun 2001; 280:196 - 203
- Jeong SW, Das PK, Jeoung SC, Song JY, Lee HK, Kim YK, et al. Ethylene suppression of sugar-induced anthocyanin pigmentation in Arabidopsis thaliana. Plant Physiol 2010; 150:1514 - 1531
- Wade HK, Bibikova TN, Valentine WJ, Jenkins GI. Interactions within a network of phytochrome, cryptochrome and UV-B phototransduction pathways regulate chalcone synthase gene expression in Arabidopsis leaf tissue. Plant J 2001; 25:675 - 685
- Shin J, Park E, Choi G. PIF3 regulates anthocyanin biosynthesis in a HY-5 dependent manner with both factors directly binding anthocyanin biosynthetic gene promoters in Arabidopsis. Plant J 2007; 49:981 - 994
- Schneider MJ, Stimson WR. Contributions of photosynthesis and phytochrome to the formation of anthocyanin in turnip seedlings. Plant Physiol 1971; 48:312 - 315
- Kim JS, Lee BH, Kim SH, Ok KH, Cho KY. Response to environmental and chemical signals for anthocyanin biosynthesis in non-chlorophyllous corn (Zea mays L.) leaf. J Plant Biol 2006; 49:16 - 25
- Drumm-Herrel H, Mohr H. Photosensitivity of seedlings differing in their potential to synthesize anthocyanin. Physiol Plant 1985; 64:60 - 66
- Burger J, Edwards GE. Photosynthetic efficiency and photodamage by UV and visible radiation in red versus green leaf Coleus varieties. Plant Cell Physiol 1996; 37:395 - 399
- Nick P, Ehmann B, Furuya M, Schäfer E. Cell communication, stochastic cell responses and anthocyanin pattern in mustard cotyledons. Plant Cell 1993; 5:541 - 552
- Bowler C, Neuhaus G, Yamagata H, Chua NH. Cyclic GMP and calcium mediate phytochrome phototransduction. Cell 1994; 77:73 - 81
- Lancaster JE, Grant JE, Lister CE, Taylor MC. Skin colour in apples—Influence of copigmentation and plastid pigments on shade and darkness of red colour in five genotypes. J Amer Soc Hort Sci 1994; 119:63 - 69
- Moscovici S, Moalem-Beno D, Weiss D. Leaf-mediated light responses in petunia flowers. Plant Physiol 1996; 110:1275 - 1282
- Weiss D, Halevy AH. The role of light reaction in the regulation of anthocyanin synthesis in Petunia corolla. Physiol Plant 1991; 81:127 - 133
- Weiss D, Schonfeld M, Halevy AH. Photosynthetic activity in the Petunia corolla. Plant Physiol 1988; 87:666 - 670
- Weiss D, van Blokland R, Kooter JM, Moi JNM, van Tunen AJ. Gibberellic acid regulates chalcone synthase gene transcription in the corolla of Petunia hybrida. Plant Physiol 1992; 98:191 - 197
- Pfannschmidt T, Brautigam K, Wagner R, Dietzel L, Schroter Y, Steiner S, Nykytenko A. Potential regulation of gene expression in photosynthetic cells by redox and energy state: approaches towards better understanding. Ann Bot 2009; 103:599 - 607
- Sugano SS, Shimada T, Imai Y, Ikawa K, Tamai A, Mori M, Hara-Nishimura I. Stomagen positively regulates stomatal density in Arabidopsis. Nature 2010; 463:241 - 244