Abstract
This review highlights a potential signaling pathway of CO2-dependent stimulation in root hair development. Elevated CO2 firstly increases the carbohydrates production, which triggers the auxin or ethylene responsive signal transduction pathways and subsequently stimulates the generation of intracellular nitric oxide (NO). The NO acts on target Ca2+ and ion channels and induces activation of MAPK. Meanwhile, reactive oxygen species (ROS) activates cytoplasmic Ca2+ channels at the plasma membrane in the apex of the root tip. This complex pathway involves transduction cascades of multiple signals that lead to the fine tuning of epidermal cell initiation and elongation. The results suggest that elevated CO2 plays an important role in cell differentiation processes at the root epidermis.
Increasing concentration of atmospheric CO2 in the 21st century will impact many aspects of the human and natural world. Elevated CO2 has some beneficial physiological effects on plants but nutrient limitation has generally been found to suppress these beneficial effects.Citation1 Therefore, under conditions of suboptimal supply of nutrients and elevated CO2, the plants need to develop adaptive mechanisms to enhance nutrient acquisition, among which the plasticity of root development is of crucial importance.
Root hairs make a significant contribution to increasing root surface area and facilitating physical anchorage to a substrate and providing a large interface for nutrient uptake.Citation2 Root-hair cells are highly polarized cellular structures resulting from tip growth of specific epidermal cells, which are controlled by multiple cellular factors and genetic processes.Citation3,Citation4 Previous studies have shown that root hair development can influenced by various environmental factors, such as nutritional status,Citation5 mycorrhizal infection and water stress,Citation6 salinityCitation7 and light intensity.Citation8 Our current research has demonstrated a profound effect of elevated CO2 on development of root hairs in Arabidopsis, which works through the well-characterized auxin signal transduction pathway.Citation9 Since root hairs are an efficient strategy to alleviate the limitation of nutrients, one promising area of future research will be to discover the pathway that control root hair differentiation in crops under elevated CO2. In this paper, we discussed a layer pathway in the interaction between CO2 and some classical signals on regulating gene regulatory network to control development of root hairs.
Process of Root Hair Development
Root-hair morphogenesis, which forms a model system for studying polarized plant cell growth, can be subdivided into three major stages: swelling formation (referred to hereafter as root-hair initiation), the transition to tip growth and tip growth.Citation10 The patterning of such a process is highly regulated. Numerous experimental observations indicate that root hair initiation and tip growth are controlled by multiple factors, such as phytohormones, ABA, cellular and extracellular signals like expansins, cytoplasmic and cell wall pH, actin cytoskeleton and microtubules.Citation11–Citation14 A tip-focussed cytoplasmic calcium (Ca2+) gradient forms during root hair growth as it does in all other tip growing cells,Citation15 These process requires tip-localized ROS produced by an NADPH oxidase through activation of hyperpolarisation-activated calcium channels.Citation16 The maintenance of this tip-focused Ca2+ gradient during hair growth is dependent on microtubules.Citation12 Besides, potassium channel is the major osmotically active ion in many plant cells and the translocation of potassium is vital for root hair growth.Citation17
Genetic analyses have resulted in identification of a number of genes that control root hair development at various stages. Genes including CPC, TRY, ETC1, TTG, GL2, GL3/EGL3, WER, RHL1, RHL2, RHL3, ERH1, ERH3 and ERH2 Citation4,Citation14 are identified to be involved in the early phase of root epidermal cell specification. SCM, a leucinerich repeat receptor-like kinase (LRR-RLK), has recently been shown to be required for position dependent pattern of epidermal cells. In scm mutants, the formation of N and H cells is not correlated to their position.Citation18 After the emergence of a bulge outside an epidermal cell (root hair initiation stage), genes RHD6, TRH1, RHD1, TIP1, AtEXP7 and AtEXP18 can affect the number of swellings on each hair cell, and hair outgrowth and elongation.Citation4,Citation13,Citation14,Citation17 Following initiation, numerous genes are activated for the correct direction and extent of root-hair-tip growth. Root hairs without functional RHD2, SHV1, SHV2, SHV3, TRH1, ROP2, KJK or AKT1 genes stop growing before this stage.Citation14,Citation17,Citation19,Citation20 These results suggest that all of these genes are important for successful establishment of hair cell elongation and tip growth. Mutations affecting the CEN1, CEN2, CEN3, SCN1, BST1 and TIP1 genes can also stop hair growth before this stage, but only in certain double-mutant combinations.Citation19 Additionally, SCN1, COW1, TIP1, CEN2, CEN1, CEN3, BST1, RHD3 or RHD4 genes can induce more branched hairs in Arabidopsis.Citation4,Citation19 LRX1, PFN1 and Sec1 protein KEULE are required for normal root hair development.Citation4,Citation14
How does Elevated CO2 Regulate Root Hairs Development?
There is accumulating evidence that elevated CO2 can accelerate plant growth and development by affecting cell division, elongation and differentiation within apical meristems.Citation21,Citation22 These cellular processes are regulated by a suite of classical signaling including auxin, ethylene, jasmonates (JAs), gibberellins (GAs), cytokinins (CKs), NO, abscisic acid (ABA), ROS, phospholipids and cytoplasmic Ca2+.Citation16,Citation23 Interestingly, elevated CO2 increases carbohydrate production,Citation24 auxin level and response in plants,Citation9,Citation25 ethylene production,Citation26 NO accumulationCitation27 and our (unpublished data) and abscisic acid concentration.Citation25 Thus, changes in levels and/or responses of these factors may play an important role in regulating the development of root hairs grown under elevated CO2.
To further discuss the pathway in which elevated CO2 affects root hair growth, we need find more convincing evidence to support the above hypothesis. In fact, many studies have shown that plants grown in elevated CO2 usually have an increased concentration of carbohydrates, such as soluble sugar and starch, in leaves because of carbohydrate assimilation in excess of consumption.Citation24 The conclusion is in accordance with the results found in many other plants. It has been recognized that an increased accumulation of carbohydrates in plants would increase the production of auxin.Citation28 Thus, elevated CO2 might thus increase concentrations of auxin in the plants via an increase in carbohydrate production. Alternatively, elevated CO2 could enhance ethylene production,Citation26 while ethylene could stimulate IAA synthesis and transport in root tips. However, Rahman et al.Citation29 reported that auxin plays a compensating role in the process of root hair development in Arabidopsis in the absence of ethylene. Both auxin and ethylene can interact on their biosynthesis and the response pathways, or sometimes independently regulate the same target genes.Citation3 The correlation between auxin and ethylene signalling in root development is complex. Moreover, it has also reported that JAs promote root hair formation in Arabidopsis, through an interaction with ethylene.Citation23 This implies that there exists interplay among phytohormones in mediating root hair development. These issues require further investigation. Recent studies have shown that elevated CO2 could increase auxin levels which then induced NO accumulation.Citation27 In addition, NO was involved in the growth and development of root hairs, of which underlying mechanisms were under the control of auxin.Citation22 Thus NO may act downstream of CO2, carbohydrates auxin, ethylene or probably JAs.
Recently, NO has been proved as a multipurpose signaling messenger that accomplishes its biological functions through its action on multiple targets. The available data illustrate that NO can directly influence the activity of target proteins through nitrosylation and has the capacity to act as a Ca2+-mobilizing intracellular messenger.Citation30 Meanwhile, NO-dependent signals can be modulated through protein phosphorylation upstream of intracellular Ca2+ release. They implicate a target for protein kinase control in ABA signalling that feeds into NO-dependent Ca2+ release.Citation31 As broadly known, a high concentration of cytoplasmic Ca2+ at the root tip is required for maintaining its growth rate. Furthermore, Samaj et al.,Citation5 have assembled these components into a model in which ROS produced by NADPH oxidase activates Ca2+ channels at the plasma membrane in the apex of the root tip, leading to a tip-focused Ca2+ concentration gradient and subsequent signaling inherent to root hair growth. The Ca2+-permeable channel modulated by ROS has been demonstrated in Vicia faba guard cells and Arabidopsis root hairs.Citation16 Additionally, root hair growth was associated with ROS production through the activation of the MAPK cascade.Citation32 Interestingly, NO has also been shown to be involved in the activation of a MAPK cascade during adventitious root formation.Citation33 These implies that NO play a fundamental role in outgrowth through MAPK cascade activation.
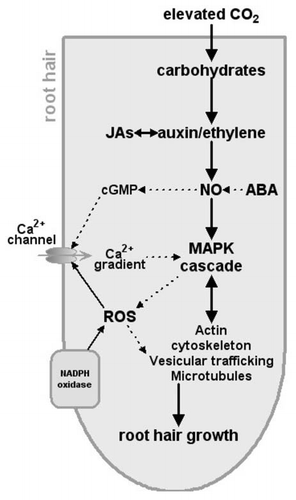
Based on previous studies and our recent observations, a model could be proposed of how CO2 regulates the root hair formation (). This model is based on that proposed by Samaj et al.,Citation5 Lombardo et al.,Citation22 and Niu et al.Citation9 Elevated CO2 firstly increases the carbohydrates production, which triggers the auxin or ethylene responsive signal transduction pathways and subsequently results in the generation of intracellular NO. NO modulate target Ca2+ and ion channels and MAPK signaling cascade that are proposed as control points of root hair development. Withal, ROS activates Ca2+ channels at the plasma membrane in the apex of the root tip. Then, these endogenous signals modulate the downstream genetic elements that control actin cytoskeleton vesicular and microtubules, which together regulate root hair development. Overall, future studies, including those focusing on molecular and physical mechanisms governing interactions among the cytoskeleton, plasma membrane and cell wall, must consider CO2 as a new and critical player to understand cell differentiation processes in the root epidermis.
Figures and Tables
Figure 1 Conceptual model showing the potential elevated-CO2-target points in the signaling events those lead to the growth of root hairs. Solid arrows indicate links established in the induction of root hair development and broken arrows represent already established links in other systems but yet to be demonstrated in the growth of root hairs. NO, nitric oxide; cGMP, cyclic GMP; JAs, jasmonates; ROS, reactive oxygen species.
Acknowledgments
We thank Dr. Caixian Tang from Department of Agricultural Sciences, La Trobe University for critical reading and revision of the manuscript. This work was financially supported by the Project of Transformation Fund for Agricultural Scientific and Technological Achievements of China (2010GB23600669), the Natural Science Foundation of China (NSFC, No. 30871590) and Doctoral Fund of Ministry of Education of China (No. 200803350117).
Addendum to:
References
- Stitt M, Krapp A. The interaction between elevated carbon dioxide and nitrogen nutrition: the physiological and molecular background. Plant Cell Environ 1999; 22:583 - 621
- Peterson RL, Farquhar ML. Root hairs: specialized tubular cells extending root surfaces. Bot Rev 1996; 62:1 - 40
- Stepanova AN, Yun J, Likhacheva AV, Alonso JM. Multilevel interactions between ethylene and auxin in Arabidopsis roots. Plant Cell 2007; 19:2169 - 2185
- Grierson C, Schiefelbein J. Genetics of root hair formation. Root Hairs Plant Cell Monogr 2009; 12:Berlin Heidelberg Springer-Verlag 1 - 15
- Samaj J, Baluška F, Menzel D. New signalling molecules regulating root hair tip growth. Trends Plant Sci 2004; 9:217 - 220
- Bibikova T, Gilroy S. Root hair development. J Plant Growth Regul 2003; 21:383 - 415
- Wang YN, Zhang WS, Li KX, Sun FF, Han CY, Wang YK, Li X. Salt-induced plasticity of root hair development is caused by ion disequilibrium in Arabidopsis thaliana. J Plant Res 2008; 121:87 - 96
- Okada K, Shimura Y. Meyerowitz EM, Somerville CR. Modulation of root growth by physical stimuli. Arabidopsis 1994; Cold Spring Harbor, NY Cold Spring Harbor Laboratory Press 665 - 684
- Niu YF, Jin CW, Jin GL, Zhou QY, Lin XY, Tang CX, Zhang YS. Auxin modulates the enhanced development of root hairs in Arabidopsis thaliana (L.) Heynh. under elevated CO2. Plant Cell Environ 2011; 34:1304 - 1317
- Dolan L, Duckett CM, Grierson C, Linstead P, Schneider K, Lawson E, et al. Clonal relationships and cell patterning in the root epidermis of Arabidopsis. Development 1994; 120:2465 - 2474
- Miller DD, de Ruijter NCA, Bisseling T, Emons AMC. The role of actin in root hair morphogenesis: studies with lipochito-oligosaccharide as a growth stimulator and cytochalasin as an actin perturbing drug. Plant J 1999; 17:141 - 154
- Bibikova TN, Blancaflor EB, Gilroy S. Microtubules regulate tip growth and orientation in root hairs of Arabidopsis thaliana. Plant J 1999; 17:657 - 665
- Cho HT, Cosgrove DJ. Regulation of root hair initiation and expansin gene expression in Arabidopsis. Plant Cell 2002; 14:3237 - 3253
- Grierson C, Schiefelbein J. Root hairs. The Arabidopsis Book First published on April 4, 2002 http://dx.doi.org/10.1199/tab.0060
- Monshausen GB, Messerli MA, Gilroy S. Imaging of the Yellow Cameleon 3.6 indicator reveals that elevations in root cytosolic Ca2+ follow oscillating increases in growth in root hairs of Arabidopsis. Plant Physiol 2008; 147:1690 - 1698
- Foreman J, Demidchik V, Bothwell JH, Mylona P, Miedema H, Torres MA, et al. Reactive oxygen species produced by NADPH oxidase regulate plant cell growth. Nature 2003; 422:442 - 446
- Rigas S, Debrosses G, Haralampidis K, Vicente-Agullo F, Feldmann KA, Grabov A, et al. TRH1 encodes a potassium transporter required for tip growth in Arabidopsis root hairs. Plant Cell 2001; 13:139 - 151
- Kwak SH, Shen R, Schiefelbein J. Positional signaling mediated by a receptor-like kinase in Arabidopsis. Science 2005; 307:1111 - 1113
- Parker JS, Cavell AC, Dolan L, Roberts K, Grierson CS. Genetic interactions during root hair morphogenesis in Arabidopsis. Plant Cell 2000; 12:1961 - 1974
- Desbrosses G, Josefsson C, Rigas S, Hatzopoulos P, Dolan L. AKT1 and TRH1 are required during root hair elongation in Arabidopsis. J Exp Bot 2003; 54:781 - 788
- Taylor G, Tricker PJ, Zhang FZ, Alston VJ, Miglietta F, Kuzminsky E. Spatial and temporal effects of free-air CO2 enrichment (POPFACE) on leaf growth, cell expansion and cell production in a closed canopy of poplar. Plant Physiol 2003; 131:177 - 185
- Lombardo MC, Graziano M, Polacco JC, Lamattina L. Nitric oxide functions as a positive regulator of root hair development. Plant Signal Behav 2006; 1:28 - 33
- Zhu CH, Gan LJ, Shen ZG, Xia K. Interactions between jasmonates and ethylene in the regulation of root hair development in Arabidopsis. J Exp Bot 2006; 57:1299 - 1308
- Long SP, Drake BG. Baker NR, Thomas H. Photosynthetic CO2 assimilation and rising atmospheric CO2 concentrations. Crop photosynthesis: spatial and temporal determinants 1992; Amsterdam, the Netherlands Elsevier 69 - 95
- Teng N, Wang J, Chen T, Wu X, Wang Y, Lin J. Elevated CO2 induces physiological, biochemical and structural changes in leaves of Arabidopsis thaliana. New Phytol 2006; 172:92 - 103
- Dhawan KR, Bassi PK, Spencer MS. Effects of carbon dioxide on ethylene production and action in intact sunflower plants. Plant Physiol 1981; 68:831 - 834
- Jin CW, Du ST, Chen WW, Li GX, Zhang YS, Zheng SJ. Elevated carbon dioxide improves plant iron nutrition through enhancing the iron-deficiency-induced responses under iron-limited conditions in tomato. Plant Physiol 2009; 150:272 - 280
- Pritchard SHG, Rogers HOH, Prior SA, Peterson CTM. Elevated CO2 and plant structure: a review. Global Change Biology 1999; 5:807 - 837
- Rahman A, Hosokawa S, Oono Y, Amakawa T, Goto N, Tsurumi S. Auxin and ethylene response interactions during Arabidopsis root hair development dissected by auxin influx modulators. Plant Physiol 2002; 130:1908 - 1917
- Courtois C, Besson A, Dahan J, Bourque S, Dobrowolska G, Pugin A, Wendehenne D. Nitric oxide signalling in plants: interplays with Ca2+and protein kinases. J Exp Bot 2008; 59:155 - 163
- Sokolovski S, Hills A, Gay R, Garcia-Mata C, Lamattina L, Blatt MR. Protein phosphorylation is a prerequisite for intracellular Ca2+ release and ion channel control by nitric oxide and abscisic acid in guard cells. Plant J 2005; 43:520 - 529
- Rentel MC, Lecourieux D, Ouaked F, Usher SL, Petersen L, Okamoto H, et al. OXI1 kinase is necessary for oxidative burst-mediated signalling in Arabidopsis. Nature 2004; 427:858 - 861
- Pagnussat GC, Lanteri ML, Lombardo MC, Lamattina L. Nitric oxide mediates the indole acetic acid induction activation of a mitogen-activated protein kinase cascade involved in adventitious root development. Plant Physiol 2004; 135:279 - 286