Abstract
Upon chronic UV treatment pavement cell expansion in Arabidopsis leaves is reduced, implying alterations in symplastic and apoplastic properties of the epidermal cells. In this study, the effect of UV radiation on microtubule patterning is analysed, as microtubules are thought to serve as guiding rails for the cellulose synthase complexes depositing cellulose microfibrils. Together with hemicelluloses, these microfibrils are regarded as the load-bearing components of the cell wall. Leaves of transgenic plants with fluorescently tagged microtubules (GFP-TUA6) were as responsive to UV as wild type plants. Despite the UV-induced reduction in cell elongation, confocal microscopy revealed that cellular microtubule arrangements were seemingly not affected by the UV treatments. This indicates an unaltered deposition of cellulose microfibrils in the presence of UV radiation. Therefore, we surmise that the reduction in cell expansion in UV-treated leaves is most probably due to changes in cell wall loosening and/or turgor pressure.
Photosynthetic functions such as solar light capture and carbon fixation are highly evolved features of plant leaves. To fulfil these functions in an optimal way, leaf development needs to be tuned to environmental conditions. Leaves are continuously exposed and subjected to environmental influences, which serve as co-regulators of leaf and plant development.Citation1 This ability of plants to adapt, secures the plant's survival, even under non-optimal conditions. An example of a regulatory environmental parameter is solar light, indispensable for photosynthesis but potentially causing photoinhibition and/or UV-radiation stress. The highly energetic ultraviolet B (UV-B) rays of short wavelengths (280–315 nm) can both cause damage, as well as induce a range of specific metabolic and morphogenic plant responses. It was reported before that exposure to low dose UV radiation reduces Arabidopsis leaf size due to a decreased cell size.Citation2 Expansion of leaf epidermal cells of Arabidopsis thaliana is the combined action of promotion and restriction of growth, resulting in the typical irregular sinuous pavement cells. It has been postulated that cellulose microfibrils are responsible for generating a force opposing isotropic expansion by creating neck regions in between outgrowing lobes.Citation3 As the microtubule cytoskeleton is believed to serve as guiding rails for the cellulose synthase complexes (CESAs),Citation4 the deposition of the cellulose fibrils is intimately linked to the cortical microtubule arrangement. We have studied the UV-effect on microtubule organisation in leaf epidermal cells whose expansion had decreased upon this UV radiation. Microtubules in the adaxial pavement cells of the fourth leaf were monitored on several successive days in a transgenic line containing GFP fused to tubulin A6.Citation5 The chronic UV treatment was started on day 0 when the plants were 2 weeks old, using UV exposure conditions as described in reference Citation2. First the responsiveness of the GFP-TUA6 plants to UV radiation was evaluated. Similar to wild type (WT) plants,Citation2 the GFP-TUA6 plants had smaller leaves following 8 days of UV treatment (t-test, p < 0.01) (). This was caused by a significant reduction in the generalized cell area average of all measured cells, irrespective of the location within the leaf (; t-test, p < 0.01). In more detail, the average cell area within the base, middle and top zones of the GFP-TUA6 leaf was systematically lower in UV-treated leaves from 8 days after the treatment started onwards (data not shown).
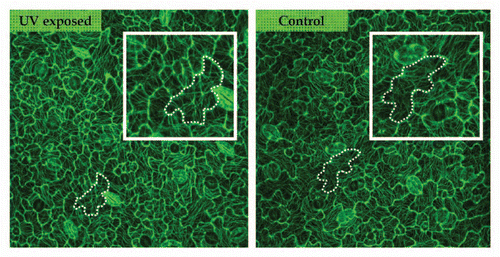
As GFP-TUA6 leaves were as responsive to UV radiation as wild type leaves, confocal microscopy was used to visualize the organisation of the cortical microtubules facing the outer periclinal wall of the adaxial epidermis. No clear difference in microtubule (re)organization could be detected during the development of pavement cells, and throughout the UV treatment period. As shown in at day 2, pavement cells with comparable areas are similarly shaped in control and UV-irradiated plants and contain similar microtubule arrangements ( and marked cells). This means that microtubule organization is not directly affected by the UV exposure and that shape development proceeds in an analoguous manner as under control conditions. This lack of alteration in the microtubule arrangement can be observed for cells at the leaf tip, which were already in the process of lobe formation at the start of the exposure period, as well as for cells at the leaf base. Under our growth conditions, and in the monitored leaf number 4, cell proliferation still took place in this part of the leaf and lobes only started to appear on the cell surface. As microtubules are linked to the deposition of cellulose microfibrils, it can be assumed that no alterations in cellulose deposition occur upon UV treatment either. We can therefore conclude that the process of lobe formation and microtubule patterning is not impeded and that only the extent of cell expansion is restricted upon UV exposure.
According to the Lockhart equation,Citation6 cell (wall) growth is modulated by wall biomechanics and turgor pressure. Concerning turgor pressure, no clear differences in this factor between UV-exposed and control plants of Lactuca sativa L.Citation7 and Pisum sativumCitation8 could be observed, reinforcing the idea that especially the modulation of cell wall properties is the main factor causing the observed UV-induced reduction in cell expansion. Some reports indicate differential expression of wall loosening enzymes like expansins or xyloglucan endotransglycosylase/hydrolases (XTHs),Citation9,Citation10 or cell wall strengthening enzymes as particular peroxidasesCitation7 after UV exposure. Another key event could involve UV-mediated changes in the phenylpropanoid pathway, which may cause changes in the lignin biosynthesis. As shown by the literatureCitation11–Citation14 lignin may well be an important modulator of cell wall architecture in Arabidopsis and therefore alterations in lignin synthesis could form the basis for morphological modifications. Further research on the cell wall properties of UV-treated plants may resolve this uncertainty.
As a general conclusion we can state that the patterning of microtubules is not altered, but that alterations in cell wall composition or arrangements are the most plausible candidates for the observed reduction in pavement cell expansion upon chronic UV treatment.
Figures and Tables
Figure 1 Effect of UV radiation on leaf and cell area after different days of UV radiation. Open asterisks indicate a statistically significant difference in leaf area between UV-treated and control plants, black asterisks indicate statistically significant difference in cell area (t-test, *p < 0.05, **p < 0.01, ***p < 0.001). Error bars indicate the standard error for five different leaves at all measured time-points and 600, 170 and 180 cells at day 0, 8 and 12 respectively.
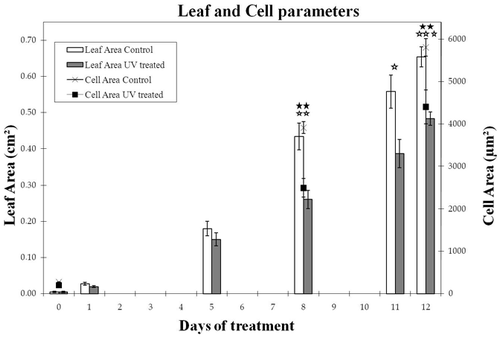
Figure 2 Microtubule pattern in control and UV-exposed leaves visualized using GFP-TUA6 and confocal microscopy. Both images are from cells at the mid zone of the fourth leaf at day 2. Microtubules are similarly arranged in equally shaped and sized cells of control and UV-exposed leaves. The marked cells show a pattern whereby the tubules are centred in the neck regions between two outgrowing lobes.
Acknowledgements
This work was supported by grants from the Research Foundation Flanders (FWO—Project G.0382.04N), the FWO Research Community (W0.038.04N) and the University of Antwerp. Authors also acknowledge the use of the CellP software provided by the department of Veterinary Sciences (University Antwerp, Professor Adriaensen).
Addendum to:
References
- Walter A, Silk WK, Schurr U. Environmental effects on spatial and temporal patterns of leaf and root growth. Annu Rev Plant Biol 2009; 60:279 - 304
- Hectors K, Jacques E, Prinsen E, Guisez Y, Verbelen JP, Jansen MAK, Vissenberg K. UV radiation reduces epidermal cell expansion in leaves of Arabidopsis thaliana. J Exp Bot 2010; 61:4339 - 4349
- Fu Y, Gu Y, Zheng Z, Wasteneys G, Yang Z. Arabidopsis interdigitating cell growth requires two antagonistic pathways with opposing action on cell morphogenesis. Cell 2005; 120:687 - 700
- Gutierrez R, Lindeboom JJ, Paredez AR, Emons AM, Ehrhardt DW. Arabidopsis cortical microtubules position cellulose synthase delivery to the plasma membrane and interact with cellulose synthase trafficking compartments. Nat Cell Biol 2009; 11:797 - 808
- Ueda K, Matsuyama T, Hashimoto T. Visualization of microtubules in living cells of transgenic Arabidopsis thaliana. Protoplasma 1999; 206:201 - 206
- Lockhart A. An analysis of irreversible plant cell elongation. J Theor Biol 1965; 8:264 - 275
- Wargent JJ, Moore JP, Ennos AR, Paul ND. Ultraviolet radiation as a limiting factor in leaf expansion and development. Photochem Photobiol 2009; 85:279 - 286
- Nogués S, Allen DJ, Morison JIL, Baker NR. Ultraviolet-B radiation effects on water relations, leaf development and photosynthesis in droughted pea plants. Plant Physiol 1998; 117:173 - 181
- Van Sandt V, Suslov D, Verbelen JP, Vissenberg K. Xyloglucan endotransglucosylase activity loosens a plant cell wall. Ann Bot 2007; 100:1467 - 1473
- Hectors K, Prinsen E, De Coen W, Jansen MAK, Guisez Y. Arabidopsis thaliana plants acclimated to low dose rates of ultraviolet B radiation show specific changes in morphology and gene expression in the absence of stress symptoms. New Phytol 2007; 175:225 - 270
- Abdulrazzak N, Pollet B, Ehlting J, Larsen K, Asnaghi C, Ronseau S, et al. A coumaroyl-ester-3-hydroxylase insertion mutant reveals the existence of nonredundant meta-hydroxylation pathways and essential roles for phenolic precursors in cell expansion and plant growth. Plant Physiol 2006; 140:30 - 48
- Ringli C, Bigler L, Kuhn BM, Leiber RM, Diet A, Santelia D, et al. The modified flavonol glycosylation profile in the Arabidopsis rol1 mutants results in alterations in plant growth and cell shape formation. Plant Cell 2008; 20:1470 - 1481
- Darley CP, Forrester AM, McQueen-Mason SJ. The molecular basis of plant cell wall extension. Plant Mol Biol 2001; 47:179 - 195
- Li X, Bonawitz ND, Weng JK, Chapple C. The growth reduction associated with repressed lignin biosynthesis in Arabidopsis thaliana is independent of flavonoids. Plant Cell 2010; 22:1620 - 1632