Abstract
Legumes can establish a symbiosis with rhizobia and form root nodules that function as an apparatus for nitrogen fixation. Nodule development is regulated by several phytohormones including auxin. Although accumulation of auxin is necessary to initiate the nodulation of indeterminate nodules, the functions of auxin on the nodulation of determinate nodules have been less characterized. In this study, the functions of auxin in nodule development in Lotus japonicus have been demonstrated using an auxin responsive promoter and auxin inhibitors. We found that the lenticel formation on the nodule surface was sensitive to the auxin defect. Further analysis indicated that failure in the development of the vascular bundle of the determinate nodule, which was regulated by auxin, was the cause of the disappearance of lenticels.
Legumes (Fabaceae) constitute the third largest plant family with around 700 genera and 20,000 species.Citation1 Legume plants form root nodules through symbiosis with a soil microbe called rhizobia. This plant-microbe symbiosis in nodules mediates an harmonized exchange of chemical signals between host plants and rhizobia.Citation2 Nodules are biologically divided into two different groups, i.e., indeterminate nodules and determinate nodules. Indeterminate nodules, represented by Trifolium repens (white clover) and Medicago truncatula, are initiated from the inner cortex to form a persistent nodule meristem, which allows continuous growth, and leads to the formation of elongated nodules, whereas in determinate legumes, nodules are mostly developed from outer cortical cells and form spherical nodules.Citation3
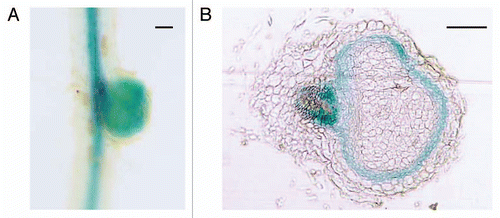
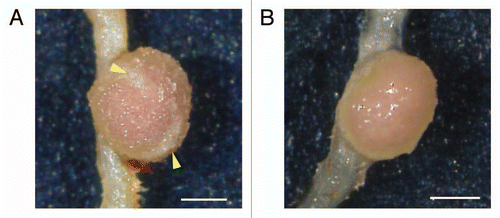
Auxin is one of the most important regulators for nodule development. Since the possible involvement of auxin in nodule formation was first reported by Thimann,Citation4 auxin distribution during nodulation has been studied in particular with indeterminate nodules.Citation5 However, little is known about auxin involvement in determinate nodule formation. To evaluate auxin functions in the determinate nodulation of legume plants, we performed an auxin-responsive promoter analysis in detail. Using GH3:GUS transformed Lotus japonicus (a kind gift from Dr. Herman P. Spaink, Leiden State University, Netherlands),Citation6 we detected auxin signals throughout the nodulation process, e.g., at the basal and front part of the nodule primordia, circumjacent to the infection zone of the young developing nodules (), and at the nodule vascular bundle in mature nodules. We also investigated the effect of several auxin inhibitors, including newly synthesized auxin antagonist PEO-IAA (kindly provided by Dr. Hayashi, Okayama University of Science, Japan),Citation7 on the nodulation of L. japonicus, and revealed that auxin was required for forming a nodule vascular bundle and lenticels ().Citation8
In indeterminate legumes, auxin is accumulated at the site of rhizobia inoculation.Citation9 This is caused by the inhibition of polar auxin transport by accumulation of flavonoids around the infection site, which are known as regulators of auxin transport. When flavonoid biosynthesis is reduced by the gene silencing of chalcone synthase, which catalyzes the first step of flavonoid synthesis, M. truncatula was unable to inhibit polar auxin transport and resulted in reduced nodule number.Citation10,Citation11 A similar phenotype was observed when the auxin transporter gene was silenced.Citation12 In addition, treatment of polar auxin transport inhibitors such as NPA and TIBA induce pseudonodule formation,Citation9 suggesting that auxin accumulation is required for nodulation of indeterminate legumes. In contrast, the treatment of polar auxin transport inhibitors in determinate nodules did not induce a nodule-like structure, suggesting a different function of auxin between indeterminate and determinate nodules. It is, however, of interest to investigate the involvement of flavonoids in determinate nodule formation, because several genes in the flavonoid biosynthesis pathway are upregulated at 2 dpi (days post inoculation) in L. japonicus.Citation13
Lenticels regulate gas permeability of nodules.Citation14 Under low oxygen or water-logged conditions, they develop more extensively, whereas they collapse, or develop very little during insufficient water conditions, or under high oxygen pressure.Citation14,Citation15 Because lenticel development on the nodule surface is accompanied with the nodule vascular bundle, growth regulators supplied from the vascular system likely facilitate lenticel development.Citation15 Our data suggests that auxin is necessary to form the nodule vascular bundle, and in fact, auxin itself is one of the candidates of growth substances that control lenticel formation. It is necessary to analyze mutants, which lack in lenticel formation, but can form a nodule vascular bundle, for clarification of further mechanisms of lenticel development.
Addendum to:
References
- Doyle JJ, Luckow MA. The rest of the iceberg. Legume diversity and evolution in a phylogenetic context. Plant Physiol 2003; 131:900 - 910
- Colebatch G, Desbrosses G, Ott T, Krusell L, Montanari O, Kloska S, et al. Global changes in transcription orchestrate metabolic differentiation during symbiotic nitrogen fixation in Lotus japonicus. Plant J 2004; 39:487 - 512
- Hirsch AM. Developmental biology of legume nodulation. New Phytol 1992; 122:211 - 237
- Thimann KV. On the physiology of the formation of nodules on legume roots. Proc Natl Acad Sci USA 1936; 22:511 - 514
- Mathesius U. Auxin: at the root of nodule development?. Funct Plant Biol 2008; 35:651 - 668
- Pacios-Bras C, Schlaman HRM, Boot K, Admiraal P, Langerak JM, Stougaard J, et al. Auxin distribution in Lotus japonicus during root nodule development. Plant Mol Biol 2003; 52:1169 - 1180
- Hayashi K, Tan X, Zheng N, Hatate T, Kimura Y, Kepinski S, et al. Small-molecule agonists and antagonists of F-box protein-substrate interactions in auxin perception and signaling. Proc Natl Acad Sci USA 2008; 105:5632 - 5637
- Takanashi K, Sugiyama A, Yazaki K. Involvement of auxin distribution in root nodule development of Lotus japonicus. Planta 2011; 234:73 - 81; PMID: 21369920; http://dx.doi.org/10.1007/s00425-011-1385-0
- Mathesius U, Schlaman HRM, Spaink HP, Sautter C, Rolfe BG, Djordjevic MA. Auxin transport inhibition precedes root nodule formation in white clover roots and is regulated by flavonoids and derivatives of chitin oligosaccharides. Plant J 1998; 14:23 - 34
- Wasson AP, Pellerone FI, Mathesius U. Silencing the f lavonoid pathway in Medicago truncatula Inhibits root nodule formation and prevents auxin transport regulation by rhizobia. Plant Cell 2006; 18:1617 - 1629
- Zhang J, Subramanian S, Stacey G, Yu O. Flavones and flavonols play distinct critical roles during nodulation of Medicago truncatula by Sinorhizobium meliloti. Plant J 2009; 57:171 - 183
- Huo XY, Schnabel E, Hughes K, Frugoli J. RNAi phenotypes and the localization of a protein::GUS fusion imply a role for Medicago truncatula PIN genes in nodulation. J Plant Growth Regul 2006; 25:156 - 165
- Kouchi H, Shimomura K, Hata S, Hirota A, Wu GJ, Kumagai H, et al. Large-scale analysis of gene expression profiles during early stages of root nodule formation in a model legume, Lotus japonicus. DNA Res 2004; 11:263 - 274
- Walsh KB. Physiology of the legume nodule and its response to stress. Soil Biol Biochem 1995; 27:637 - 655
- Pankhurst CE, Sprent JI. Surface features of soybean root nodules. Protoplasma 1975; 85:85 - 98