Abstract
Plants activate defense responses through the recognition of microbe-associated molecular patterns (MAMPs). Recently, several pattern-recognition receptors (PRRs) have been identified in plants, paving the way for manipulating MAMP signaling. CEBiP is a receptor for the chitin elicitor (CE) identified in the rice plasma membrane and XA21 is a member of the receptor-like protein kinase (RLK) family that confers disease resistance to rice bacterial leaf blight expressing the sulfated protein Ax21. To improve resistance to rice blast, the most serious fungal disease of rice, we aimed to create a defense system that combines high affinity of CEBiP for CE and the ability of XA21 to confer disease resistance. Cultured rice cells expressing the chimeric receptor CRXA, which consists of CEBiP and the intracellular region of XA21, induced cell death accompanied by an increased production of reactive oxygen and nitrogen species after exposure to CE. Rice plants expressing the chimeric receptor exhibited more resistance to rice blast. Engineering PRRs may be a new strategy in molecular breeding for achieving disease resistance.
Time for Manipulating Pattern-recognition Receptors (PRRs) has Come
The annual global loss due to crop disease amounts to 220 billion dollars;Citation1 thus, enhancement of crop disease resistance is one of the most important objectives in agriculture. Plants encounter various microbes in the field, but resist infection. This means that plants have an effective self-defense system and that only microbes that have evolved a pathogenicity system have become plant pathogens. Plants activate defense systems upon recognizing microbe-associated molecular patterns (MAMPs; also known as pathogenassociated molecular patterns) through PRRs.Citation2 Recently, various PRRs have been identified in plants. FLS2Citation3 and EFRCitation4 of Arabidopsis and XA21Citation5,Citation6 of rice are receptor-like protein kinases (RLKs) possessing an extracellular leucine-rich repeat domain (LRR). These PRRs recognize the flg22 peptide derived from bacterial flagellin, the elf18 peptide derived from elongation factor Tu (EF-Tu), and a sulfated peptide derived from Ax21 secreted from Xanthomonas oryzae pv. oryzae (Xoo). CEBiPCitation7 of rice and CERK1/LysM RLK1Citation8,Citation9 of Arabidopsis possess extracellular LysM domains and perceive chitin fragments, which are a representative fungal MAMP. EIX1 and EIX2Citation10 of tomato are PRRs for fungal xylanase, and the soluble protein GEBPCitation11 of soybean binds 1,6-1,3-β-glucans from the oomycete Phytophthora sojae. WAK1 of Arabidopsis is a receptor for damage-associated molecular patterns.Citation12 WAK1 recognizes oligogalacturonides, which are released from plant cell walls by bacterial infection, and activates defense responses.
Recent studies have shown that recognition of pathogens and activation of defense responses through PRRs contribute to the basal resistance of plant.Citation13 Because MAMPs are essential for survival or pathogenicity, approaches directed at manipulating the PRR-mediated defense system may achieve more durable and broad-spectrum resistance. While conventional breeding or genetic engineering of rice has used Xa21 to confer robust resistance to diverse strains of Xoo,Citation14 studies on increasing disease resistance in plants using PRR genes are still in their infancy. Introduction of Arabidopsis EFR into Nicotiana benthamiana and tomato brought about broad-spectrum defense against bacterial pathogens, indicating that activity of a PRR is retained after transfer between plant families.Citation15 Brutus et al.Citation12 showed that overexpression of WAK1 increases the resistance against the fungal pathogen Botrytis cinerea in Arabidopsis.
Chimeras of PRRs: A New Approach to Enhance Disease Resistance
Knockdown of CEBiP expression in rice plants led to increased spread of the infection hyphae of rice blast fungus, Magnaporthe oryzae, suggesting the generation of CE during the early infection stages.Citation16 Pretreatment with CE delays hyphal growth of M. oryzae; however, the effect on enhancement of disease resistance is subtle.Citation17 CE shows only weak cell death-inducing activity, whereas effective defense reactions are often accompanied by hypersensitive-response (HR) cell death.Citation18 Therefore, we aimed to convert CE signals to signals leading to HR cell death.
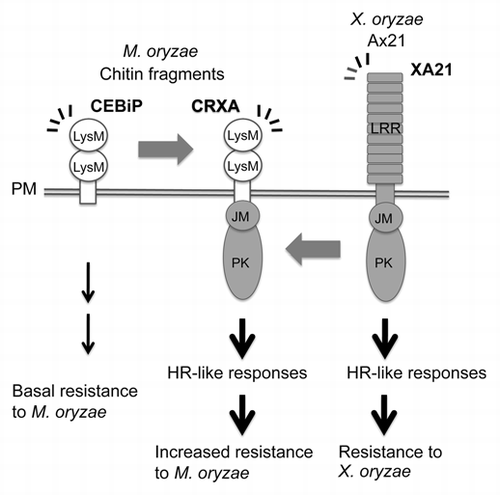
CEBiP has been identified as a CE-binding plasma membrane glycoprotein in rice cultured cells.Citation7,Citation19 CEBiP possesses two LysM domains and a putative transmembrane domain (TM).Citation7 XA21 consists of an extracellular LRR domain, a TM, an intracellular juxta-membrane domain (JM), and a Ser/Thr protein kinase domain.Citation6 Although XA21 is a PRR, it confers effective resistance to Xoo producing Ax21, and the interaction between XA21 and Ax21 leads to HR-like cell death in cultured rice cells.Citation20 To enhance defense responses upon sensing CE, we designed a defense system that combines high affinity of CEBiP for CECitation7 and the ability of XA21 that effectively avoids the disease (). We produced transgenic rice expressing a chimeric gene encoding CRXA, which is composed of the extracellular portion of CEBiP and the intracellular portion of XA21 via a TM.
Cell death induction and transient generation of reactive oxygen species (ROS) and reactive nitrogen species (RNS) are characteristic phenotypes of HR.Citation21,Citation22 Treatment with CE of the suspension-cultured rice cells expressing CRXA caused increased cell death accompanied by an elevated production of ROS and RNS.Citation16 Expression of several genes was also altered in the transformed cells. These results strongly suggest that CE signals were converted to cell death-inducing signals through the introduced CRXA fusion protein, as expected.
Rice plants expressing CRXA showed significantly increased resistance to M. oryzae. Lesion formation and proliferation of M. oryzae was decreased. Resistance to Xoo, which has no chitin, was not altered, strongly suggesting that the increased resistance to M. oryzae was due to the recognition of CE and subsequent induction of HR by the introduced CRXA protein.Citation16
Other studies concerning the chimeric receptor have been reported. Rice cells expressing a chimeric receptor composed of the extracellular domain of BRI1, a receptor for brassinosteroids and the intracellular domain of XA21 initiate HR-like responses upon treatment with brassinosteroid. This indicates that the brassinosteroid signal was converted to defense signaling downstream of XA21 through the chimeric receptor.Citation20 More recently, chimeric receptors composed of the extracellular LRR region from EFR and intracellular kinase regions from FLS2 or WAK1 have been shown to respond to elf18 in Arabidopsis.Citation12 It will be interesting to determine whether other combinations of PRRs also work as chimeric receptors.
Prospects of Manipulating PRRs
Durability and a broad-spectrum of action are expected to be part of the strategy aimed at enhancing disease resistance by manipulating PRRs. In achieving this strategy, several issues have emerged.
Simple overexpression of a PRR may not be sufficient. Overexpression of OsFLS2, which functions in Arabidopsis as the flg22 receptor, enhances rice defense responses involving cell death in cultured cells but does not affect the resistance against an Acidovorax avenae-compatible strain in rice plants.Citation23 We also demonstrated that, although overexpression of CEBiP enhances ROS production in suspension-cultured cells treated with CE and suppresses the spread of infection hyphae to some extent, levels of RNS generation, extent of cell death and disease resistance are not altered.Citation16 Overexpression of a PRR seems to increase the recognition efficiency of the corresponding MAMP. Whereas rice cells respond to CE at subnanomolar levels, some plants, like Arabidopsis, require higher concentrations of CE.Citation8 Thus, upregulation of CEBiP in such plants may be effective. In addition, as Lacombe et al.Citation15 have demonstrated with EFR,Citation15 the introduction of a PRR into plants in which the PRR is absent may also be effective.
In general, MAMP-induced resistance shows only a certain effect on suppressing disease. Thus, we have attempted the chimeric receptor approach to introduce XA21 activity downstream of CE signaling. However, unlike a result of the gene-for-gene interaction,Citation24 rice blast was not completely avoided, indicating that there is room for improving the molecularly engineered defense system. The efficacy of the present system likely depends on the level of the MAMPs generated during infection and the HR-inducing ability of the receptor. Using other RLKs that induce HR more efficiently than XA21 may be helpful in achieving better resistance. On the other hand, an increase in CE-induced cell death could promote infection of necrotrophic fungal pathogens because infection by necrotrophic fungi requires host cell death.Citation25 Although susceptibility of rice plants constitutively expressing CRXA against the rice necrotrophic pathogen Cochliobolus miyabeanus was not affected,Citation16 effects on necrotrophic pathogens should be reassessed when more CE-induced cell death occurs.
Manipulating PRRs could cause undesirable results due to the wide distribution of MAMPs. Although rice plants constitutively expressing CRXA grew normally in a green house,Citation16 whether rice plants also react to beneficial fungi possessing chitin and the effect on agronomic traits in the field should be examined more thoroughly.
Is MAMPs-induced resistance really durable? Pathogens have acquired a pathogenicity system that overcomes MAMPs-inducing resistance in the host. It might be possible that manipulation of MAMP signaling leads to further evolution of pathogenicity factors.
Discovery and development of new PRRs, including molecularly engineered ones with different specification, could provide a more effective strategy for enhancing disease resistance in plants.
Abbreviations
| LysM | = | lysin-motif |
Addendum to:
References
- Agrios GN. Plant Pathology 2005; fifth edition Academic Press
- Boller T, He SY. Innate immunity in plants: an arms race between pattern recognition receptors in plants and effectors in microbial pathogens. Science 2009; 324:742 - 744
- Chinchilla D, Bauer Z, Regenass M, Boller T, Felix G. The Arabidopsis receptor kinase FLS2 binds flg22 and determines the specificity of flagellin perception. Plant Cell 2006; 18:465 - 476
- Zipfel C, Kunze G, Chinchilla D, Caniard A, Jones JD, Boller T, et al. Perception of the bacterial PAMP EF-Tu by the receptor EFR restricts Agrobacterium-mediated transformation. Cell 2006; 125:749 - 760
- Lee SW, Han SW, Sririyanum M, Park CJ, Seo YS, Ronald PC. A type I-secreted, sulfated peptide triggers XA21-mediated innate immunity. Science 2009; 326:850 - 853
- Song WY, Wang GL, Chen LL, Kim HS, Pi LY, Holsten T, et al. A receptor kinase-like protein encoded by the rice disease resistance gene, Xa21. Science 1995; 270:1804 - 1806
- Kaku H, Nishizawa Y, Ishii-Minami N, Akimoto-Tomiyama C, Dohmae N, Takio K, et al. Plant cells recognize chitin fragments for defense signaling through a plasma membrane receptor. Proc Natl Acad Sci USA 2006; 103:11086 - 11091
- Miya A, Albert P, Shinya T, Desaki Y, Ichimura K, Shirasu K, et al. CERK1, a LysM receptor kinase, is essential for chitin elicitor signaling in Arabidopsis. Proc Natl Acad Sci USA 2007; 104:19613 - 19618
- Wan J, Zhang XC, Neece D, Ramonell KM, Clough S, Kim SY, et al. A LysM receptor-like kinase plays a critical role in chitin signaling and fungal resistance in Arabidopsis. Plant Cell 2008; 20:471 - 481
- Ron M, Avni A. The receptor for the fungal elicitor ethylene-inducing xylanase is a member of a resistance-like gene family in tomato. Plant Cell 2004; 16:1604 - 1615
- Umemoto N, Kakitani M, Iwamatsu A, Yoshikawa M, Yamaoka N, Ishida I. The structure and function of a soybean beta-glucan-elicitor-binding protein. Proc Natl Acad Sci USA 1997; 94:1029 - 1034
- Brutus A, Sicilia F, Macone A, Cervone F, De Lorenzo G. A domain swap approach reveals a role of the plant wall-associated kinase 1 (WAK1) as a receptor of oligogalacturonides. Proc Natl Acad Sci USA 2010; 107:9452 - 9457
- Boller T, Felix G. A renaissance of elicitors: perception of microbe-associated molecular patterns and danger signals by pattern-recognition receptors. Annu Rev Plant Biol 2009; 60:379 - 406
- Wang GL, Song WY, Ruan DL, Sideris S, Ronald PC. The cloned gene, Xa21, confers resistance to multiple Xanthomonas oryzae pv. oryzae isolates in transgenic plants. Mol Plant Microbe Interact 1996; 9:850 - 855
- Lacombe S, Rougon-Cardoso A, Sherwood E, Peeters N, Dahlbeck D, van Esse HP, et al. Interfamily transfer of a plant pattern-recognition receptor confers broad-spectrum bacterial resistance. Nat Biotechnol 2010; 28:365 - 369
- Kishimoto K, Kouzai Y, Kaku H, Shibuya N, Minami E, Nishizawa Y. Perception of the chitin oligosaccharides contributes to disease resistance to blast fungus Magnaporthe oryzae in rice. Plant J 2010; 64:343 - 354
- Tanabe S, Okada M, Jikumaru Y, Yamane H, Kaku H, Shibuya N, et al. Induction of resistance against rice blast fungus in rice plants treated with a potent elicitor, N-acetylchitooligosaccharide. Biosci Biotechnol Biochem 2006; 70:1599 - 1605
- Jones JD, Dangl JL. The plant immune system. Nature 2006; 444:323 - 329
- Ito Y, Kaku H, Shibuya N. Identification of a high-affinity binding protein for N-acetylchitooligosaccharide elicitor in the plasma membrane of suspension-cultured rice cells by affinity labeling. Plant J 1997; 12:347 - 356
- He Z, Wang ZY, Li J, Zhu Q, Lamb C, Ronald P, et al. Perception of brassinosteroids by the extracellular domain of the receptor kinase BRI1. Science 2000; 288:2360 - 2363
- Delledonne M, Zeier J, Marocco A, Lamb C. Signal interactions between nitric oxide and reactive oxygen intermediates in the plant hypersensitive disease resistance response. Proc Natl Acad Sci USA 2001; 98:13454 - 13459
- Levine A, Tenhaken R, Dixon R, Lamb C. H2O2 from the oxidative burst orchestrates the plant hypersensitive disease resistance response. Cell 1994; 79:583 - 593
- Takai R, Isogai A, Takayama S, Che FS. Analysis of flagellin perception mediated by flg22 receptor OsFLS2 in rice. Mol Plant Microbe Interact 2008; 21:1635 - 1642
- Laugé R, De Wit PJ. Fungal avirulence genes: structure and possible functions. Fungal Genet Biol 1998; 24:285 - 297
- Wolpert TJ, Dunkle LD, Ciuffetti LM. Host-selective toxins and avirulence determinants: what's in a name?. Annu Rev Phytopathol 2002; 40:251 - 285