 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Plants are able to discriminately allocate greater biomass to organs that grow under higher resource levels. Recent evidence demonstrates that split-root plants also discriminately allocate more resources to roots that grow under dynamically improving nutrient levels, even when their other roots grow in richer patches. Here, we further tested whether, besides their responsiveness to the direction of resource gradients, plants are also sensitive to the steepness of environmental trajectories. Split-root Pisum sativum plants were grown so that one of their roots developed under constantly-high nutrient levels and the other root was subjected to dynamically improving nutrient levels of variable steepness. As expected, plants usually allocated a greater proportion of their biomass to roots that developed under constantly high resource availability; however, when given a choice, they allocated greater biomass to roots that initially experienced relatively low but steeply improving nutrient availabilities than to roots that developed under continuously-high nutrient availability. Such discrimination was not observed when the roots in the poor patch experienced only gentler improvements in nutrient availability. The results are compatible with the notion that responsiveness to the direction and steepness of environmental gradients could assist annual plants to increase their performance by anticipating resource availabilities foreseeable before the end of their growing season. The results exemplify the ability of plants to integrate and utilize environmental information and execute adaptive behaviours which, until recently, were attributed only to animals with central nervous systems.
Introduction
From the perspective of any living organism, environmental conditions are invariably heterogeneous in both space and timeCitation1–Citation3 and select for a wide spectrum of both genetically determined and environmentally induced adaptations.Citation4,Citation5 Coping with environmental variability is especially challenging for sessile organisms, where both stress avoidance and opportunistic behavior largely hinge on phenotypic plasticity rather than on motility.Citation6,Citation7 Although underlying myriad adaptations, phenotypic plasticity is often limited and costly.Citation8 Environmental responses, especially those involving the development of new organs, might lag behind environmental change and thus could result in costly functional phenotype-environment mismatches.Citation8,Citation9 Accordingly, selection is expected to promote responsiveness to cues that bear reliable information regarding the probable future environment.Citation10,Citation11 For example, plants have been shown to be able to take advantage of red/far-red (R/FR) spectral cues to anticipate light competition,Citation12–Citation14 preempt probable future herbivory using volatile cues emitted from damaged neighborsCitation15 and adapt to forthcoming nitrogen limitationCitation16 and drought.Citation17 Previous studies have further suggested that animalsCitation18–Citation21 and plantsCitation22–Citation24 are able to grow in accordance with anticipated future growth conditions, based on the perception of environmental gradients. In a recent study, Pisum sativum plants have been demonstrated to allocate greater biomass to roots growing under dynamically improving nutrient levels than to roots that grew under higher, yet constant or deteriorating, nutrient availabilities.Citation25 Responsiveness to the direction and steepness of spatial and temporal trajectories of environmental variables might enable plants to increase their performance by anticipating future resource availabilities in their immediate proximity.Citation10,Citation26
Here, we further test whether, besides the general responsiveness to the direction of resource gradients, plants are also sensitive to the steepness of environmental trajectories. In short-living annuals, gradient responsiveness is expected to be especially important where resource gradients are sufficiently steep and thus indicative of temporal changes in patch quality, which are expected to take place before the termination of the growing season. In contrast, such plants are expected to be less responsive to relatively gentle gradients, despite their potential predictive value, as they indicate expected benefits of discriminatory growth at time horizons which are longer than the expected lifespan of the plants.Citation10,Citation26
We tested these hypotheses using a “choice experiment,” whereby one of the roots of a split-root Pisum sativum plant was grown under constantly high-nutrient levels and the other root was subjected to dynamically improving nutrient availabilities of variable steepness. We predicted that due to the time required for the perception of the differential growth conditions and nutrient gradients, plants would initially allocate greater biomass to roots growing under the continuously high nutrients, but would later shift their allocation to roots growing under improving nutrient levels, which were sufficiently steep to indicate performance benefits before the termination of the growing season.
Results
Whole-plant performance.
Despite the large range of nutrient availabilities provided to the DI patches, no significant treatment effects were found on either whole-plant biomass or its vegetative and reproductive components (Table S1 and Figs. S2–5).
Relative growth rates and biomass allocation.
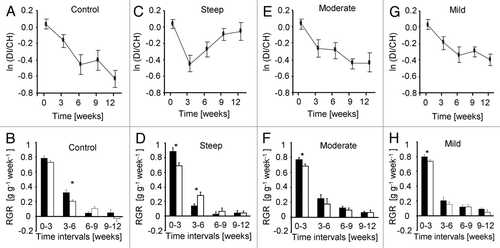
Patch preference significantly varied with time in all four treatments (Time effect: F4,338 = 23.610, p < 0.001). As expected, in the MODERATE, MILD and CONTROL treatments, the relative growth rates of the CH roots were initially higher than those of the DI roots. These differences in growth rates resulted in a ca. 2-fold greater biomass allocation to CH roots than to roots that developed under continuously low nutrient availability in the CONTROL, with similar trends in the MODERATE and MILD treatments (). In the STEEP treatment, the relative growth rates of roots in the CH patches were also initially higher than those of the DI roots; however, starting from week four and onwards, this trend was reversed, with significantly greater relative growth rates of the DI than their CH counterparts (Wilcoxon signed-rank test, Z = 3.12, N = 15, p = 0.002; Table S2). This shift in growth rates resulted in an increased allocation to the DI patch, which by the end of the experiment translated into equal biomasses of the CD and the DI root systems (Treatment × Time interaction; F12,338 = 2.740, p = 0.001), despite the 100% greater average nutrient supply provided throughout the experiment to the CH roots.
Discussion
Correlations within and among spatiotemporal trajectories bear valuable environmental information.Citation31–Citation34 The perception and integration of the direction and steepness of persistent trajectories allow the anticipation of extrapolated states and positions,Citation35,Citation36 and the preemption of various risksCitation37 and opportunitiesCitation38 in both biological and human-made systems. In plants, gradient responsiveness has been shown to increase the efficiency of shade avoidance,Citation22,Citation23 exploitation of rooting opportunitiesCitation24 and nutrient foraging.Citation25,Citation26 Our results further suggest that plants are not only responsive to the direction of environmental gradients but also to their steepness. In agreement with previous studies, plants usually allocated greater proportion of their biomass to roots that developed under constantly high resource availability ( and B),Citation39,Citation40 however, root allocation also depended on the dynamics of nutrient availability. When given a choice, plants allocated greater biomass to roots that initially experienced relatively low but sharply improving nutrient availabilities than to roots that developed under continuously high-nutrient availability. Following the dynamics of root growth revealed that these plants initially allocated more biomass to roots growing in the richer patch, but later shifted their allocation to the roots which developed under the lower but steeply improving nutrient levels (). Interestingly, this resulted in 100% greater growth rates of the DI roots, despite their experiencing a 50–75% lower nutrient availability at the time of the shift in allocation ().
The results are compatible with the notion that responsiveness to environmental gradients could assist plants to anticipate expected values of soil patches.Citation26 In both the STEEP and the MODERATE treatments, the DI root experienced 50–75% lower nutrient availability than its CH counterpart, between weeks 3–6 and 5–10, respectively. The fact that the experimental plants only increased their allocation to the DI roots when experiencing the steepest improvement in nutrient availability (STEEP) supports our hypothesis that plants are not merely responsive to the absolute nutrient availabilities, or to the direction of resource trajectories, but also to the steepness of environmental gradients. Such a response is hypothesized to be especially crucial in short-living annuals, where only steep gradients indicate significant changes in growth conditions before the end of the growing season.Citation25,Citation26 In contrast, although the gentler gradients (i.e., MODERATE, MILD) might also reliably indicate anticipated increases in nutrient availability, they are indicative of extrapolated spatiotemporal horizons, not relevant to the life span of such short-living annuals.
The results reiterate the notion that rudimentary life forms, such as bacteriaCitation18 and plants, are capable of integrating and utilizing environmental information and executing adaptive behaviors which, until recently, were only attributed to animals with central nervous systems.Citation41,Citation42 The results call for additional studies to test the particular ecological and evolutionary circumstances under which such capabilities are selected, and the prevalence of these abilities amongst wild taxa. Pursuing the mechanism underlying sophisticated behaviors in organisms without a central nervous system is not only expected to shed more light on information processingCitation43,Citation44 and learningCitation45 in biological systems, but can also facilitate the development of novel approaches in machine learning, automation and robotics.
Methods
Pisum sativum L. cv Kelvedon Wonder was used throughout. Young “split-root” seedlings were grown following Gersani & Sachs.Citation27 Pisum seedlings were grown so that they developed two equal roots following removal of the tip of the seminal root. Eight-day old seedlings, with symmetrical 3 ± 0.5 cm-long roots, were grown so each of their roots was separately planted in a 400 mL well-drained polyethylene pot filled with grade-3 vermiculite. Nutrient solutions were prepared using a 20-20-20 NPK fertilizer with microelements (Poly-Feed; Haifa Chemicals, Haifa, Israel).
Treatments were initiated one day after planting. One of the two individual roots of each plant was provided with a constantly high (CH) nutrient solution comprising 0.2 g fertilizer L−1, previously proven non-toxic, to elicit high growth rates in these plants,Citation25 while the other root of the same plant was provided with dynamically increasing (DI) nutrient levels, starting at a low nutrient concentration of 0.001 gL−1 for the first 84 h, and gradually increasing nutrient availability by replacing the nutrient solutions every 84 h according to the following schedules:
STEEP-nutrient supply increased in 0.0085 gL−1 increments, which reached the nutrient level supplied to the other root on the same plant in 12 weeks (). Across the entire experiment, the average nutrient availability was 50% lower for the DI root compared to the CH root.
MODERATE-nutrient supply increased in 0.00425 gL−1 increments, which would have reached the nutrient level supplied to the other (CH) root on the same plant in 24 weeks (). The nutrient level at the final harvest was 0.1 gL.−1 Across the entire experiment, the average nutrient availability was 75% lower for the DI root compared to the CH root.
MILD-nutrient supply increased in 0.002125 gL−1 increments, which would have reached the nutrient level supplied to the other (CH) root on the same plant in 48 weeks (). Across the entire experiment, the average nutrient availability was 87.5% lower for the DI root compared to the CH root.
CONTROL-nutrient levels were sustained at 0.001 gL−1 throughout the experiment, which was 99.5% lower than the nutrient concentration available to the CH root ().
Each pot was flushed twice a week by 300 ml (600 ml per plant) of the designated nutrient solution. The experiment was conducted in a glasshouse on the campus of Ben-Gurion University, Beer-Sheva, Israel (31°14′ N, 34°48′ E), under 70% of natural sunlight.
All plants were grown for 12 weeks (). Plants were assigned to blocks according to their initial leaf number. In order to enable intermediate harvests, each block included four plants from each treatment, one replication per treatment per harvest. The experiment included 15 blocks and a total of 240 plants. An additional 30 plants were harvested and quantified for their initial sizes before the commencement of the experimental treatments to allow the calculation of relative plant growth rates. Destructive harvests were conducted 3, 6, 9 and 12 weeks after the initiation of the experiment ().
At harvest, the two root systems of each plant were separated and the dry biomass of plant parts was estimated after drying the plants in a ventilated oven at 60°C for 72 h.
Data analyses.
Whole-plant response variables such as total plant, shoot, root and reproductive biomasses were analyzed using one-way ANOVAs, with treatment as an independent factor. Because the focus of the study was on allocation behavior rather than on overall plant performance, these data are presented as Supplemental materials (Table S1 and Figs. S2–5).
Patch preference was calculated, within each plant, using the ratio of the biomasses of the DI and the CH roots. Ratios were Log transformed to give equal weight to cases where either root system was larger than its counterpart.Citation28 Average ln(DI/CH) ratios were analyzed using a two-way ANOVA with Time and Treatment as the independent variables.
As the absolute values of total biomass ratios cannot clearly depict the allocation dynamics, relative growth rates of the CH and DI root systems were also analyzed. Relative growth rates were calculated, according to the convention,Citation29 using the following equation:
where mt was root biomass at time t and τ was the period between two consecutive harvests. Because the growth of the two root systems of each plant was interdependent, their relative growth rates were analyzed using split plot ANOVAs, with the two root systems serving as the within-subject factor and time and treatment as the between-subject factor.Citation30 Accordingly, a significant root system effect (within subject factor) indicated an overall difference between the two roots of the same plant, regardless of treatment. A significant treatment effect (between subject factor) indicated that the total root mass (i.e., the two root systems in the aggregate) varied among treatments. A significant root system by treatment interaction indicated that root allocation significantly varied between treatments.
All analyses were conducted using SYSTAT 11 (Systat Software, Inc., CA). Out of 270 harvested plants, seven plants from the four different treatments were excluded from the analyses as outliers (Fig. S1) and three plants died. Identical qualitative trends were obtained from analyses which included or excluded the outliers.
Figures and Tables
Figure 1 Experimental design testing the responsiveness of plants to steepness of resource gradients. One of the roots of split-root plants was subjected to constantly high nutrient availability while the other root experienced constantly low or variably improving resource availabilities. Arrows represent the timing of harvests.
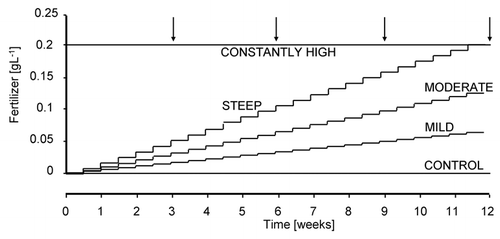
Figure 2 The ln(DI/CH) ratio (ln(dynamically increasing root mass/constantly high root mass)) was used as an estimator for patch preference. Changes in the ln(DI/CH) for the four different treatments are presented along time (A, C, E and G). The corresponding relative growth rates (RGR) of the two different root systems are presented below (B, D, F and H). RGRs are displayed for 3 week intervals. Black and empty bars represent the constantly high and dynamically increasing root systems respectively. All data presented are Mean ± 1 SE. Pairwise comparisons were done using Wilcoxon Signed Rank Tests (*p < 0.05).
Additional material
Download Zip (89.6 KB)Acknowledgments
The authors wish to thank Mordechai Gersani for his insightful comments and Yael Teleman-Rosen and Yael Ziv for their technical assistance. This is publication no. 749 of the Mitrani Department of Desert Ecology.
References
- Levins R. Evolution in changing environments 1968; New Jersey Princeton University Press
- Wijesinghe DK, Hutchings MJ. The effects of spatial scale of environmental heterogeneity on the growth of a clonal plant: An experimental study with Glechoma hederacea. J Ecol 1997; 85:17 - 28
- Qian H, Kissling WD. Spatial scale and cross-taxon congruence of terrestrial vertebrate and vascular plant species richness in China. Ecology 2010; 91:1172 - 1183
- Alpert P, Simms EL. The relative advantages of plasticity and fixity in different environments: when is it good for a plant to adjust?. Evol Ecol 2002; 16:285 - 297
- Valladares F, Gianoli E, Gomez JM. Ecological limits to plant phenotypic plasticity. New Phytol 2007; 176:749 - 763
- Sultan SE. Phenotypic plasticity and plant adaptation. Acta Botanica Neerlandica 1995; 44:363 - 383
- Bradshaw AD. Evolutionary significance of phenotypic plasticity in plants. Adv Genet 1965; 13:115 - 155
- DeWitt TJ, Sih A, Wilson DS. Costs and limits of phenotypic plasticity. Trends Ecol Evol 1998; 13:77 - 81
- Padilla DK, Adolph SC. Plastic inducible morphologies are not always adaptive: The importance of time delays in a stochastic environment. Evol Ecol 1996; 10:105 - 117
- Novoplansky A. Picking battles wisely: plant behavior under competition. Plant Cell Environ 2009; 32:726 - 741
- Ballare CL, Sanchez RA, Scopel AL, Casal JJ, Ghersa CM. Early detection of neighbor plants by phytochrome perception of spectral changes in reflected sunlight. Plant Cell Environ 1987; 10:551 - 557
- Smith H. Phytochromes and light signal perception by plants—an emerging synthesis. Nature 2000; 407:585 - 591
- Novoplansky A. Developmental responses of portulaca seedlings to conflicting spectral signals. Oecologia 1991; 88:138 - 140
- Ballare CL, Scopel AL, Sanchez RA. Far-Red radiation reflected from adjacent leaves—an early signal of competition in plant canopies. Science 1990; 247:329 - 332
- Heil M, Karban R. Explaining evolution of plant communication by airborne signals. Trends Ecol Evol 2010; 25:137 - 144
- Forde B, Zhang HM. …response: Nitrate and root branching. Trends Plant Sci 1998; 3:204 - 205
- Novoplansky A, Goldberg D. Interactions between neighbour environments and drought resistance. J Arid Environ 2001; 47:11 - 32
- Eisenbach M. A hitchhiker's guide through advances and conceptual changes in chemotaxis. J Cell Physiol 2007; 213:574 - 580
- Denver RJ, Mirhadi N, Phillips M. Adaptive plasticity in amphibian metamorphosis: Response of Scaphiopus hammondii tadpoles to habitat desiccation. Ecology 1998; 79:1859 - 1872
- Owen JP, Mullens BA, Justus KA, Carde RT. Northern fowl mite orientation in a thermal gradient and evidence for idiothetic course control. Physiol Entomol 2005; 30:293 - 302
- Spieler M, Linsenmair KE. Choice of optimal oviposition sites by Hoplobatrachus occipitalis (Anura: Ranidae) in an unpredictable and patchy environment. Oecologia 1997; 109:184 - 199
- Weijschede J, Martinkova J, de Kroon H, Huber H. Shade avoidance in Trifolium repens: costs and benefits of plasticity in petiole length and leaf size. New Phytol 2006; 172:655 - 666
- Leeflang L, During HJ, Werger MJA. The role of petioles in light acquisition by Hydrocotyle vulgaris L. in a vertical light gradient. Oecologia 1998; 117:235 - 238
- Nyanumba SM. Developmental strategies of plants under temporal variation in growth conditions 2007; Life Sci: Ben-Gurion University of the Negev
- Shemesh H, Arbiv A, Gersani M, Ovadia O, Novoplansky A. The Effects of Nutrient Dynamics on Root Patch Choice. PLoS One 2010; 5
- Shemesh H, Ovadia O, Novoplansky A. Anticipating future conditions via trajectory sensitivity. Plant Signal Behav 2010; 5:1501 - 1503
- Gersani M, Sachs T. Development correlations between roots in heterogeneous environments. Plant Cell Environ 1992; 15:463 - 469
- Novoplansky A. Hierarchy establishment among potentially similar buds. Plant Cell Environ 1996; 19:781 - 786
- Hunt R. Plant growth curves 1982; London Edward Arnold
- Zar JH. Biostatistical analysis 1999; Upper Saddle River, New Jersey Prentice-Hall Inc
- Keshavarz B, Landwehr K, Baures R, Oberfeld D, Hecht H, Benguigui N. Age-correlated incremental consideration of velocity information in relative time-to-arrival judgments. Ecol Psychol 22:212 - 221
- Dommes A, Cavallo V. The role of perceptual, cognitive and motor abilities in street-crossing decisions of young and older pedestrians. Ophthalmic Physiol Opt 31:292 - 301
- Wohl S, Schuster S. Hunting archer fish match their take-off speed to distance from the future point of catch. J Exp Biol 2006; 209:141 - 151
- Rossel S, Corlija J, Schuster S. Predicting three-dimensional target motion: how archer fish determine where to catch their dislodged prey. J Exp Biol 2002; 205:3321 - 3326
- Livingston RB. Neurophysiology 1990; Baltimore Williams & Wilkins
- Blanke M, Pourzanjani M, Vukic Z. Manoeuvring and control of marine craft 2000; Oxford, UK Pergamon
- Melillo JM, McGuire AD, Kicklighter DW, Moore B, Vorosmarty CJ, Schloss AL. Global climate-change and terrestrial net primary production. Nature 1993; 363:234 - 240
- Veale SR. Stocks, bonds, options, futures: investments and their markets 1991; New York Prentice Hall Trade
- Drew MC, Saker LR. Nutrient supply and growth of seminal root-system in barley. 3. compensatory increases in growth of lateral roots, and in rates of phosphate uptake, in response to a localized supply of phosphate. J Exp Bot 1978; 29:435 - 451
- Hodge A. The plastic plant: root responses to heterogeneous supplies of nutrients. New Phytol 2004; 162:9 - 24
- Trewavas A. Aspects of plant intelligence. Ann Bot (Lond) 2003; 92:1 - 20
- Trewavas A. What is plant behavior?. Plant Cell Environ 2009; 32:606 - 616
- Masi E, Ciszak M, Stefano G, Renna L, Azzarello E, Pandolfi C, et al. Spatiotemporal dynamics of the electrical network activity in the root apex. Proc Natl Acad Sci USA 2009; 106:4048 - 4053
- Baluska F, Mancuso S, Volkmann D, Barlow PW. Root apex transition zone: a signalling-response nexus in the root. Trends Plant Sci 2010; 15:402 - 408
- Bose I, Karmakar R. Simple models of plant learning and memory. Phys Scr 2003; 106:9 - 12