Abstract
Pre-mRNA splicing occurs in two chemical steps that are catalyzed by a large, dynamic RNA-protein complex called the spliceosome. Initially assembled in a catalytically inactive form, the spliceosome undergoes massive compositional and conformational remodeling, through which disparate RNA elements are re-configured and juxtaposed into a functional catalytic center. The intricate construction of the catalytic center requires the assistance of spliceosomal proteins. Recent structure-function analyses have demonstrated that the yeast-splicing factor Cwc2 is a main player that contacts and shapes the catalytic center of the spliceosome into a functional conformation. With this advance, corroborated by the atomic structure of the evolutionarily related group IIC introns, our understanding of the organization and formation of the spliceosomal catalytic center has progressed to a new level.
Introduction
Pre-mRNA splicing is an essential step in gene expression in eukaryotes, in which non-coding sequences (introns) are removed from the pre-mRNA and the coding sequences (exons) are ligated to form a mature mRNA ready for translation. The chemical splicing reaction comprises two consecutive phosphoryl-transfer steps that involve reactive groups located in three distinct pre-mRNA regions: the two splice sites (SS) flanking the intron (5′SS and 3′SS) and the branch-point sequence (BPS). Pre-mRNA splicing is performed by the spliceosome, a multi-megadalton machine that is assembled from five small nuclear ribonucleoprotein particles (snRNPs) and non-snRNP proteins. Each of the snRNPs is composed of one specific RNA molecule (U1, U2, U4, U5 or U6) and tightly associated proteins.Citation1
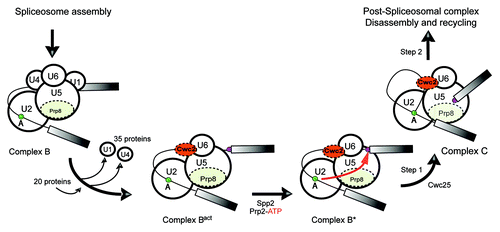
Spliceosome dynamics follows a cyclic pathway (reviewed by Will and Lührmann),Citation2 that starts with the binding of U1 snRNP to the 5′SS, which is followed by the recognition of the BPS by the U2 snRNP. Thereafter, the preformed U4/U6.U5 tri-snRNP is recruited, generating a catalytically inactive spliceosome called complex B. At this stage, all five snRNAs and the pre-mRNA are base-paired in an intricate RNA–RNA network stabilized by proteins. The formation of the catalytic center occurs during the conversion of B to the B* complex. This transition can be experimentally dissected into two successive stages: B to Bact, followed by Bact to B* (). The most dramatic change in the composition and conformation of the spliceosome, called activation, occurs at the B to Bact transition. Thus in yeast, U1 and U4 snRNAs along with 35 proteins dissociate, while 20 new proteins are recruited (). Under the influence of the RNA helicase Prp2, the Bact complex is further converted into B*, which is able to catalyze the first reaction upon recruitment of the protein Cwc25 ().Citation3 After further recruitments and conformational remodeling, the spliceosome catalyzes the second phosphoryl-transfer reaction and finally disassembles.
Figure 1. Remodeling events at the spliceosome from the pre-catalytic stage to the first step of splicing. The proteins known to be important for formation of the catalytic center are highlighted. The nucleophilic attack of the BPS at the 5′SS is represented by a red arrow. The BPS and 5′SS are shown as green and purple circles, respectively.
The spliceosome is a metalloenzymeCitation4-Citation7 that catalyzes the two phosphoryl-transfer steps by a two-metal-ion mechanism, similarly to the self-splicing ribozymes termed “group II introns.”Citation5 Owing to the resemblance between the spliceosomal RNA-RNA network and group II introns, it has been assumed that the catalysis of pre-mRNA splicing is RNA-based.Citation8 Remarkably, the recent detection of homology between group II intron maturases and the spliceosomal protein Prp8 provides additional evidence that the two splicing systems share a common ancestry.Citation9
Significant effort has been made to identify the components of the catalytic center of the spliceosome—chemical groups that coordinate the catalytic metal ions and the ones which position the reactive groups of the pre-mRNA for phosphoryl transfer. The 5′SS and BPS are positioned through the specific base pairing within the RNA-RNA network as well as through their recognition by proteins, as has been suggested for the RNase H-like domain of Prp8.Citation10,Citation11 The catalytic metal ions are coordinated by two distinct regions of the U6 snRNA: a bulged-out uracil from the intramolecular stem-loop (ISL)Citation12,Citation13 and a three-base sequence from helix Ib (AGC) named the catalytic triad.Citation14 Parallels with the crystal structures of group II introns suggest how a third region of the U6 snRNA, called the ACAGAGA box, integrates the bulge and the triad tightly into the catalytic center, by inducing tertiary interactions ( and ).Citation15
Figure 2. Comparative view of secondary structures of the RNA-RNA network of the Bact spliceosomes and group II introns according to Madhani and GuthrieCitation16 and Keating et al.,Citation15 respectively. (A) The U2/U6/pre-mRNA nucleotides important for formation of the catalytic center and for catalysis, as well as the ones contacted by Cwc2, are shown.Citation17 Nucleotides crosslinked to Cwc2 are marked by blue “lightning” symbols. Bases protected from chemical modification in the presence of Cwc2 are indicated by blue bars. The last two bases of the ACAGAGA box, the catalytic triad and the bulge (from left to right in the diagram) are shown in red. The blue arrows suggest RNA interactions with the Cwc2 molecule (represented as a surface in the background and color-coded as in ). The broad arrow indicates how Cwc2 might induce tertiary interactions among the catalytic elements (purple arrows). The catalytic reactants of the first step of splicing (5′SS and BPS adenosine) are shown in green. (B) Group II introns: the functional equivalents of the spliceosomal elements are depicted and color-coded as in A. Bases involved in tertiary interactions are indicated by blue bars. Tertiary interactions between domains I and V are indicated by blue arrows, while the ones between the catalytic regions J2/3, the triad and the bulge (from left to right) are shown in red. (Adapted, with modifications, from Keating et al.)Citation15
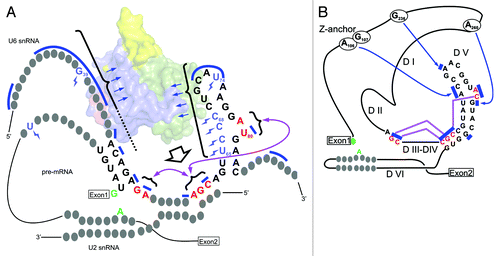
The formation of the spliceosomal catalytic center requires RNA–RNA and RNA–protein rearrangements through which the disparate and sequestered catalytic components, along with the reactants, are liberated and juxtaposed to catalyze the phosphoryl-transfer reaction. Thus, after the dissociation of the U1 and U4 snRNAs, the U6 snRNA adopts a different conformation and takes part in new duplexes. The central U6 snRNA region, previously base-paired to U4 snRNA, forms the catalytic ISL, while other regions form duplexes with the U2 snRNA (helices Ia and Ib) and with the 5′ end of the intron via the ACAGAGA box ().Citation16 Although the remodeling of the RNA-RNA interactions that underlies activation is the major prerequisite for formation of the catalytic center, additional events are required for catalysis to occur. Recent structure-function analyses have demonstrated that, after activation, the RNA-binding protein Cwc2 is required to shape further the active configuration of the catalytic center.Citation17,Citation18
The mapping of the contacts of Cwc2 with the U6 snRNA in several spliceosomal complexes (Bact, B* and C)Citation17 and in the binary Cwc2–U6 snRNA complexes,Citation18 correlated with the crystal structures of Cwc2 and group II intronsCitation18-Citation20 leads to valuable insight into the three-dimensional organization of the spliceosomal catalytic center.
The Evolutionary Background of Prp8 Brings Additional Evidence to the Common Ancestry Shared by Spliceosomes and Group II Introns
It was earlier suggested that the five snRNA molecules of the spliceosome might be regarded as originating from a self-splicing RNA ancestor that divided into independent pieces of spliceosomal RNA, which act in trans on the pre-mRNA instead of on itself.Citation21 Group II introns have the ability to self-splice by using functional equivalents of the spliceosomal snRNA regions involved in substrate recognition and catalysis. The secondary structure of the group II intron consists of six RNA domains, named I–VI.Citation20 Domain V is functionally the most important for catalysis, as it carries the catalytic bulge and triad, as does the spliceosomal counterpart U6-ISL together with the U6-U2 helix Ib. Experiments with chimeric constructs showed that Domain V could replace the U6 ISL in a splicing assay of the minor spliceosome, demonstrating their functional equivalence.Citation22 However, all similarities between spliceosomes and group II introns refer exclusively to the reaction chemistry and RNA components. Because spliceosomes are dominated by proteins, it was difficult to judge whether the two splicing systems share a common ancestry or their resemblance is a result of convergent evolution impelled by the requirement to catalyze the same type of reaction chemistry.
A recent finding concerning the evolutionary origin of the spliceosomal protein Prp8 seems to solve this dilemma. With more than 2400 residues in yeast, Prp8 is the largest and the most highly conserved spliceosomal protein (62% identity between human and yeast). Despite the fact that Prp8 is large enough to accommodate a modular architecture, its domain structure is unclear. Until recently, only two domains—together representing about 20% of the entire protein—had been identified, one of them only by determining its crystal structure.Citation23-Citation26 Recent bioinformatic analyses led to the identification of central Prp8 domains that unequivocally share the highest conservation with reverse transcriptases encoded by group II introns (RT).Citation9 Group II introns are mobile retroelements, and in addition to self-splicing they can catalyze their own incorporation into DNA through reverse splicing, followed by reverse transcription with the help of self-encoded reverse transcriptases. These enzymes also possess “maturase” function, interacting extensively with the RNA domains to induce and stabilize the catalytic conformation required for self-splicing.Citation26,Citation27 In the absence of maturases, the self-splicing process is inefficient.Citation28
The parallels between maturases and Prp8 are remarkable, the latter having a crucial role in dealing with RNA transactions within the spliceosomal RNA-RNA network. This finding is of the utmost importance, since it provides the missing link between the spliceosomal proteins and the proteins associated with group II introns, lending support to the postulate of a common evolutionary origin of the two splicing systems.Citation9
The Catalytic Center is Shaped by Proteins Within the Spliceosome, and by Specific RNA Domains in Group II Introns
The crystal structures of group IIC introns (spanning Domains I–V) allow the visualization of the catalytic components contained by Domain V, both in the pre-catalytic state and in the post-catalytic state that follows exon ligation and precedes exon release.Citation20,Citation29 The structures reveal how the massive Domain I acts as a main scaffold that surrounds Domain V, inducing its functional conformation by several tertiary interactions.
As the RNA scaffold from group II introns does not have an equivalent in the spliceosome, it is likely that its role has been taken over by some of the numerous spliceosomal proteins. This would explain the very low efficiency of a splicing-like reaction catalyzed by a protein-free RNA construct, consisting of the U6 and U2 regions that can form base-pair interactions identical to the ones in the spliceosome.Citation30,Citation31 As RNA can adopt numerous alternative conformations, the presence of protein components restricts its freedom of folding, stabilizing a functional structure.Citation32
Prp8 interactions with several catalytically important RNA regions are consistent with a role of this protein as a scaffold and a master regulator of the remodeling events from the RNA–RNA network (reviewed in Grainer and Beggs).Citation33 Thus, Prp8 was crosslinked to the 5′SS before the first step of splicing as well as to the 3′SS and BPS after this step.Citation17,Citation33,Citation34
X-ray crystallography revealed that a Prp8 fragment, previously crosslinked to the GU from the 5′SS in the B complex,Citation35 adopts the fold of an RNase H-like domain.Citation23-Citation25 This finding unified a large body of biochemical and genetic data and suggested how Prp8 recognizes the 5′SS in the RNA-RNA network.Citation10,Citation11,Citation35 Moreover, point mutations in this domain suppress defects in the 5′SS, 3′SS and BPS, indicating that this Prp8 domain is involved in functional interactions with the reactive groups of the pre-mRNA during both steps of splicing.Citation35
Despite the evidence that places Prp8 in close proximity to the catalytic center, no crosslinks have been detected between Prp8 and the U6 snRNA in Bact complexes.Citation17 Very surprisingly, it was found that the protein Cwc2, previously unsuspected of having a central function, plays a major role: it recognizes U6 snRNA after activation, inducing the active conformation of the catalytic center.Citation17
Extensive Interactions between Cwc2 and the U6 snRNA are Essential for the Formation of a Functional Catalytic Center
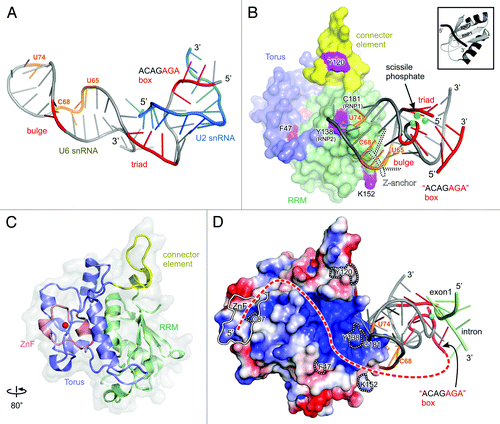
In contrast to Prp8, Cwc2 is far smaller (40 kDa), it exhibits much lower sequence conservation (26% identity between yeast and human), and it is recruited to the spliceosome at a later point, during the transition from the B to the Bact complex.Citation36 Even more strikingly, phylogenetic analysis shows that the homology between the yeast Cwc2 and its human counterpart Rbm22 is limited to a central region comprising the zinc finger (ZnF), the recently identified Torus domain and an RNA-recognition motif (RRM) domain ().Citation17,Citation18
Figure 3. Structural analysis of the spliceosomal catalytic center. (A) Solution structure of the yeast U6/U2 snRNAs complex (pdb 2LKR).Citation37 Bases crosslinked to Cwc2 in intact spliceosomes are shown in orange. Catalytically important regions are shown in red and labeled accordingly. (B) Hypothetical model of the complex between yeast Cwc2 and U6 snRNA in the catalytically competent conformation. The position of the catalytic metal ions (cyan) and scissile phosphate and the conformation of U6 snRNA are assumed to be identical to those of their respective equivalents from the crystal structure of group IIC intron (pdb 3IGI), except for the U6 snRNA pentaloop, which was modeled on the basis of the Domain V tetraloop. The Z-anchor—specific to the group II introns—is depicted by a dotted outline. The Cwc2 structure is colored according to its domains and labeled accordingly. Residues crosslinked to the U6 snRNA in binary complexes are colored purple and labeled. A typical RRM domain in complex with RNA is showed in the upper right corner (pdb 1FHT).Citation43 The distance between Cwc2 and the bulge, triad and ACAGAGA box is of about 18, 19 and 27 Å, respectively (C) Ribbon plot of the crystal structure of yeast Cwc2 functional core rotated about the y axis as indicated (pdb 3TP2).Citation18 (D) The proposed model is oriented as in , where the electrostatic potential is mapped on the surface of Cwc2. A putative interacting tract of the protected U6 snRNA region, located upstream of the ACAGAGA box, is depicted as a red dashed line. All labels are as in . The position of the exon 1-intron junction is depicted in the same way as the one from group II introns in the pre-catalytic conformation (pdb 4DS6).Citation29
Cwc2 is essential for the viability of yeast cells, and spliceosomes lacking Cwc2 are not able to catalyze the first step of splicing. Catalysis can be effectively rescued when Cwc2 is restored to the Bact spliceosomal complexes, along with the downstream factors Prp2, Spp2 and Cwc25.Citation17 Current data indicate that the crucial role of Cwc2 for catalysis originates in its recognition of the U6 snRNA and not, for example, in merely allowing recruitment of subsequent proteins to the spliceosome. Thus, Cwc2 depletion does not affect the recruitment of Prp2 and Spp2 to the spliceosome or their activity in converting Bact to B*.Citation17 Finally, the recruitment of Cwc25 does not seem to be mediated by direct contacts with Cwc2, since an interaction between these two proteins could not be detected by a pull-down assay in vitro (our unpublished results).
Crosslinking experiments from the Lührmann laboratory have led to the accurate identification of several bases within the Bact, B* and C complexes that are in contact with Cwc2.Citation17 Except for one, from the pre-mRNA, most of them belong to the U6 snRNA: one upstream of the ACAGAGA box and five in the ISL (four in the stem and one in the pentaloop, ). The crosslink to G39 is recorded with the same intensity in Bact, B* and C complexes, indicating a firm and constant contact during the spliceosomal transitions. The crosslinks from the ISL exhibit minor differences in the three complexes, suggesting that ISL accommodation by Cwc2 occurs gradually, from a loose binding in the Bact to a firm one in C complex.Citation17 Furthermore, chemical treatment of Bact and of a Bact lacking Cwc2 with reagents that modify the Watson-Crick edge of the bases demonstrates that the influence of Cwc2 on U6 snRNA is much more extensive. Thus, numerous bases located along the entire U6 snRNA strand are protected from chemical modification only in the presence of Cwc2 (). On the basis of these data and the analogy with the 3D model of the group II introns, Rasche et al.Citation17 concluded that Cwc2 recognizes U6 snRNA, and the binding of these induces a fold in U6 snRNA that brings together elements of the catalytic center.
Cwc2’s Compact Architecture is Required to Recognize Regions of U6 snRNA and to Induce the Functional Conformation of the Catalytic Center
Guided by the crystal structures of Cwc2 and group IIC introns, the interactions described above can lead to insights that include predictions about the 3D organization of the spliceosomal catalytic center. The detection of Cwc2-U6 snRNA crosslinks indicates that the extensive protection of the U6 snRNA results from direct contacts with Cwc2 in some regions and, possibly, from induced conformational modifications in other regions. The overlap between protection and crosslinking can give an approximate idea of two main U6 snRNA regions that interact with Cwc2: the extended one around the crosslinked G39 and one in the ISL, encompassing the crosslinked and protected bases from the pentaloop, plus the crosslinked ones from the stem ().Citation17
The other regions of U6 snRNA do not contain any bases crosslinked to Cwc2, and their inaccessibility in structure-probing might reflect a Cwc2-induced restructuring either through the formation of tertiary interactions or by the establishment of new contacts with other spliceosomal components. Remarkably, these regions include the putative components of the catalytic center: the bulge, the catalytic triad and the ACAGAGA box (). Analysis of available atomic structures containing these elements helps to interpret their Cwc2-induced restructuring of their 3D organization.
The recently determined solution structure of the yeast U2/U6 snRNA complex shows the absence of any tertiary interactions between the catalytic triad, the bulge and the ACAGAGA box, all three elements being located remote of each other ().Citation37 The previously reported structures of constructs encompassing the ISL and helix Ib show a similar separation between the bulge and the triad.Citation38 These structures reflect conformations adopted in isolation, in the absence of any constraints from other spliceosomal components. Consistently with this, the bulge and triad from group II introns are also remote from each other in the solution structure of the isolated Domain V.Citation39
The crystal structures of group II introns reveal a compact packing of the RNA domains that encapsulate and anchor Domain V in a central position. Constrained by an elaborate network of tertiary interactions, Domain V adopts a folding very different from the one observed in isolation, with the bulge and catalytic triad juxtaposed to coordinate jointly the two catalytic metal ions (). It is noteworthy that two of the three bases in the J2/3 region—the junction between Domains II and III—interact with the triad and the bulge, stabilizing them in the active conformation. In spliceosomes, the ACAGAGA box comes into proximity with the triad through tertiary interactions as well,Citation40,Citation41 and its last three nucleotides were proposed to be the equivalent of J2/3.Citation15 Considering the common origin of the two splicing systems, the spliceosomal ISL-helix Ib region would be expected to adopt a conformation similar to that of Domain V, at least in respect of the juxtaposition of the bulge, triad and ACAGAGA box to form the catalytic center. Therefore, Domain V in the active conformation would be suitable for crude modeling of docking with the Cwc2 protein.
The crystal structure of Cwc2 revealed how the CCCH-type ZnF and the RRM domain are fastened by a specific Torus domain in one compact and specific fold.Citation18 UV irradiation of the Cwc2-U6 snRNA binary complexes allowed the identification of three main regions that possess RNA-binding affinity: in the RRM domain, in the ZnF and in the connector element (). Mutagenic analysis further confirmed the importance of these regions for RNA binding and for splicing in vitro.Citation18,Citation42 Out of the three RNA binding regions, only residues from the RRM could be crosslinked to a construct encompassing the ISL and catalytic triad.Citation18 Two of these residues, C181 and Y138, belong to the two canonical RNA-binding motifs—RNP1 and RNP2—respectively.
On the basis of the consideration that Cwc2 binding to U6 snRNA induces a new conformation of the ISL, comparable to the catalytic one of Domain V, and further taking into account the identity of the ISL bases with which Cwc2 makes contact in activated spliceosomes as well as the crosslinked amino acid residues of RRM, we propose, as a working hypothesis, a model of U6-ISL in complex with Cwc2 in the catalytically competent conformation ( and ). Thus, the relative orientation of the U6-ISL and Cwc2 is dictated by the highly restrictive manner in which canonical RNP motifs interact with RNA molecules: only single-stranded RNA is bound, and the direction of the RNA strand (5′ to 3′) always corresponds to the orientation (RNP2 to RNP1) of the two motifs (). The three protected bases CAU74 in the pentaloop are the only single-stranded RNA regions of ISL that can be conceived of as making, and retaining, contact with the RNP motifs, while the four bases from the stem may be expected to come to lie on the RRM region that contains the crosslinked K152. In this manner, the amino-acid residues in contact with the pentaloop would play the role of the G236 that interacts with Domain V in group II introns ( and ). Furthermore, the contacts between Cwc2 and the stem might contribute to an effect similar to the one of the Z-anchor on Domain V conformation ( and ). Despite the role of Cwc2 in inducing the active conformation of the U6-ISL, a notable prediction of the model is that the 5′SS and the catalytic center are remote of the Cwc2 surface (about 20 Å, ). Consistently, no crosslinks were observed between any of these RNA elements and Cwc2.Citation17 In addition, this configuration leaves enough space for the RNase H-like domain of Prp8 that was suggested to make direct contact with the 5′SS during the first step of catalysis.Citation22,Citation23,Citation24,Citation35
The crosslinked G39 cannot be placed unambiguously on the basis of the available data. However, the position and direction of the ACAGAGA box in this model indicate the relative orientation of the protected region containing G39 with respect to the other residues crosslinked to RNA (). Thus, consistently with its notable length, this RNA strand may come to lie in the positively charged depression delimited by the Torus and the connector element, continuing toward the ZnF and even beyond it (). This putative recognition path of the RNA strand is flanked by all the residues of Cwc2 that were crosslinked to the U6 snRNA and not to the ISL (C87, F47, Y120; ). The resulting model illustrates the role of Cwc2 in bringing the ACAGAGA box into the proximity of the ISL, where its last three nucleotides engage the bulge and the triad and bring them together into a unique structural entity—the catalytic center of the spliceosome. A validation of this principle requires methods suitable to evidence changes in interatomic distances from U6 snRNA upon Cwc2 addition, in isolation or within intact spliceosome (e.g., NMR, fluorescence spectroscopy or paramagnetic resonance).
Conclusion and Future Challenges
In view of the similarities between the spliceosomal RNA network and the group II introns of the self-splicing ribozymes, it has long been suggested that the catalytic center of the spliceosome is RNA-based—if not entirely, then at least mainly. Recently discovered homology between Prp8 and the maturases encoded by the group II introns provides notable additional support for the common ancestry of the two splicing systems.
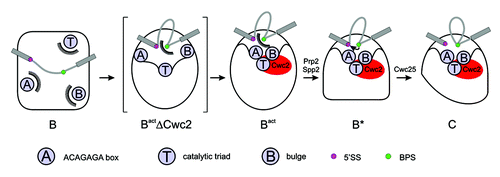
Elaborate biochemical experiments have brought the spliceosomal protein Cwc2 into the spotlight as a main organizer of the U6 snRNA components of the catalytic center. Thus, a more detailed scenario of the catalytic center’s formation and organization has emerged. After re-configuration of the base pairing of the U6 snRNA during activation, a second profound change in the U6 snRNA's conformation occurs when it is recognized and bound by Cwc2. The most likely effect of this event is that the key U6 snRNA components of the catalytic center—the bulge, triad and ACAGAGA box—are juxtaposed into a unique structural entity able to coordinate metal ions for catalysis ( and and summarized in ). Taking together the atomic structures of Cwc2 and group II introns as well as the biochemical data on the Cwc2–U6 snRNA interaction, a model Cwc2-U6 snRNA complex in the catalytically competent configuration is proposed ( and ).
Figure 4. Spliceosomal remodeling events that lead to formation of the catalytic center and to catalysis. The three important components of the catalytic center (ACAGAGA box, catalytic triad, bulge; labeled A, T, B) are sequestered and remote from each other in the B complex. Afterwards, they are re-organized in the proximity of the reactive groups during activation, forming the Bact ΔCwc2 complex (Bact lacking Cwc2), depicted as an artificial snapshot intermediate. The action of Cwc2 makes A, T and B further associate, to form the active center within the Bact complex. The further remodeling of the spliceosome under Prp2/Spp2 action allows the BPS access to the catalytic center.Citation3 Finally, Cwc25 triggers the first step of splicing.
The newly evidenced role of Cwc2 complements the long-studied one of Prp8 at the catalytic center: while Cwc2 shapes the U6 snRNA to create the co-ordination site for metal ions, Prp8 positions the reactive groups of the pre-mRNA in proximity to the metal ions for catalysis. Considering the highly complex nature of the spliceosomal proteome and its inner RNA–RNA network, Cwc2 and Prp8 may act synergetically at the catalytic center, perhaps even involving other spliceosomal proteins.
Because Cwc2 represents only a piece of a large puzzle, a future challenge will be to widen our picture of the catalytic center by identifying other spliceosomal proteins that contact the RNA-RNA network at various spliceosomal stages and by mapping their interactions. This should lead to the reconstitution of functionally relevant complexes that contain even more subunits and then to the use of these complexes in further structural and functional studies.
| Abbreviations: | ||
| snRNA | = | small nuclear ribonucleic acid |
| snRNP | = | small nuclear ribonucleoprotein particle |
| BPS | = | branch-point sequence |
| 5′SS | = | 5′ splice site |
| ISL | = | intramolecular stem-loop |
| DMS | = | Dimethylsulfat |
| CMCT | = | 1-cyclohexyl-(2-morpholinoethyl) carbodiimide metho-p-toluene sulfonate |
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
We are very grateful to Olexander Dybkov and Kum-Loong Boon for helpful comments and critical reading of the manuscript.
References
- Will CL, Luhrmann R. Spliceosome structure and function. The RNA World, Third Edition 2006:369-400.
- Will CL, Lührmann R. Spliceosome structure and function. Cold Spring Harb Perspect Biol 2011; 3:3; http://dx.doi.org/10.1101/cshperspect.a003707; PMID: 21441581
- Warkocki Z, Odenwälder P, Schmitzová J, Platzmann F, Stark H, Urlaub H, et al. Reconstitution of both steps of Saccharomyces cerevisiae splicing with purified spliceosomal components. Nat Struct Mol Biol 2009; 16:1237 - 43; http://dx.doi.org/10.1038/nsmb.1729; PMID: 19935684
- Moore MJ, Sharp PA. Evidence for two active sites in the spliceosome provided by stereochemistry of pre-mRNA splicing. Nature 1993; 365:364 - 8; http://dx.doi.org/10.1038/365364a0; PMID: 8397340
- Steitz TA, Steitz JA. A general two-metal-ion mechanism for catalytic RNA. Proc Natl Acad Sci U S A 1993; 90:6498 - 502; http://dx.doi.org/10.1073/pnas.90.14.6498; PMID: 8341661
- Gordon PM, Sontheimer EJ, Piccirilli JA. Metal ion catalysis during the exon-ligation step of nuclear pre-mRNA splicing: extending the parallels between the spliceosome and group II introns. RNA 2000; 6:199 - 205; http://dx.doi.org/10.1017/S1355838200992069; PMID: 10688359
- Sontheimer EJ. The spliceosome shows its metal. Nat Struct Biol 2001; 8:11 - 3; http://dx.doi.org/10.1038/82979; PMID: 11135658
- Cech TR. The generality of self-splicing RNA: relationship to nuclear mRNA splicing. Cell 1986; 44:207 - 10; http://dx.doi.org/10.1016/0092-8674(86)90751-8; PMID: 2417724
- Dlakić M, Mushegian A. Prp8, the pivotal protein of the spliceosomal catalytic center, evolved from a retroelement-encoded reverse transcriptase. RNA 2011; 17:799 - 808; http://dx.doi.org/10.1261/rna.2396011; PMID: 21441348
- Abelson J. Is the spliceosome a ribonucleoprotein enzyme?. Nat Struct Mol Biol 2008; 15:1235 - 7; http://dx.doi.org/10.1038/nsmb1208-1235; PMID: 19050716
- Reyes JL, Gustafson EH, Luo HR, Moore MJ, Konarska MM. The C-terminal region of hPrp8 interacts with the conserved GU dinucleotide at the 5′ splice site. RNA 1999; 5:167 - 79; http://dx.doi.org/10.1017/S1355838299981785; PMID: 10024169
- Yean SL, Wuenschell G, Termini J, Lin RJ. Metal-ion coordination by U6 small nuclear RNA contributes to catalysis in the spliceosome. Nature 2000; 408:881 - 4; http://dx.doi.org/10.1038/35048617; PMID: 11130730
- Villa T, Pleiss JA, Guthrie C. Spliceosomal snRNAs: Mg(2+)-dependent chemistry at the catalytic core?. Cell 2002; 109:149 - 52; http://dx.doi.org/10.1016/S0092-8674(02)00726-2; PMID: 12007401
- Hilliker AK, Staley JP. Multiple functions for the invariant AGC triad of U6 snRNA. RNA 2004; 10:921 - 8; http://dx.doi.org/10.1261/rna.7310704; PMID: 15146076
- Keating KS, Toor N, Perlman PS, Pyle AM. A structural analysis of the group II intron active site and implications for the spliceosome. RNA 2010; 16:1 - 9; http://dx.doi.org/10.1261/rna.1791310; PMID: 19948765
- Madhani HD, Guthrie C. A novel base-pairing interaction between U2 and U6 snRNAs suggests a mechanism for the catalytic activation of the spliceosome. Cell 1992; 71:803 - 17; http://dx.doi.org/10.1016/0092-8674(92)90556-R; PMID: 1423631
- Rasche N, Dybkov O, Schmitzová J, Akyildiz B, Fabrizio P, Lührmann R. Cwc2 and its human homologue RBM22 promote an active conformation of the spliceosome catalytic centre. EMBO J 2012; 31:1591 - 604; http://dx.doi.org/10.1038/emboj.2011.502; PMID: 22246180
- Schmitzová J, Rasche N, Dybkov O, Kramer K, Fabrizio P, Urlaub H, et al. Crystal structure of Cwc2 reveals a novel architecture of a multipartite RNA-binding protein. EMBO J 2012; 31:2222 - 34; http://dx.doi.org/10.1038/emboj.2012.58; PMID: 22407296
- Sharp PA. “Five easy pieces”. Science 1991; 254:663; http://dx.doi.org/10.1126/science.1948046; PMID: 1948046
- Toor N, Keating KS, Taylor SD, Pyle AM. Crystal structure of a self-spliced group II intron. Science 2008; 320:77 - 82; http://dx.doi.org/10.1126/science.1153803; PMID: 18388288
- Shukla GC, Padgett RA. A catalytically active group II intron domain 5 can function in the U12-dependent spliceosome. Mol Cell 2002; 9:1145 - 50; http://dx.doi.org/10.1016/S1097-2765(02)00505-1; PMID: 12049749
- Pena V, Rozov A, Fabrizio P, Lührmann R, Wahl MC. Structure and function of an RNase H domain at the heart of the spliceosome. EMBO J 2008; 27:2929 - 40; http://dx.doi.org/10.1038/emboj.2008.209; PMID: 18843295
- Ritchie DB, Schellenberg MJ, Gesner EM, Raithatha SA, Stuart DT, Macmillan AM. Structural elucidation of a PRP8 core domain from the heart of the spliceosome. Nat Struct Mol Biol 2008; 15:1199 - 205; http://dx.doi.org/10.1038/nsmb.1505; PMID: 18836455
- Yang K, Zhang L, Xu T, Heroux A, Zhao R. Crystal structure of the beta-finger domain of Prp8 reveals analogy to ribosomal proteins. Proc Natl Acad Sci U S A 2008; 105:13817 - 22; http://dx.doi.org/10.1073/pnas.0805960105; PMID: 18779563
- Pena V, Liu S, Bujnicki JM, Lührmann R, Wahl MC. Structure of a multipartite protein-protein interaction domain in splicing factor prp8 and its link to retinitis pigmentosa. Mol Cell 2007; 25:615 - 24; http://dx.doi.org/10.1016/j.molcel.2007.01.023; PMID: 17317632
- Matsuura M, Noah JW, Lambowitz AM. Mechanism of maturase-promoted group II intron splicing. EMBO J 2001; 20:7259 - 70; http://dx.doi.org/10.1093/emboj/20.24.7259; PMID: 11743002
- Lambowitz AM, Zimmerly S. Group II introns: mobile ribozymes that invade DNA. Cold Spring Harb Perspect Biol 2011; 3:a003616; http://dx.doi.org/10.1101/cshperspect.a003616; PMID: 20463000
- Cui X, Matsuura M, Wang Q, Ma H, Lambowitz AM. A group II intron-encoded maturase functions preferentially in cis and requires both the reverse transcriptase and X domains to promote RNA splicing. J Mol Biol 2004; 340:211 - 31; http://dx.doi.org/10.1016/j.jmb.2004.05.004; PMID: 15201048
- Chan RT, Robart AR, Rajashankar KR, Pyle AM, Toor N. Crystal structure of a group II intron in the pre-catalytic state. Nat Struct Mol Biol 2012; 19:555 - 7; http://dx.doi.org/10.1038/nsmb.2270; PMID: 22484319
- Valadkhan S, Manley JL. Splicing-related catalysis by protein-free snRNAs. Nature 2001; 413:701 - 7; http://dx.doi.org/10.1038/35099500; PMID: 11607023
- Valadkhan S, Mohammadi A, Jaladat Y, Geisler S. Protein-free small nuclear RNAs catalyze a two-step splicing reaction. Proc Natl Acad Sci U S A 2009; 106:11901 - 6; http://dx.doi.org/10.1073/pnas.0902020106; PMID: 19549866
- Schroeder R, Barta A, Semrad K. Strategies for RNA folding and assembly. Nat Rev Mol Cell Biol 2004; 5:908 - 19; http://dx.doi.org/10.1038/nrm1497; PMID: 15520810
- Grainger RJ, Beggs JD. Prp8 protein: at the heart of the spliceosome. RNA 2005; 11:533 - 57; http://dx.doi.org/10.1261/rna.2220705; PMID: 15840809
- Teigelkamp S, Newman AJ, Beggs JD. Extensive interactions of PRP8 protein with the 5′ and 3′ splice sites during splicing suggest a role in stabilization of exon alignment by U5 snRNA. EMBO J 1995; 14:2602 - 12; PMID: 7781612
- Siatecka M, Reyes JL, Konarska MM. Functional interactions of Prp8 with both splice sites at the spliceosomal catalytic center. Genes Dev 1999; 13:1983 - 93; http://dx.doi.org/10.1101/gad.13.15.1983; PMID: 10444596
- Fabrizio P, Dannenberg J, Dube P, Kastner B, Stark H, Urlaub H, et al. The evolutionarily conserved core design of the catalytic activation step of the yeast spliceosome. Mol Cell 2009; 36:593 - 608; http://dx.doi.org/10.1016/j.molcel.2009.09.040; PMID: 19941820
- Burke JE, Sashital DG, Zuo X, Wang YX, Butcher SE. Structure of the yeast U2/U6 snRNA complex. RNA 2012; 18:673 - 83; http://dx.doi.org/10.1261/rna.031138.111; PMID: 22328579
- Sashital DG, Cornilescu G, McManus CJ, Brow DA, Butcher SE. U2-U6 RNA folding reveals a group II intron-like domain and a four-helix junction. Nat Struct Mol Biol 2004; 11:1237 - 42; http://dx.doi.org/10.1038/nsmb863; PMID: 15543154
- Sigel RK, Sashital DG, Abramovitz DL, Palmer AG, Butcher SE, Pyle AM. Solution structure of domain 5 of a group II intron ribozyme reveals a new RNA motif. Nat Struct Mol Biol 2004; 11:187 - 92; http://dx.doi.org/10.1038/nsmb717; PMID: 14745440
- Madhani HD, Bordonné R, Guthrie C. Multiple roles for U6 snRNA in the splicing pathway. Genes Dev 1990; 4:12B 2264 - 77; http://dx.doi.org/10.1101/gad.4.12b.2264; PMID: 2149118
- de Lencastre A, Pyle AM. Three essential and conserved regions of the group II intron are proximal to the 5′-splice site. RNA 2008; 14:11 - 24; http://dx.doi.org/10.1261/rna.774008; PMID: 18039742
- Lu P, Lu G, Yan C, Wang L, Li W, Yin P. Structure of the mRNA splicing complex component Cwc2: insights into RNA recognition. Biochem J 2012; 441:591 - 7; http://dx.doi.org/10.1042/BJ20111385; PMID: 21957909
- Oubridge C, Ito N, Evans PR, Teo CH, Nagai K. Crystal structure at 1.92 A resolution of the RNA-binding domain of the U1A spliceosomal protein complexed with an RNA hairpin. Nature 1994; 372:432 - 8; http://dx.doi.org/10.1038/372432a0; PMID: 7984237