Abstract
CMT1B is the second most frequent autosomal dominant inherited neuropathy and is caused by assorted mutations of the myelin protein zero (MPZ) gene. MPZ mutations cause neuropathy gain of function mechanisms that are largely independent MPZs normal role of mediating myelin compaction. Whether there are only a few or multiple pathogenic mechanisms that cause CMT1B is unknown. Arg98Cys and Ser63Del MPZ are two CMT1B causing mutations that have been shown to cause neuropathy in mice at least in part by activating the unfolded protein response (UPR). We have recently treated Arg98Cys mice with derivatives of curcumin that improved the neuropathy and reduced UPR activation.Citation1 Future studies will address whether manipulating the UPR will be a common or rare strategy for treating CMT1B or other forms of inherited neuropathies.
Charcot Marie Tooth disease (CMT) is the most common genetic neuromuscular disorder afflicting approximately 1:2500.Citation2 CMT is genetically heterogeneous as mutations in more than 50 genes cause the various types of CMT. These can be inherited as autosomal dominant (AD) disorders that affect myelin (CMT1) or axons (CMT2) in the peripheral nervous system (PNS). Autosomal recessive (AR) (CMT4) and X-linked (CMTX) forms of CMT also exist. CMT is also pathogenically heterogeneous. Many AD forms are caused by “gain of function” mutations in which the mutated protein causes deleterious effects to the Schwann cell or neuron that are independent of the protein’s normal function. Alternatively, many of the AR forms of CMT are “loss of function” disorders that are caused by the loss of function of the mutated protein. The large numbers of genes that cause CMT enable investigators to focus on identifying molecular pathways that cause demyelination or axonal degeneration since the cause of the neuropathy is known. In our studies we have focused on pathogenic gain of function mechanisms that cause CMT1B, the second most common form of CMT1.
CMT1B is caused by mutations in the Myelin Protein Zero (MPZ) gene.Citation3,Citation4 MPZ comprises more than 50% of the myelin proteins in the peripheral nervous system and is responsible for myelin compaction.Citation5 More than 120 different disease-causing mutations in MPZ have been identified (http://www.molgen.ua.ac.be/CMTMutations/default.cfm). Most patients with CMT1B present in two phenotypic groups: one with extremely slow nerve conduction velocities and onset of symptoms during the period of motor development; and a second with normal or near normal nerve conduction velocities and the onset of symptoms as adults.Citation6 Different mutations cause mild or severe phenotypes by at least several gain of function mechanisms.
One of these mechanisms results occurs when CMT1B mutations cause mutated MPZ to be retained in the endoplasmic reticulum (ER) instead of being transported to the cell membrane or myelin sheath. Examples include MpzS51ΔW57,Citation7 506delT and 550del3insGCitation8 studied in vitro and Ser63del and R98C mice, studied in vivo.Citation9-Citation11 ER stress in both Ser63del and R98C mice contributed to the neuropathy by activating a canonical unfolded protein response (UPR).Citation9,Citation10 UPR activation aims to reduce the load of unfolded proteins through upregulation of chaperones, attenuation of protein synthesis and increased protein degradation. The UPR is mediated initially by three molecules located in the ER membrane: IRE1, ATF6 and PERK. Three parameters may be used to detect UPR activation: (1) XBP1 splicing as an indicator of IRE1 pathway activation, (2) ATF6 cleavage and (3) increase in the levels of the transcription factor CHOP and its translocation to the nucleus, as an indicator of PERK pathway. R98C Mpz activates all three arms of the UPR.Citation10 These mice thus serve as a model to test therapies directed at relieving ER stress in CMT1B and in related neuropathies.Citation10
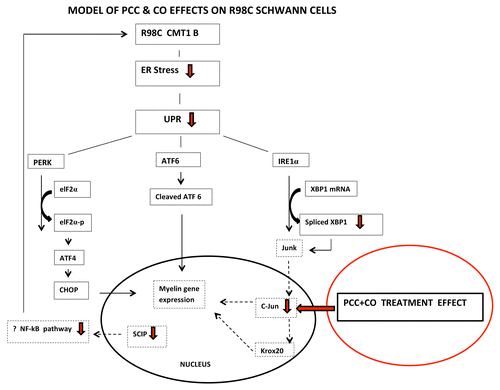
Sarcoplasmic/endoplasmic reticulum calcium pump (SERCA) inhibitors reduce ER stress and consequently UPR activation by inhibiting calcium binding and disrupting calnexin function.Citation12 Curcumin, derived from the curry spice turmeric, has multiple cellular targets and pleiotropic biological effects including activity as a low affinity SERCA inhibitor.Citation13 It has been shown to relieve ER stress and ameliorate the phenotype in several in vitro and in vivo models of UPR activation. Therefore, we treated R98C mice with orally administered curcumin. We did not obtain significant benefits in mice treated with either curcumin dissolved in alimentum (CA)—the form used to treat TrJ miceCitation14—or with a fluorinated curcumin derivative (CDF).Citation15 However, a phosphatidylcholine curcumin derivative (PCC) designed to increase bioavailabilityCitation16 and curcumin dissolved in sesame oil (CO) improved the R98C/R98C animals by neurophysiological and morphological criteria and the R98C/+ mice by clinical, neurophysiological and morphological criteria. PCC or CO treatment attenuated the UPR. Of particular interest were findings that treatment with PCC increased percentages of fully myelinated preterminal internodes and decreased length of demyelinated segments approaching the NMJ of treated animals. We hypothesized that these changes at the NMJ may contribute to the clinical improvement in the mice and perhaps also to the increased numbers of large diameter axons in the treated nerves. Also of interest were the findings that R98C mice treated with sesame oil showed a trend toward increased CMAP amplitudes and improved Rotarod performance. The recent study in which cholesterol treatment improved transgenic mice with the leukodystrophy Pelizaeus-Merzbacher disease (PMD) may provide an explanation for these results.Citation17 The most common form of PMD is caused by a duplication of the major CNS myelin protein proteolipid protein 1 (PLP1).Citation18 Dietary supplementation with cholesterol improved Plp1 overexpressing mice clinically and increased their myelin content, at least in part by facilitating the incorporation of PLP1 into CNS myelin membranes through lipid rafts.Citation17 Moreover, a previous study by the same group showed that cholesterol might act similarly on Mpz.Citation19 Sesame oil contains negligible cholesterol but it does contain multiple plant sterols that function through similar pathways in humans and that presumably contribute to cholesterol-lowering properties of sesame and other plant oils.Citation20 Thus, we hypothesize that sesame oil may act like cholesterol to promote trafficking of MpzR98C into myelin and contribute to the improvement of R98C mice, a hypothesis that is supported by the fact that CO treatment was as effective as PCC in treating the animals. We also speculate that treatment regulates Schwann cell transcription to promote myelination (see ).
Figure 1. Model of PCC and CO effects on R98C Schwann cells. We propose that PCC or CO treatment promotes a myelinating phenotype in Schwann cells by decreasing the expression of transcription factors c-Jun or SCIP that inhibit myelination when upregulated.
The potential significance of our findings was summarized in a commentary that accompanied our article in Brain.Citation21 As was pointed out in the commentary, we are currently entering an era in which therapeutic strategies for genetic diseases, including CMT, are being increasingly undertaken. However, it will be important to determine whether the results from our study will be applicable to patients with only the particular Arg98Cys MPZ mutation or whether UPR activation is one of a limited number of dysfunctional pathways that contribute to gain of function deficits in CMT.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- Patzkó A, Bai Y, Saporta MA, Katona I, Wu X, Vizzuso D, et al. Curcumin derivatives promote Schwann cell differentiation and improve neuropathy in R98C CMT1B mice. Brain 2012; 135:3551 - 66; http://dx.doi.org/10.1093/brain/aws299; PMID: 23250879
- Skre H. Genetic and clinical aspects of Charcot-Marie-Tooth’s disease. Clin Genet 1974; 6:98 - 118; http://dx.doi.org/10.1111/j.1399-0004.1974.tb00638.x; PMID: 4430158
- Nelis E, Van Broeckhoven C, De Jonghe P, Löfgren A, Vandenberghe A, Latour P, et al. Estimation of the mutation frequencies in Charcot-Marie-Tooth disease type 1 and hereditary neuropathy with liability to pressure palsies: a European collaborative study. Eur J Hum Genet 1996; 4:25 - 33; PMID: 8800924
- Saporta ASD, Sottile SL, Miller LJ, Feely SM, Siskind CE, Shy ME. Charcot-Marie-Tooth disease subtypes and genetic testing strategies. Ann Neurol 2011; 69:22 - 33; http://dx.doi.org/10.1002/ana.22166; PMID: 21280073
- Trapp BD, Pfeiffer SE, Anitei A, Kidd GJ. Cell Biology and myelin assembly. In: Lazzarini RA ed, Myelin Biology and Disorders. San Diego/London: Elsevier Academic Press; 2003:29-56.
- Shy ME, Jáni A, Krajewski KM, Grandis M, Lewis RA, Li J, et al. Phenotypic clustering in MPZ mutations. Brain 2004; 127:371 - 84; http://dx.doi.org/10.1093/brain/awh048; PMID: 14711881
- Grandis M, Vigo T, Passalacqua M, Jain M, Scazzola S, La Padula V, et al. Different cellular and molecular mechanisms for early and late-onset myelin protein zero mutations. Hum Mol Genet 2008; 17:1877 - 89; http://dx.doi.org/10.1093/hmg/ddn083; PMID: 18337304
- Khajavi M, Inoue K, Wiszniewski W, Ohyama T, Snipes GJ, Lupski JR. Curcumin treatment abrogates endoplasmic reticulum retention and aggregation-induced apoptosis associated with neuropathy-causing myelin protein zero-truncating mutants. Am J Hum Genet 2005; 77:841 - 50; http://dx.doi.org/10.1086/497541; PMID: 16252242
- Pennuto M, Tinelli E, Malaguti M, Del Carro U, D’Antonio M, Ron D, et al. Ablation of the UPR-mediator CHOP restores motor function and reduces demyelination in Charcot-Marie-Tooth 1B mice. Neuron 2008; 57:393 - 405; http://dx.doi.org/10.1016/j.neuron.2007.12.021; PMID: 18255032
- Saporta MA, Shy BR, Patzko A, Bai Y, Pennuto M, Ferri C, et al. MpzR98C arrests Schwann cell development in a mouse model of early-onset Charcot-Marie-Tooth disease type 1B. Brain 2012; 135:2032 - 47; http://dx.doi.org/10.1093/brain/aws140; PMID: 22689911
- Wrabetz L, D’Antonio M, Pennuto M, Dati G, Tinelli E, Fratta P, et al. Different intracellular pathomechanisms produce diverse Myelin Protein Zero neuropathies in transgenic mice. J Neurosci 2006; 26:2358 - 68; http://dx.doi.org/10.1523/JNEUROSCI.3819-05.2006; PMID: 16495463
- Egan ME, Pearson M, Weiner SA, Rajendran V, Rubin D, Glöckner-Pagel J, et al. Curcumin, a major constituent of turmeric, corrects cystic fibrosis defects. Science 2004; 304:600 - 2; http://dx.doi.org/10.1126/science.1093941; PMID: 15105504
- Epstein J, Sanderson IR, Macdonald TT. Curcumin as a therapeutic agent: the evidence from in vitro, animal and human studies. Br J Nutr 2010; 103:1545 - 57; http://dx.doi.org/10.1017/S0007114509993667; PMID: 20100380
- Khajavi M, Shiga K, Wiszniewski W, He F, Shaw CA, Yan J, et al. Oral curcumin mitigates the clinical and neuropathologic phenotype of the Trembler-J mouse: a potential therapy for inherited neuropathy. Am J Hum Genet 2007; 81:438 - 53; http://dx.doi.org/10.1086/519926; PMID: 17701891
- Padhye S, Banerjee S, Chavan D, Pandye S, Swamy KV, Ali S, et al. Fluorocurcumins as cyclooxygenase-2 inhibitor: molecular docking, pharmacokinetics and tissue distribution in mice. Pharm Res 2009; 26:2438 - 45; http://dx.doi.org/10.1007/s11095-009-9955-6; PMID: 19714451
- Marczylo TH, Verschoyle RD, Cooke DN, Morazzoni P, Steward WP, Gescher AJ. Comparison of systemic availability of curcumin with that of curcumin formulated with phosphatidylcholine. Cancer Chemother Pharmacol 2007; 60:171 - 7; http://dx.doi.org/10.1007/s00280-006-0355-x; PMID: 17051370
- Saher G, Rudolphi F, Corthals K, Ruhwedel T, Schmidt KF, Löwel S, et al. Therapy of Pelizaeus-Merzbacher disease in mice by feeding a cholesterol-enriched diet. Nat Med 2012; 18:1130 - 5; http://dx.doi.org/10.1038/nm.2833; PMID: 22706386
- Inoue K, Osaka H, Sugiyama N, Kawanishi C, Onishi H, Nezu A, et al. A duplicated PLP gene causing Pelizaeus-Merzbacher disease detected by comparative multiplex PCR. Am J Hum Genet 1996; 59:32 - 9; PMID: 8659540
- Saher G, Quintes S, Möbius W, Wehr MC, Krämer-Albers EM, Brügger B, et al. Cholesterol regulates the endoplasmic reticulum exit of the major membrane protein P0 required for peripheral myelin compaction. J Neurosci 2009; 29:6094 - 104; http://dx.doi.org/10.1523/JNEUROSCI.0686-09.2009; PMID: 19439587
- Abidi SL. Chromatographic analysis of plant sterols in foods and vegetable oils. J Chromatogr A 2001; 935:173 - 201; http://dx.doi.org/10.1016/S0021-9673(01)00946-3; PMID: 11762774
- Roberts RC. The Charcot-Marie-Tooth diseases: how can we identify and develop novel therapeutic targets?. Brain 2012; 135:3527 - 8; http://dx.doi.org/10.1093/brain/aws311; PMID: 23250878