Abstract
Nrat1 is a plasma membrane-localized aluminum transporter recently identified in rice, which is a member of Nramp family. Here, we further characterized this transporter in terms of transport substrate specificity. Heterologous assay in yeast showed that Al transport activity by Nrat1 was unaffected by the presence of high concentration of Ca, but significantly inhibited by trivalent ions including Yb and Ga, analogs of Al. Knockout of Nrat1 did not affect the uptake of Cd and Mn in rice. On the other hand, over-expression of Nrat1 led to enhanced Al uptake by rice roots compared with wild-type rice, but did not affect Cd uptake. These results provide further evidence that unlike other Nramp members, Nrat1 is an influx transporter for trivalent Al ion.
Aluminum ion (mainly Al3+) inhibits root growth and functions, which toxicity is the major limiting factor of crop production on acid soils.Citation1 However, there is a wide variation in the tolerance to Al toxicity between species and cultivars within a species.Citation2–Citation4 Species or cultivars with high Al tolerance have evolved various strategies to detoxify Al externally and/or internally.Citation2–Citation4 Rice has been known as a highly Al-tolerance species. Recent identification of a transcription factor (ART1) for Al tolerance has revealed that multiple genes are involved in high Al tolerance in rice.Citation5 One of the genes regulated by ART1 is Nrat1.
Nrat1 belongs to Nramp family, but shares low similarity (<60%) with other members.Citation6 A detailed functional analysis of Nrat1 showed that unlike other Nramp members, which are transporters of divalent metals, Nrat1 is a transporter of trivalent Al ion (Al3+).Citation6 Nrat1 is localized to the plasma membrane of all root cells. Knockout of Nrat1 resulted in decreased Al uptake, but increased cell wall-binding Al and Al sensitivity.Citation6 We therefore concluded that Nrat1 is required for prior step of final Al detoxification through sequestration of Al into vacuoles. In this report, we further characterize Nrat1 in terms of transport substrate specificity.
It has been reported that Ca2+ can alleviate Al toxicity by decreasing Al accumulation in the roots.Citation7,Citation8 Therefore, there is a possibility that Ca2+ affects Al uptake through Nrat1. To test this possibility, we examined the effect of Ca on Al uptake in yeast expressing Nrat1 or not. In the presence of Ca up to 10 times of Al, the Al uptake by Nrat1 was unaffected (), indicating that Ca2+ does not affect Al uptake mediated by Nrat1 at least in yeast.
Previous results have showed that Nrat1 transports trivalent Al ion, but not other Al forms.Citation6 Presence of divalent metals including Mn and Cd did not afftect the Al uptake by Nrat1 in yeast.Citation6 To examine whether Nrat1 is able to transport other trivalent ions including Yb3+ and Ga3+, analogs of Al, we performed a competition experiment between Al and these two trivalent cations. In the presence of equimolar concentration of Yb or Ga, the Al uptake by yeast expressing Nrat1 was almost inhibited half (). This result indicates that Nrat1 is also able to transport Yb3+ and Ga3+. However, considering that Al is the most abundant metal in the earth's crust, it is likely that Nrat1 mainly functions as an Al transporter.
In the yeast system, Nrat1 did not show transport activity for Fe2+, Cd2+ and Mn2+.Citation6 To confirm this result in rice, we compared the uptake of Cd and Mn between wild-type and two independent knockout lines of Nrat1. Knockout of Nrat1 significantly resulted in decreased Al uptake into the root cells.Citation6 In contrast, there were no differences in the concentration of both Cd and Mn in the root-cell sap between wild-type and knockout lines ( and B). These results further show that Nrat1 is not capable of transporting divalent metal ions as observed in yeast.
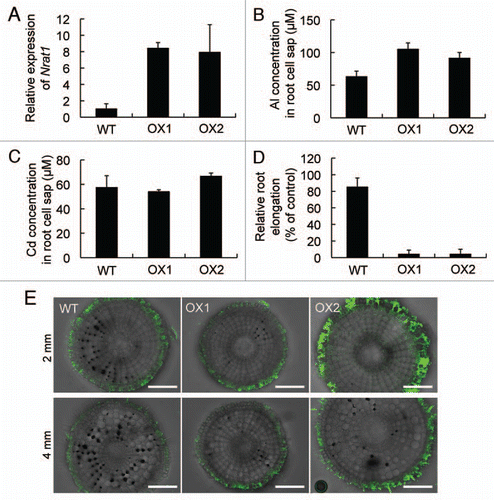
To further confirm that Nrat1 is a transporter of trivalent Al in rice, we overexpressed this gene in rice under control of maize ubiquitin1 promoter. In two independent transgenic lines, the expression level of Nrat1 was enhanced by about 9 times under Al treatment condition (). Overexpression of Nrat1 resulted in increased Al concentration in the root cell sap compared with wild type rice (). However, there was no difference in the Cd concentration of root cell sap between overexpressed and wildtype lines (). These results again indicate that Nrat1 is a transporter for trivalent Al rather than Cd. Overexpression of Nrat1 also resulted in increased Al sensitivity (). Morin staining, an Al specific fluorescent dye, showed that the overexpressed lines exhibited enhanced signal intensity compared with wildtype rice (). Morin can detect Al inside the cells but cannot detect cell wall-bound Al.Citation9 This result is consistent with Al concentration in the root cell sap of overexpressed lines (). Therefore, increased Al sensitivity in the Nrat1-overexpressed lines is likely caused by a high Al concentration in the cytosol due to enhanced Al uptake (). Aluminum entering into the cells may be detoxified by chelation with organic acid anions and/or sequestration into the vacuoles.Citation4 An ABC transporter ALS1 in Arabidopsis has been suggested to be involved in sequestration of Al into vacuoles.Citation10 There is a homolog of ALS1 in rice, which also has been suggested to be involved in Al toleranceCitation5 although the function of these genes have not been characterized in both Arabidopsis and rice. In the overexpressed line, these capacities for final detoxification were not enhanced simultaneously, resulting in toxic level of Al in the cytosol. It will be interesting to enhance both Nrat1 and internal detoxification capacity in the future.
In conclusion, our findings further demonstrated that Nrat1 is an influx transporter for trivalent Al. It remains to be examined whether similar transporters are present in other plant species and also other organisms.
Figures and Tables
Figure 1 Effect of Ca, Yb and Ga on Al uptake by Nrat1 in yeast. (A) Effect of Ca2+ on the transport activity of Al ion by Nrat1. Yeast cells expressing Nrat1 were exposed for two hours to a solution (pH 4.2) containing 50 µM AlCl3 in the absence or presence of different concentrations of Ca (50, 150, 250 or 500 µM as CaCl2). (B) Effect of Yb and Ga on Al transport activity by Nrat1. Yeast cells expressing Nrat1 were exposed for two hours to a solution containing 50 µM AlCl3 at pH 4.2 in the absence or presence of equal concentration of Yb or Ga. The Al concentration in the yeast was determined by atomic absorption spectrophotometer after digested with 2 N HCl. Data are means ± SD of three biological replicates.
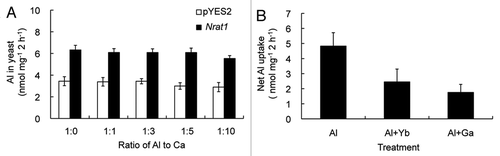
Figure 2 Effect of Nrat1 knockout on transport of Cd and Mn in rice roots. (A and B) Concentration of Cd (A) and Mn (B) in the root cell sap of wild-type rice and two knockout lines of Nrat1 (NE 7009 and NF7046). The roots were exposed to a 0.5 mM CaCl2 solution (pH 4.5) containing 30 µM Cd or 30 µM Mn for eight hours. The concentration of Cd and Mn in the cell sap of root tips (0–1 cm) was determined by atomic absorption spectrophotometer. Data are means ± SD of three biological replicates.
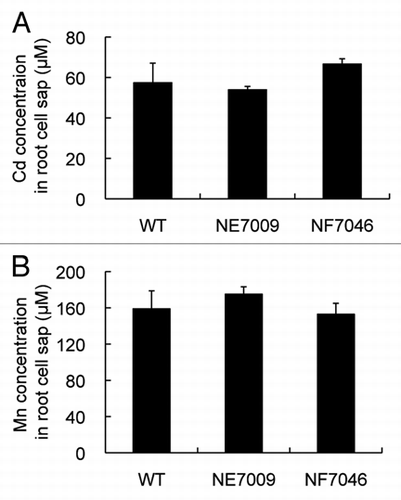
Figure 3 Charaterization of Nrat1-overexpressed lines. (A) Expression of Nrat1 in two independent Nrat1 overexpressed lines. Both wild-type rice and overexpressed lines (OX1 and OX2) were exposed to a 0.5 mM CaCl2 solution (pH 4.5) containing 30 µM AlCl3 for six hours. The expression level of Nrat1 in the roots were determined by quantitative real-time PCR and Histone H3 was used as an internal standard. Expression relative to the wild-type rice is shown. Data are means ± SD (n = 3). (B and C) Concentration of Al (B) or Cd (C) in the root cell sap of wild-type rice and two Nrat1 overexpressed lines (OX1 and OX2). The roots were exposed to a 0.5 mM CaCl2 solution (pH 4.5) containing 30 µM Al or 30 µM Cd for eight hours. The concentration of Al or Cd in the cell sap of root tips (0–1 cm) was determined by atomic absorption spectrophotometer. Data are means ± SD of three biological replicates. (D) Al sensitivity of Nrat1 overexpressed lines. Seedlings of wild-type rice (WT) and two Nrat1 overexpressed lines (OX1 and OX2) were exposed to a 0.5 mM CaCl2 solution (pH 4.5) containing 30 µM Al for 24 hours. The root length was measured before and after the treatment and the elongation relative to the root growth without Al was shown. Data are means ± SD (n = 8). (E) Morin staining of Nrat1 overexpressed lines. Seedlings of wild-type and the overexpressed lines were exposed to a 0.5 mM CaCl2 solution (pH 4.5) containing 30 µM Al for 24 hours and then stained with 0.1% Morin (green). Roots were cross-sectioned by hands. Scale bar = 100 µm.
Addendum to:
References
- von Uexkull HR, Mutert E. Global extent, development and economic impact of acid soils. Plant Soil 1995; 171:1 - 15
- Ryan PR, Delhaize E, Jones DL. Function and mechanism of organic anion exudation from plant roots. Annu Rev Plant Physiol Plant Mol Biol 2001; 52:527 - 560
- Kochian LV, Pineros MA, Hoekenga OA. The physiology, genetics and molecular biology of plant aluminum resistance and toxicity. Plant Soil 2005; 274:175 - 195
- Ma JF. Syndrome of aluminum toxicity and diversity of aluminum resistance in higher plants. Int Rev Cytol 2007; 264:225 - 252
- Yamaji N, Huang CF, Nagao S, Yano M, Sato Y, Nagamura Y, Ma JF. A Zn-finger transcription factor ART1 regulates multiple genes implicated in aluminum tolerance in rice. Plant Cell 2009; 21:3339 - 3349
- Xia JX, Yamaji N, Kasai T, Ma JF. Plasma membrane-localized transporter for aluminum in rice. Proc Natl Acad Sci USA 2010; 107:18381 - 18385
- Ryan PR, Reid RJ, Smith FA. Direct evaluation of the Ca2+-displacement hypothesis for Al toxicity. Plant Physiol 1997; 113:1351 - 1357
- Kinraide TB. Three mechanisms for the calcium alleviation of mineral toxicities. Plant Physiol 1998; 118:513 - 520
- Eticha D, Stass A, Horst WJ. Localization of aluminium in the maize root apex: Can morin detect cell wall-bound aluminium?. J Exp Bot 2005; 56:1351 - 1357
- Larsen PB, Cancel J, Rounds M, Ochoa V. Arabidopsis ALS1 encodes a root tip and stele localized half type ABC transporter required for root growth in an aluminum toxic environment. Planta 2007; 225:1447 - 1458