Abstract
Plant cells experience a tremendous amount of mechanical stress caused by turgor pressure. Because cells are glued to their neighbors by the middle lamella, supracellular patterns of physical forces are emerging during growth, usually leading to tension in the epidermis. Cortical microtubules have been shown to reorient in response to these mechanical stresses, and to resist them, indirectly via their impact on the anisotropic structure of the cell wall. In a recent study, we show that the polar localization of the auxin efflux carrier PIN1 can also be under the control of physical forces, thus linking cell growth rate and anisotropy by a common mechanical signal. Because of the known impact of auxin on the stiffness of the cell wall, this suggests that the mechanical properties of the extracellular matrix play a crucial signaling role in morphogenesis, notably controlling the polarity of the cell, as observed in animal systems.
The current development of high throughput analyses of gene regulatory networks is feeding a very complex view of growth control and shape changes. To go beyond the accumulation of data, the identification of universal and parsimonious mechanisms explaining the robustness of morphogenesis becomes a central issue in today's developmental biology.Citation1–Citation3 Among them, the coupling between molecular and mechanical signals has the strong advantage of providing a simple way to coordinate cell behavior synchronously and over long distances. The role of such signals has been investigated in different systems and the contribution of mechanical forces to animal development is now widely accepted, as the expression of key genes (e.g., TWISTCitation4) and key cellular events (e.g., mitotic spindle orientationCitation5) have been shown to depend on the mechanical environment of the tissue. Several mechanosensors have also been identified.Citation6
In an earlier study, we showed that the orientation of the cortical microtubular cytoskeleton in plant shoot meristems depends on the principal direction of mechanical stress. Cortical microtubules are known to guide the deposition of the cellulose microfibrils in the cell wall and thus to control the main direction of growth, and consequently, shape. Evidence indicates that the epidermis is under tension, and therefore the shape of the tissue can influence the pattern of mechanical stress. In this framework, multicellular shape is transposed into a map of stress directions in the epidermis that can act as a supracellular instructional signal. By applying mechanical constraints on a meristem with GFP-marked microtubules, we were able to close the feedback loop: microtubule orientation became parallel to the externally applied stress, supporting a view in which mechanical stress controls cell behavior.Citation7
In a more recent study we showed that in addition to the cortical microtubules, the polar localization of the auxin efflux carrier PIN1 can also be controlled by its mechanical environment. In particular, we observed that, when viewed from the top, PIN1 is usually concentrated on the membrane that is parallel to the microtubule orientation. Furthermore, a single cell ablation, which induces both a circumferential pattern of stress around the wound and a circumferential orientation of microtubules, also induced a relocalization of PIN1 away from the wound on the circumferential membrane, consistent with the hypothesis that PIN1 would be preferentially recruited on the membrane undergoing the most tensile stress.Citation8 Mechanistically, it is unclear how this could be achieved, but the PIN1 vesicle recycling machinery is likely to play a major role, since it is now well established that membrane tension inhibits endocytosis and favors exocytosis.Citation9 In such a scenario, PIN1 would be trapped in a membrane as long as the tension of the membrane is higher than that of its neighbours.
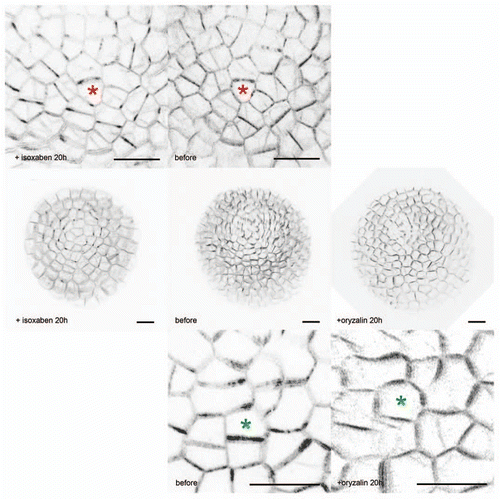
To further test the response of PIN1 to mechanical forces, we used a pharmacological approach. highlights the correlation between the predicted opposable impacts of isoxaben and oryzalin on stress and the response of PIN1. In the presence of isoxaben, a well known inhibitor of cellulose synthesis, the thickness of the cell wall is supposed to decrease. Knowing that mechanical stress is here defined as a force divided by the area of a section of the wall, stress is expected to increase after isoxaben treatment. When we treated PIN1-GFP meristems with isoxaben, we observed a “hyper” localization of PIN1, with in most cases a preferential localization of PIN1 along the supracellular stress patterns, and within the cell, a concentration of the signal at cell corners, predicted sites of stress maxima. In contrast, in the presence of oryzalin, which by depolymerising the microtubules leads to isotropic growth and thus isotropic stresses, PIN1 localization became more homogeneous.
It seems therefore plausible that mechanical stress acts as a common instructional signal for both microtubule-dependent cell anisotropy and PIN1/auxin-dependent growth rate. Mathematical modeling further supported this proposal. Several successful models for the generation of organ patterns in the meristem assume an ability of individual cells to sense auxin concentration in their neighbours.Citation10–Citation16 However to date no mechanism had been proposed to explain how one cell could measure the concentration of auxin in its vicinity. One of the main implications of our study is that, if PIN1 can respond to the mechanical status of the wall, then it also integrates auxin concentration of the neighboring cells, indirectly, as auxin loosens the cell wall, allowing cell expansion. Using such a hypothesis, computer simulations were able to reproduce the stereotyped pattern of organogenesis in the shoot further confirming the plausibility of the model.
It must be noted however that our work does not exclude other hypotheses. In particular, it has recently been proposed that the ROP2 and ROP6 proteins, well known effectors of cell polarity, could respond differently to ABP1-dependent auxin signaling, thus providing a model in which cell-cell communication via ROP could “measure” local differences in auxin between neighbors.Citation17 These different scenarios could actually be reconciled some day, especially knowing that Rho proteins in animals have been involved in the responses to mechanical forces.Citation18 Last, the control of PIN1 polar localization by its mechanical environment could actually reveal a more universal response of cells to the stiffness and tension of the extracellular matrix. Similarly, animal motile (and polar) cells can sense the rigidity of their substrateCitation19–Citation21 and respond by reinforcing the cytoskeleton at the cell cortex.Citation22–Citation25
Figures and Tables
Figure 1 Impact of isoxaben and oryzalin on the localization of PIN1 in meristematic cells. The PIN1-GFP signal (in black) is very heterogenous in the control meristematic cells, consistent with the preferential localization of PIN1 to one side of the cells. Sometimes the signal is even restricted to one cell corner. After microtubule depolymerization with oryzalin, cell growth becomes more isotropic, and while PIN1 localization remains heterogenous, the signal becomes more widespread on each plasma membranes and thus tends to homogeneity. In contrast, after isoxaben treatment (which inhibits cellulose synthesis and thus is predicted to increase stress levels), the PIN1-GFP protein concentrates at the corners of the cells.Citation8
Addendum to:
References
- Jaeger J, Irons D, Monk N. Regulative feedback in pattern formation: towards a general relativistic theory of positional information. Development 2008; 135:3175 - 3183
- Cotterell J, Sharpe J. An atlas of gene regulatory networks reveals multiple three-gene mechanisms for interpreting morphogen gradients. Mol Syst Biol 2010; 6:425
- Oates AC, Gorfinkiel N, Gonzalez-Gaitan M, Heisenberg CP. Quantitative approaches in developmental biology. Nat Rev Genet 2009; 10:517 - 530
- Farge E. Mechanical induction of twist in the Drosophila foregut/stomodeal primordium. Curr Biol 2003; 13:1365 - 1377
- Thery M, Jimenez-Dalmaroni A, Racine V, Bornens M, Julicher F. Experimental and theoretical study of mitotic spindle orientation. Nature 2007; 447:493 - 496
- Vogel V, Sheetz M. Local force and geometry sensing regulate cell functions. Nat Rev Mol Cell Biol 2006; 7:265 - 275
- Hamant O, Heisler MG, Jonsson H, Krupinski P, Uyttewaal M, Bokov P, et al. Developmental patterning by mechanical signals in Arabidopsis. Science 2008; 322:1650 - 1655
- Heisler MG, Hamant O, Krupinski P, Uyttewaal M, Ohno C, Jonsson H, et al. Alignment between PIN1 polarity and microtubule orientation in the shoot apical meristem reveals a tight coupling between morphogenesis and auxin transport. PLoS Biol 2010; 8:1000516
- Morris CE, Homann U. Cell surface area regulation and membrane tension. J Membr Biol 2001; 179:79 - 102
- Reinhardt D, Pesce ER, Stieger P, Mandel T, Baltensperger K, Bennett M, et al. Regulation of phyllotaxis by polar auxin transport. Nature 2003; 426:255 - 260
- Heisler MG, Ohno C, Das P, Sieber P, Reddy GV, Long JA, et al. Patterns of auxin transport and gene expression during primordium development revealed by live imaging of the Arabidopsis inflorescence meristem. Curr Biol 2005; 15:1899 - 1911
- de Reuille PB, Bohn-Courseau I, Ljung K, Morin H, Carraro N, Godin C, et al. Computer simulations reveal properties of the cell-cell signaling network at the shoot apex in Arabidopsis. Proc Natl Acad Sci USA 2006; 103:1627 - 1632
- Smith RS, Guyomarc'h S, Mandel T, Reinhardt D, Kuhlemeier C, Prusinkiewicz P. A plausible model of phyllotaxis. Proc Natl Acad Sci USA 2006; 103:1301 - 1306
- Jonsson H, Heisler MG, Shapiro BE, Meyerowitz EM, Mjolsness E. An auxin-driven polarized transport model for phyllotaxis. Proc Natl Acad Sci USA 2006; 103:1633 - 1638
- Stoma S, Lucas M, Chopard J, Schaedel M, Traas J, Godin C. Flux-based transport enhancement as a plausible unifying mechanism for auxin transport in meristem development. PLoS Comput Biol 2008; 4:1000207
- Bayer EM, Smith RS, Mandel T, Nakayama N, Sauer M, Prusinkiewicz P, et al. Integration of transport-based models for phyllotaxis and midvein formation. Genes Dev 2009; 23:373 - 384
- Xu T, Wen M, Nagawa S, Fu Y, Chen JG, Wu MJ, et al. Cell surface- and rho GTPase-based auxin signaling controls cellular interdigitation in Arabidopsis. Cell 2010; 143:99 - 110
- Burridge K, Wennerberg K. Rho and Rac take center stage. Cell 2004; 116:167 - 179
- Lo CM, Wang HB, Dembo M, Wang YL. Cell movement is guided by the rigidity of the substrate. Biophys J 2000; 79:144 - 152
- Paszek MJ, Zahir N, Johnson KR, Lakins JN, Rozenberg GI, Gefen A, et al. Tensional homeostasis and the malignant phenotype. Cancer Cell 2005; 8:241 - 254
- Mitrossilis D, Fouchard J, Pereira D, Postic F, Richert A, Saint-Jean M, et al. Real-time single-cell response to stiffness. Proc Natl Acad Sci USA 2010; 107:16518 - 16523
- Choquet D, Felsenfeld DP, Sheetz MP. Extracellular matrix rigidity causes strengthening of integrin-cytoskeleton linkages. Cell 1997; 88:39 - 48
- Riveline D, Zamir E, Balaban NQ, Schwarz US, Ishizaki T, Narumiya S, et al. Focal contacts as mechanosensors: externally applied local mechanical force induces growth of focal contacts by an mDia1-dependent and ROCK-independent mechanism. J Cell Biol 2001; 153:1175 - 1186
- Yonemura S, Wada Y, Watanabe T, Nagafuchi A, Shibata M. alpha-Catenin as a tension transducer that induces adherens junction development. Nat Cell Biol 2010; 12:533 - 542
- Fletcher DA, Mullins RD. Cell mechanics and the cytoskeleton. Nature 2010; 463:485 - 492