Abstract
Thioredoxin (NTR/TRX) and glutathione (GSH/GRX) are the two major systems which play a key role in the maintenance of cellular redox homeostasis. They are essential for plant development, cell division or the response to environmental stresses. In a recent article,1 we studied the interplay between the NADP-linked thioredoxin and glutathione systems in auxin signaling genetically, by associating TRX reductase (ntra ntrb) and glutathione biosynthesis (cad2) mutations. We show that these two thiol reduction pathways interfere with developmental processes. This occurs through modulation of auxin activity as shown by genetic analyses of loss of function mutations in a triple ntra ntrb cad2 mutant. The triple mutant develops almost normally at the rosette stage but fails to generate lateral organs from the inflorescence meristem, producing almost naked stems that are reminiscent of mutants affected in PAT (polar auxin transport) or biosynthesis. The triple mutant exhibits other defects in processes regulated by auxin, including a loss of apical dominance, vasculature defects and reduced secondary root production. Furthermore, it has lower auxin (IAA) levels and decreased capacity for PAT, suggesting that the NTR and glutathione pathways influence inflorescence meristem development through regulation of auxin transport and metabolism.
Exposure of living organisms to environmental stresses triggers various defense and developmental responses. Redox signaling is involved in many aspects of these responses.Citation2–Citation6 The key players in these responses are the NADPH-dependent glutathione/glutaredoxin system (NGS) and the NADPH-dependent thioredoxin system (NTS). TRX and GRX play key roles in the maintenance of cellular redox homeostasis.Citation7–Citation10 Genetic approaches aiming to identify functions of TRX and GRX in knock-out plants have largely been limited by the absence of phenotypes of single mutants, presumably due to functional redundancies among members of the multigene families of TRX and GRX.Citation11 Interplay between NTS and NGS pathways have been studied in different organismsCitation12–Citation17 and association of mutants involved in these two pathways have recently revealed new functions in several aspects of plant development.Citation4–Citation6
A Cross between ntra ntrb and gsh1 Mutants Reveals New Functions of these Pathways
Previously, Reichheld et al.Citation4 constructed a double ntra ntrb mutant in the two genes encoding cytosolic and mitochondrial thioredoxin reductases (NTR). The viability of this mutant results from implication of the GSH-dependent reduction system as an alternative pathway for the thioredoxins, as shown pharmacologically by the high sensitivity of the ntra ntrb mutant to Buthionine sulfoximine (BSO), a specific inhibitor of GSH1, the first enzyme in glutathione synthesis. This has been confirmed by crossing the ntra ntrb mutant with the rootmeristemless1 (rml1) mutant. The triple ntra ntrb rml1 mutant shows an additive shootmeristemless phenotype as compared to the phenotype of rml1.Citation4 Crossing of the ntra ntrb mutant with a weaker allele of the gsh1 mutation, cadmiumsensitive2 (cad2), revealed a new phenotype related to flower meristem development. However, in contrast to the ntra ntrb rml1 mutant, vegetative meristem development is not perturbed. This developmental difference may be related to divergence in the inactivation of the GSH1 enzyme in the distinct mutants and therefore to the threshold of GSH availability. This implies that the floral meristem is more sensitive to redox perturbations than the vegetative meristem. Vernoux et al.Citation2 have suggested that the root meristem phenotype of rml1 is due to a higher sensitivity to glutathione perturbation of the root meristem, as compared to the shoot meristem. This may be partially due to the fact that the first step of glutathione biosynthesis is mainly performed in the chloroplast and accumulation of glutathione in the root meristem is probably dependent on the trafficking of its precursors from the shoot to the root.Citation18
Auxin-related Phenotypes of the ntra ntrb cad2 Mutants
The flowerless phenotype of the ntra ntrb cad2 mutant is reminiscent of mutants affected in polarized auxin transport (PAT). In agreement with this observation, we found PAT to be affected to the same degree as in the pin1 mutant, which is inactivated in the PIN1 auxin efflux carrier.Citation19 This suggests the intriguing possibility that perturbed PAT may be responsible for the pin-like phenotype of the ntra ntrb cad2 mutant. However, phenotypic analyses in different mutants suggests additive effects of the two thiol reduction pathways in different aspects of auxin metabolism. As represented in , cad2, ntra ntrb and ntra ntrb cad2 mutants are affected in polarized auxin transport (PAT) and root development. However, only the triple ntra ntrb cad2 mutant is affected in auxin level and shows a pin-like phenotype. These results suggest that the pin-like phenotype of ntra ntrb cad2 mutant is due to a combination of perturbed auxin synthesis and auxin transport while the root phenotype is due to perturbation of polar auxin transport. The picture is slightly different in the pin1 mutant (). While ntra ntrb cad2 and pin1 mutants harbour similar inflorescence meristem and PAT perturbations, the auxin level is not perturbed in the pin1 inflorescence meristem, in contrast to the ntra ntrb cad2 mutant.Citation1 Moreover, root growth in pin1 was shown to be poorly affected, presumely due to overlap with other PIN proteins.Citation20 Interestingly, the expression of several auxin efflux (PIN1, 2, 3 and 4) and influx (AUX1) carriers is perturbed in the ntra ntrb cad2 mutant, suggesting that the decreased PAT is mediated by modified PIN expression. In contrast to the pin1 mutant, root growth cannot be rescued by overlapping functions of PIN proteins.
It is known that the polarized membrane localization of PIN proteins is subjected to complex intracellular trafficking and that cellular internalization of the proteins is observed in many conditions (reviewed in ref. Citation21) Both our dataCitation1 and those recently published by Koprivova et al,Citation22 suggest that glutathione play a major role in the expression of PIN genes. BSO treatments of pPIN:PIN-GFP reporter plants considerably reduces the accumulation of PIN1, PIN2, PIN3 and PIN7 GFP fusion proteins. The cause of this effect is still unclear but it is apparently not due to mis-localization of the proteins at the plasma membrane. This suggests that BSO does not perturb intracellular vesicular trafficking, in contrast to the previously-described effect of brefeldin A on PIN localizationCitation23 or PINOID-dependent phosphorylation required for PIN1 polar localization.Citation24 Our data show that the steady-state expression levels of PIN genes are reduced by BSO treatment and that the in mRNA levels decrease in parallel with glutathione synthesis inhibition. Nevertheless, we do not know whether the decrease of PIN mRNAs is due to downregulation of transcription or to additional post-transcriptional mechanisms.
The phenotype of the ntra ntrb cad2 mutant is probably relayed by GRX and TRX (). CC-type glutaredoxins and TRXm were recently shown to act in flower development,Citation3,Citation25,Citation26 phytohormone signalingCitation27 and meristem developmentCitation6 through interaction with transcription factors.Citation28 Future investigations will focus on identification of the disulfide reductases involved in the auxin-dependent developmental phenotype of the ntra ntrb cad2 mutant.
Figures and Tables
Figure 1 Hypothetical model explaining the link between cellular redox status and auxin signaling in controlling inflorescence development in Arabidopsis. Modification of redox homeostasis triggered by environmental stress or by developmental signals is relayed by glutathione and NTR. The triple ntra ntrb cad2 mutant generated by crossing ntra ntrb and cad2 mutants (blue double-headed arrow) is mimicking the redox perturbation. Inactivation of NTR and decreased GSH availability is relayed by mis-reduction of GRX and TRXh and subsequently of TRXh/GRX target protein(s). These redox regulated target protein(s) are involved in meristem activity or/and auxin metabolism and their mis-reduction in the ntra ntrb cad2 leads to perturbation of inflorescence development and auxin metabolism. Black arrows refer to the direction of reduction, crossing is marked by a blue double-headed arrow and inefficient reduction by a horizontal line. The ntra ntrb cad2 developmental defect is caused by meristematic activity, auxin level, auxin transport or a combination of the three.
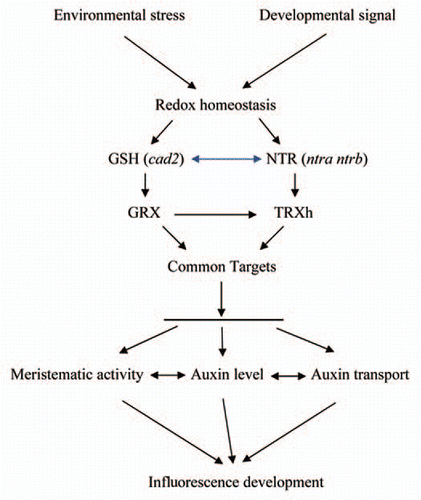
Table 1 Summary of the auxin-related phenotype observed in the mutants
Addendum to:
References
- Bashandy T, Guilleminot J, Vernoux T, Caparros-Ruiz D, Ljung K, Meyer Y, et al. Interplay between the NADP-linked thioredoxin and glutathione systems in Arabidopsis auxin signaling. Plant Cell 2010; 22:376 - 391
- Vernoux T, Wilson RC, Seeley KA, Reichheld JP, Muroy S, Brown S, et al. The ROOT MERISTEMLESS1/CADMIUM SENSITIVE2 gene defines a glutathione-dependent pathway involved in initiation and maintenance of cell division during postembryonic root development. Plant Cell 2000; 12:97 - 110
- Xing S, Rosso MG, Zachgo S. ROXY1, a member of the plant glutaredoxin family, is required for petal development in Arabidopsis thaliana. Development 2005; 132:1555 - 1565
- Reichheld JP, Khafif M, Riondet C, Droux M, Bonnard G, Meyer Y. Inactivation of thioredoxin reductases reveals a complex interplay between thioredoxin and glutathione pathways in Arabidopsis development. Plant Cell 2007; 19:1851 - 1865
- Marty L, Siala W, Schwarzländer M, Fricker MD, Wirtz M, Sweetlove LJ, et al. The NADPH-dependent thioredoxin system constitutes a functional backup for cytosolic glutathione reductase in Arabidopsis. Proc Natl Acad Sci USA 2009; 106:9109 - 9114
- Benitez-Alfonso Y, Cilia M, San Roman A, Thomas C, Maule A, Hearn S, et al. Control of Arabidopsis meristem development by thioredoxin-dependent regulation of intercellular transport. Proc Natl Acad Sci USA 2009; 106:3615 - 3620
- Arnér ES, Holmgren A. Physiological functions of thioredoxin and thioredoxin reductase. Eur J Biochem 2000; 267:6102 - 6109
- Buchanan BB, Balmer Y. Redox regulation: A broadening horizon. Annu Rev Plant Biol 2005; 56:187 - 220
- Rouhier N, Lemaire SD, Jacquot J. The role of glutathione in photosynthetic organisms: Emerging functions for glutaredoxins and glutathionylation. Annu Rev Plant Biol 2008; 59:143 - 166
- Montrichard F, Alkhalfioui F, Yano H, Vensel WH, Hurkman WJ, Buchanan BB. Thioredoxin targets in plants: The first 30 years. J Proteomics 2009; 72:452 - 474
- Meyer Y, Siala W, Bashandy T, Riondet C, Vignols F, Reichheld JP. Glutaredoxins and thioredoxins in plants. Biochim Biophys Acta 2008; 1783:589 - 600
- Carmel-Harel O, Storz G. Roles of the glutathione- and thioredoxin-dependent reduction systems in the Escherichia coli and Saccharomyces cerevisiae responses to oxidative stress. Annu Rev Microbiol 2000; 54:439 - 461
- Kanzok SM, Fechner A, Bauer H, Ulschmid JK, Müller HM, Botella-Munoz J, et al. Substitution of the thioredoxin system for glutathione reductase in Drosophila melanogaster. Science 2001; 291:643 - 646
- Gelhaye E, Rouhier N, Jacquot JP. Evidence for a subgroup of thioredoxin h that requires GSH/Grx for its reduction. FEBS Lett 2003; 555:443 - 448
- Trotter EW, Grant CM. Non-reciprocal regulation of the redox state of the glutathione-glutaredoxin and thioredoxin systems. EMBO Rep 2003; 4:184 - 188
- Trotter EW, Grant CM. Overlapping roles of the cytoplasmic and mitochondrial redox regulatory systems in the yeast Saccharomyces cerevisiae. Eukaryot Cell 2005; 4:392 - 400
- Koh CS, Navrot N, Didierjean C, Rouhier N, Hirasawa M, Knaff DB, et al. An atypical catalytic mechanism involving three cysteines of thioredoxin. J Biol Chem 2008; 283:23062 - 23072
- Li Y, Dankher OP, Carreira L, Smith AP, Meagher RB. The shoot-specific expression of gamma-glutamylcysteine synthetase directs the long-distance transport of thiol-peptides to roots conferring tolerance to mercury and arsenic. Plant Physiol 2006; 141:288 - 298
- Gälweiler L, Guan C, Müller A, Wisman E, Mendgen K, Yephremov A, et al. Regulation of polar auxin transport by AtPIN1 in Arabidopsis vascular tissue. Science 1998; 282:2226 - 2230
- Blilou I, Xu J, Wildwater M, Willemsen V, Paponov I, Friml J, et al. The PIN auxin efflux facilitator network controls growth and patterning in Arabidopsis roots. Nature 2005; 433:39 - 44
- Gao X, Nagawa S, Wang G, Yang Z. Cell polarity signaling: focus on polar auxin transport. Mol Plant 2008; 1:899 - 909
- Koprivova A, Mugford ST, Kopriva S. Arabidopsis root growth dependence on glutathione is linked to auxin transport. Plant Cell Rep 2010; 29:1157 - 1167
- Geldner N, Friml J, Stierhof YD, Jürgens G, Palme K. Auxin transport inhibitors block PIN1 cycling and vesicle trafficking. Nature 2001; 413:425 - 428
- Christensen SK, Dagenais N, Chory J, Weigel D. Regulation of auxin response by the protein kinase PINOID. Cell 2000; 100:469 - 478
- Xing S, Zachgo S. ROXY1 and ROXY2, two Arabidopsis glutaredoxin genes, are required for anther development. Plant J 2008; 53:790 - 801
- Li S, Lauri A, Ziemann M, Busch A, Bhave M, Zachgo S. Nuclear activity of ROXY1, a glutaredoxin interacting with TGA factors, is required for petal development in Arabidopsis thaliana. Plant Cell 2009; 21:429 - 441
- Ndamukong I, Abdallat AA, Thorow C, Fode B, Zander M, Weigel R, et al. SA-inducible Arabidopsis glutaredoxin interacts with TGA factors and suppresses JA-responsive PDF1.2 transcription. Plant J 2007; 50:128 - 139
- Murmu J, Bush MJ, DeLong C, Li S, Xu M, Khan M, et al. Arabidopsis bZIP transcription factors TGA9 and TGA10 interact with floral glutaredoxins ROXY1 and ROXY2 and are redundantly required for anther development. Plant Physiol 2010; 154:1492 - 1504