Abstract
Activation of NB-LRR-related UNI proteins by uni-1D mutation, a gain-of-function mutation of the UNI gene, induces some pathogenesis-related responses and also affects morphology through modulation of meristem activities. In a recent study we reported that the uni-1D phenotypes require cooperative action of ERECTA (ER) receptor kinase family members in UNI-expressing cells, suggesting that an intracellular signaling crosstalk between ER-family-dependent and UNI-triggered signaling pathways plays a significant role in the phenotypes. Further we recently succeeded in the establishment of a methodology for rapid identification of factors involved in the UNI function. EMS-induced causal mutations that suppress the uni-1D phenotypes could be identified using whole-genome-sequencing technologies with much less labor compared with the conventional map-based cloning method that is generally time-consuming and labor-intensive. Thus it would be now possible to intensively identify factors that play significant roles in regulation of UNI proteins and/or UNI-related signaling pathways.
UNI Activation Affects Meristem Activities in a Non-Cell-Autonomous Manner
The uni-1D mutant of Arabidopsis thaliana harbors a gain-of-function and dominant mutation in the UNI gene, which encodes a member of the CC-NB-LRR (coiled-coil-nucleotide-binding-site-leucine-rich-repeat) family.Citation1 Because NB-LRR-type proteins are known to function as molecular sensors to trigger intracellular signaling pathways upon stimulation by ligands/elicitors,Citation2–Citation4 the uni-1D mutation probably converts UNI proteins into an active state without an activation event, which triggers downstream intracellular signaling pathways; however, such a ligand or an elicitor for UNI activation have not yet been identified.
The heterozygous uni-1D/+ plants show morphological alterations including early termination of inflorescence stem growth due to rapid consumption of stem cells with the shoot apical meristem (SAM) and formation of extra axillary meristems (AMs) at leaf axils.Citation1,Citation5 Interestingly, the UNI promoter is active only outside the meristems though uni-1D/+ plants display meristem abnormalitiesCitation5 (), suggesting that the SAM maintenance and AM formation might be modulated by non-cell-autonomous effects triggered by UNI activation. Expression of WUSCHEL (WUS), a central player in stem cell maintenance in SAMs,Citation6 is severely reduced in uni-1D/+ plants.Citation5 Because CLAVATA3 (CLV3) controls the expression pattern and level of WUSCitation7 and there is a regulatory feedback loop between WUS and CLV3, UNI activation might affect the homeostasis of the feedback loop. Cytokinin is one of players affecting the WUS-CLV3 regulatory loopCitation8–Citation10 and cytokinin-related regulation is modulated in uni-1D/+ mutants,Citation1,Citation5 suggesting that cytokinin might be involved in the non-cell-autonomous effects observed in uni-1D/+ mutants. We will describe the relationship between UNI signaling and cytokinin-related regulation in the next section.
Intracellular Signaling Crosstalk between UNI-Triggered and ERECTA-Family-Dependent Signaling Pathways Modulates Cytokinin-Related Regulation
Trans-zeatin (tZ)-type cytokinins accumulate in uni-1D/+ plants and, correspondingly, expression of the cytokinin- responsive genes is upregulated in uni-1D/+ plants.Citation1 Artificial reduction of cytokinins in uni-1D/+ mutants suppresses the abnormal morphologies of uni-1D/+ plants, suggesting the significance of cytokinin pathways for the morphological alterations of uni-1D/+ mutants.Citation1 tZ-type cytokinin production requires the CYP735A enzymeCitation11 and CYP735A2 expression is upregulated in uni-1D/+ mutants.Citation1 We recently reported that cooperative action of ERECTA (ER) family members in UNI-expressing cells is required for uni-1D/+ morphological alterations and also upregulation of cytokinin response and CYP735A2 expression.Citation5 The ER family consists of ER, ER-LIKE 1 (ERL1) and ERL2 Citation12 and encodes leucine-rich repeat receptor-like kinases in a subfamily of transmembrane-type signaling receptors in plants.Citation12,Citation13 Although it has been inferred that ER-family functions in receptor complexes through associations with multiple partners,Citation14 information about such partners and about ligands for the receptor complexes in UNI-related signaling is still lacking. Because both ER-family members and UNI have protein structures that can act as starting points to trigger intracellular signaling pathways (), it would be interesting to investigate intracellular signaling cross-talk between signaling pathways triggered by ER-family proteins and those triggered by UNI proteins and also to analyze how these signaling pathways are connected to cytokinin-related regulation including regulation of CYP735A2 expression.
Methodology for Rapid Identification of Factors Involved in UNI-Related Regulation by Whole Genome Sequencing
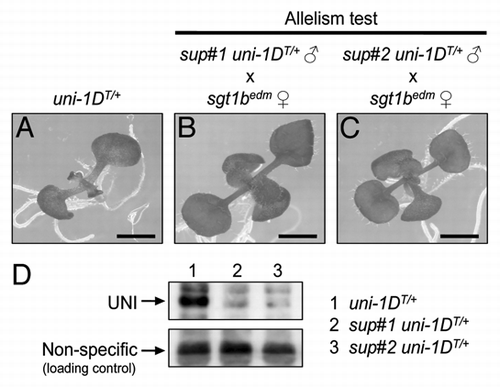
We recently established a methodology to rapidly identify the EMS-induced causal mutations that suppress the morphological alterations of uni-1D/+ mutants using one round of whole genome sequencing.Citation15 This methodology requires much less labor compared with the conventional mapbased cloning method that is generally time-consuming and labor-intensive. In our recent paper, we identified independent recessive mutations in SGT1b gene as candidates of causal mutations of two distinct uni-1D/+ suppressor mutants (termed sup#1 mutant and sup#2 mutant, respectively).Citation15 SGT1b is a core member of the chaperone complex that plays a significant role for NB-LRR-type proteins to exert their proper functionsCitation16–Citation25 and loss-of-function mutations in the SGT1b gene render NB-LRR proteins non-functional mainly through their destabilization. Here, as shown in , we confirmed the sup#1 and sup#2 are actually allelic to a reported sgt1b loss-of-function mutant. Also, as shown in , immunoblot analysis using anti-UNI antibody showed that UNI proteins are unable to stably exist in sup#1 and sup#2 mutants. These results demonstrate that causal mutations of sup#1 and sup#2 mutants are loss-of-function mutations in SGT1b gene, affecting the stability of UNI proteins.
Final Remarks
There are still a lot of unrevealed points about regulation of UNI protein functions and signaling pathways triggered by UNI activation.Citation5 However it would be now possible to intensively identify factors that play significant roles in UNI-related regulation by our new methodology using next generation sequencing technologies.Citation15 Many NB-LRR-type proteins have been reported to play significant roles in plant immunity as sensors for pathogen-derived factors to activate defense responsesCitation2–Citation4 and uni-1D plants also show some aspects of pathogenesis responses.Citation1 Though so far there has been no evidence that UNI is actually involved in plant immunity, UNI is, at least partly, regulated by mechanisms common to immunity-related NB-LRR proteins.Citation1,Citation15,Citation26 Identification of causal mutations of yet unanalyzed uni-1D suppressor mutants would contribute not only to analysis of the UNI function but also to further exploration into general mechanisms that regulate NB-LRR proteins in plant immunity. In addition, it would be interesting to dissect relationships between immune responses and morphological regulation through such studies using uni-1D mutants.Citation26
Figures and Tables
Figure 1 UNI activation non-cell-autonomously affects activities of both the SAM and axillary meristem. UNI promoter is active only outside the meristems. Activities of the SAMs, which are located at the top of stems, are largely attenuated in uni-1D/+ plants and extra axillary meristems are formed at leaf axils of uni-1D/+ plants. See the text for further explanation.
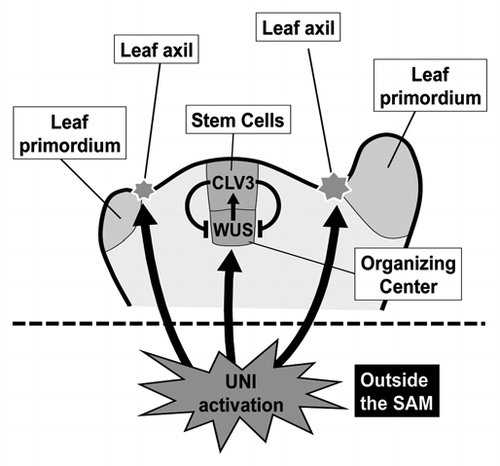
Figure 2 Intracellular signaling crosstalk between UNI-triggered and ER-family-dependent signaling pathways modulates cytokinin-related pathway. UNI harbors a CC (coiled-coil) domain, an NB (nucleotide-binding-site) domain and an LRR (leucine-rich repeat) domain. A ligand or an elicitor for activation of UNI proteins have not yet been identified. The ERECTA (ER) family members encode LRR (leucine-rich-repeat) receptor-like kinases in a subfamily of transmembrane-type receptors. Information about co-receptors that might form receptor complexes with ER-family members in UNI-related signaling and also about ligands for such receptor complexes is still lacking. Activities of ER-family members in UNI-expressing cells are required for uni-1D/+ phenotypes and also for modulation of cytokinin-related pathway.
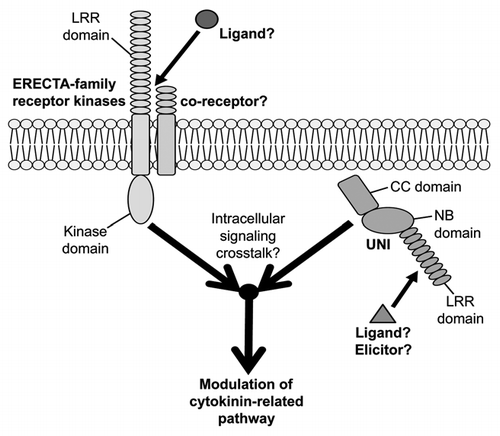
Figure 3 sup#1 and sup#2 mutants are allelic to sgt1b loss-of-function mutant and UNI proteins are unable to stably exist in both mutants. (A) 10-d-old seedling of the hemizygous transgenic plant harboring a uni-1D genomic fragment (hereafter, uni-1DT/+)Citation1,Citation15 shows growth defects such as slower leaf formation and narrow leaf shape. Allelism test was performed by crossing edm1,Citation22 an sgt1b loss-of-function mutant, with (B) sup#1 uni-1DT/+ or (C) sup#2 uni-1DT/+ plants. The resulting F1 seeds were sown on kanamycin-containing plates to ensure the presence of uni-1DT transgene that contains a kanamycin-resistant gene. edm seeds were kindly gifted from Dr. Ken Shirasu (RIKEN). Bars = 1 mm. (D) Protein extracts from 10-d-old seedlings were separated by SDS-PAGE and then immunoblot using anti-UNI antibody was performed. Lane 1: uni-1DT/+. Lane 2: sup#1 uni-1DT/+. Lane 3: sup#2 uni-1DT/+. Plants were grown on kanamycin-containing plates to ensure the presence of uni-1DT transgene. Upper part shows UNI proteins and lower part shows non-specific bands detected on the same membrane as loading controls.
Addendum to: and
References
- Igari K, Endo S, Hibara K, Aida M, Sakakibara H, Kawasaki T, et al. Constitutive activation of a CC-NB-LRR protein alters morphogenesis through the cytokinin pathway in Arabidopsis. Plant J 2008; 55:14 - 27
- Bent AF, Mackey D. Elicitors, effectors and R genes: the new paradigm and a lifetime supply of questions. Annu Rev Phytopathol 2007; 45:399 - 436
- Jones JD, Dangl JL. The plant immune system. Nature 2006; 444:323 - 329
- Padmanabhan M, Cournoyer P, Dinesh-Kumar SP. The leucine-rich repeat domain in plant innate immunity: a wealth of possibilities. Cell Microbiol 2009; 11:191 - 198
- Uchida N, Igari K, Bogenschutz NL, Torii KU, Tasaka M. Arabidopsis ERECTA-family Receptor Kinases Mediate Morphological Alterations Stimulated by Activation of NB-LRR-type UNI proteins. Plant Cell Physiol 2011; 52:804 - 814
- Mayer KF, Schoof H, Haecker A, Lenhard M, Jurgens G, Laux T. Role of WUSCHEL in regulating stem cell fate in the Arabidopsis shoot meristem. Cell 1998; 95:805 - 815
- Schoof H, Lenhard M, Haecker A, Mayer KF, Jurgens G, Laux T. The stem cell population of Arabidopsis shoot meristems in maintained by a regulatory loop between the CLAVATA and WUSCHEL genes. Cell 2000; 100:635 - 644
- Gordon SP, Chickarmane VS, Ohno C, Meyerowitz EM. Multiple feedback loops through cytokinin signaling control stem cell number within the Arabidopsis shoot meristem. Proc Natl Acad Sci USA 2009; 106:16529 - 16534
- Leibfried A, To JP, Busch W, Stehling S, Kehle A, Demar M, et al. WUSCHEL controls meristem function by direct regulation of cytokinin-inducible response regulators. Nature 2005; 438:1172 - 1175
- Zhao Z, Andersen SU, Ljung K, Dolezal K, Miotk A, Schultheiss SJ, et al. Hormonal control of the shoot stem-cell niche. Nature 2010; 465:1089 - 1092
- Takei K, Yamaya T, Sakakibara H. Arabidopsis CYP735A1 and CYP735A2 encode cytokinin hydroxylases that catalyze the biosynthesis of trans-Zeatin. J Biol Chem 2004; 279:41866 - 41872
- Shpak ED, Berthiaume CT, Hill EJ, Torii KU. Synergistic interaction of three ERECTA-family receptor-like kinases controls Arabidopsis organ growth and flower development by promoting cell proliferation. Development 2004; 131:1491 - 1501
- Torii KU, Mitsukawa N, Oosumi T, Matsuura Y, Yokoyama R, Whittier RF, et al. The Arabidopsis ERECTA gene encodes a putative receptor protein kinase with extracellular leucine-rich repeats. Plant Cell 1996; 8:735 - 746
- Shpak ED, Lakeman MB, Torii KU. Dominant-negative receptor uncovers redundancy in the Arabidopsis ERECTA Leucine-rich repeat receptor-like kinase signaling pathway that regulates organ shape. Plant Cell 2003; 15:1095 - 1110
- Uchida N, Sakamoto T, Kurata T, Tasaka M. Identification of EMS-induced causal mutations in a non-reference Arabidopsis thaliana accession by whole genome sequencing. Plant Cell Physiol 2011; 52:716 - 722
- Austin MJ, Muskett P, Kahn K, Feys BJ, Jones JD, Parker JE. Regulatory role of SGT1 in early R gene-mediated plant defenses. Science 2002; 295:2077 - 2080
- Azevedo C, Betsuyaku S, Peart J, Takahashi A, Noel L, Sadanandom A, et al. Role of SGT1 in resistance protein accumulation in plant immunity. EMBO J 2006; 25:2007 - 2016
- Azevedo C, Sadanandom A, Kitagawa K, Freialdenhoven A, Shirasu K, Schulze-Lefert P. The RAR1 interactor SGT1, an essential component of R gene-triggered disease resistance. Science 2002; 295:2073 - 2076
- Boter M, Amigues B, Peart J, Breuer C, Kadota Y, Casais C, et al. Structural and functional analysis of SGT1 reveals that its interaction with HSP90 is required for the accumulation of Rx, an R protein involved in plant immunity. Plant Cell 2007; 19:3791 - 3804
- Hubert DA, Tornero P, Belkhadir Y, Krishna P, Takahashi A, Shirasu K, et al. Cytosolic HSP90 associates with and modulates the Arabidopsis RPM1 disease resistance protein. EMBO J 2003; 22:5679 - 5689
- Stuttmann J, Parker JE, Noel LD. Staying in the fold: The SGT1/chaperone machinery in maintenance and evolution of leucine-rich repeat proteins. Plant Signal Behav 2008; 3:283 - 285
- Tor M, Gordon P, Cuzick A, Eulgem T, Sinapidou E, Mert-Turk F, et al. Arabidopsis SGT1b is required for defense signaling conferred by several downy mildew resistance genes. Plant Cell 2002; 14:993 - 1003
- Yang H, Shi Y, Liu J, Guo L, Zhang X, Yang S. A mutant CHS3 protein with TIR-NB-LRR-LIM domains modulates growth, cell death and freezing tolerance in a temperature-dependent manner in Arabidopsis. Plant J 2010; 63:283 - 296
- Zhang M, Kadota Y, Prodromou C, Shirasu K, Pearl LH. Structural basis for assembly of Hsp90-Sgt1-CHORD protein complexes: implications for chaperoning of NLR innate immunity receptors. Mol Cell 2010; 39:269 - 281
- Zhou F, Mosher S, Tian M, Sassi G, Parker J, Klessig DF. The Arabidopsis gain-of-function mutant ssi4 requires RAR1 and SGT1b differentially for defense activation and morphological alterations. Mol Plant Microbe Interact 2008; 21:40 - 49
- Uchida N, Tasaka M. Intersections between immune responses and morphological regulation in plants. J Exp Bot 2010; 61:2539 - 2547