Abstract
Type I toxin-antitoxin loci consist of two genes: one encodes a small, toxic protein and the second encodes a small RNA antitoxin that represses toxin gene expression. These pairs were first described on plasmids where they regulate plasmid maintenance. However, recent discoveries have found novel type I loci, with no homology to plasmid sequences, in the chromosome of Escherichia coli and closely related species. The Ibs-Sib, ShoB-OhsC and Zor-Orz loci are examples of these new loci. For these toxic proteins, much more is known about how their expression is regulated than their biological function. Although all are found in E. coli and closely related bacteria, there is great variation among species as to which loci they possess. Herein, I discuss how these sRNA antitoxins prevent toxin production and how the distribution of these loci across species may be providing insights into their true function.
Introduction
In bacteria, type I toxin-antitoxin loci consist of two genes: one encoding a small (under 60 amino acids), hydrophobic, potentially toxic protein and the second, a small, regulatory RNA (sRNA; antitoxin) that represses expression of the toxic protein. The sRNA has sequence complementarity to the toxin mRNA that allows it to base pair with and repress translation of the target and/or cause its degradation. The first type I toxin loci described were found on plasmids in the model organism Escherichia coli as well as the Gram-positive bacterium Enterococcus faecalis. These loci regulate plasmid stability within a population. The gene pairs have a very specific orientation: for E. coli systems including Hok/Sok, the genes are oriented such that the antitoxin is opposite the 5′ untranslated region (UTR) of the toxin mRNA; for the par locus of E. faecalis plasmid pAD1, the antitoxin gene overlaps the 3′ UTR of the toxin mRNA.Citation1-Citation5
In recent years, numerous type I loci have been identified within the chromosome of E. coli and closely related species.Citation6-Citation8 These chromosomal loci had no discernable homology to any plasmid sequence or any other extrachromosomal DNA element at the time of their discovery. An aspect that separates the chromosomally encoded loci from the plasmid-encoded systems is the genetic orientation of the toxin and the antitoxin. Many of these newly described chromosomal loci are oriented such that the antitoxin does not overlap the toxin UTR. For example, the Ibs-Sib gene pairs are oriented such that the sib antitoxin gene is directly antisense to the coding region of the ibs toxin gene (); this is the first description of such an orientation for a type I locus.Citation6 In other recently identified loci, the antitoxin is transcribed divergently from the toxin gene. The genes for these novel pairs do not overlap and have far less base pairing potential compared with other systems. The first of these divergent systems identified was the Tis-IstR pair (see article by Wagner E and Unoson C in this issue), and this was subsequently followed by the discovery ( and ) of the ShoB-OhsC and Zor-Orz gene pairs.Citation6-Citation8 These discoveries broadened our views as to how toxin-antitoxin systems could be oriented; consequently, they have helped advance cross-species searches for new loci. Additionally, they raise questions as to how these gene pairs evolved over time.
Figure 1. Ibs-Sib loci in E. coli MG1655. (A) Genetic organization of the loci. (B) Alignment of the five sib gene sequences from E. coli MG1655. The Target Recognition Domains (TRD1 and TRD2) are indicated as determined by Han, et al.Citation16
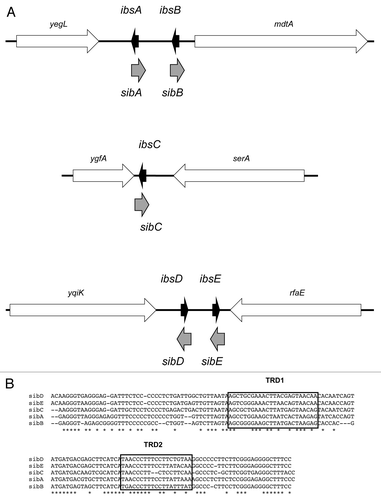
Figure 2. ShoB-OhsC locus of E. coli MG1655. (A) Genetic organization of the locus. Note the arrow for shoB indicates the mRNA; the box within the arrow indicates the coding region of the sequence. (B) Base pairing between shoB and OhsC. (C) Predicted secondary structure of shoB.Citation28 The start codon is boxed; the predicted ribosome binding site is indicated by the dashed bracket; the region of base pairing to OhsC is indicated by the bracket.
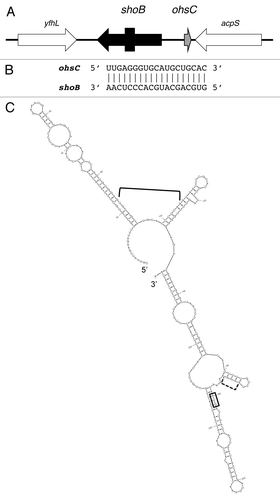
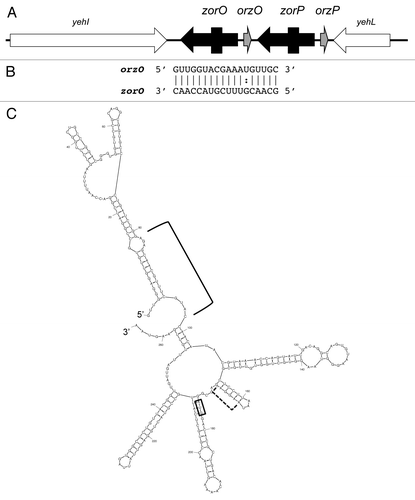
Figure 3. Zor-Orz locus of E. coli O157:H7 str. EDL933. (A) Genetic organization of the locus. Note the arrows for zorO and zorP indicate the mRNAs; the boxes within the arrows indicate the coding region of the sequences. (B) Base pairing between zorO and OrzO. (C) Predicted secondary structure of zorO.Citation28 The start codon is boxed; the predicted ribosome binding site is indicated by the dashed bracket; the region of base pairing to OhsC is indicated by the bracket.
The loci that are the focus of this review—Ibs-Sib, ShoB-OhsC, Zor-Orz, are interesting not only because of their genetic organization and its consequences for regulation by the small RNA, but also because of their distribution across bacterial species. The distribution of each locus can vary greatly in terms of copy number in individual species as well their presence among “wild” (isolated recently from natural habitats) vs. laboratory strains. The significance of the variability in the presence or absence of the genes among different strains has not been fully elucidated; however, recent and ongoing research may shed some light on this.
Directly Antisense: Ibs-Sib
The Ibs-Sib locus was discovered serendipitously. After completion of the genomic sequence of E. coli MG1655, Rudd noted several large repeat sequences in the chromosome.Citation9 One particular sequence, referred to as QUAD, was repeated four times within the chromosome with a tandem duplication in the intergenic region between yegL and mdtA (). Within each repeat were strong promoter elements, but no potential open reading frame; thus he proposed that these repeats might encode stable RNAs.
Following Rudd’s initial observation, active searches to identify sRNAs in E. coli and other bacteria exploded. Several groups did indeed detect expression of sRNAs from the QUAD repeats, confirming Rudd’s prediction.Citation10-Citation13 In general, it was noted that the RNAs were expressed in both rich and minimal media, and consisted of two forms approximately 140 and 110 nts in length, respectively.
Further sequence refinement revealed the presence of a fifth QUAD repeat missing from the original annotation of the MG1655 genome. Homology searches across other organisms indicated that although the QUAD repeat was present in closely related bacteria, the number of repeats could vary greatly.Citation6,Citation7 Given this variation in copy number, the name QUAD was replaced with SIB (Short, Intergenic, aBundant sequence; Fozo E et al., 2008).Citation6 Studies in MG1655 confirmed that all five Sib sRNAs (referred to as SibA, SibB, SibC, etc.) were expressed in both rich and minimal media, and are highly homologous to each other ().
Most E. coli sRNAs function to control the expression of target RNAs, and often are dependent on the RNA chaperone Hfq for function.Citation14 Studies of the Sib RNAs showed no difference in their stability in the presence or absence of Hfq, nor did they have apparent Hfq-binding regions.Citation6 Also, there was no evidence that the Sib sRNAs regulated any of the putative target mRNAs identified by software predictions (Fozo E, unpublished observations). Thus, it seemed that the Sibs were not functioning as typical E. coli base pairing sRNAs.
Deletion strains of the individual sib genes showed no growth defects in rich or in minimal media, or during growth in a variety of temperatures or carbon sources. However, attempts to overexpress the Sibs from plasmids gave some unexpected results which led us to reexamine the gene sequence.Citation6 Closer examination of the sib gene sequence revealed the presence of small open reading frames (18–19 amino acids long) encoded on the opposite strand of DNA directly antisense to each sib gene (). These open reading frames were extremely hydrophobic and reminiscent of type I toxins; thus, we proposed that the Sibs were the antitoxins to these putative toxins. To test this, these small proteins were overexpressed from multicopy plasmids. As predicted, their overproduction caused cell stasis (hence the name ibs: induction brings stasis), and if overproduced at high levels, cell death occurred.Citation6 To confirm that the Sib RNA functioned to repress toxicity of the Ibs protein, a rescue experiment was designed. Each sib gene was cloned behind an inducible promoter (PLac: IPTG inducible) on one plasmid, and its antisense ibs gene was cloned behind a different inducible promoter (PBAD; arabinose inducible) on a second plasmid; both plasmids were transformed into E. coli. Expression of the ibs gene alone halted cell growth; cell growth could continue if the corresponding Sib sRNA was co-expressed. Thus, the Sib sRNA represses Ibs-induced toxicity. Furthermore, this repression was shown to be specific: only SibC could repress IbsC-induced toxicity; SibE could not repress IbsC-toxicity.Citation6 This confirmed that despite the high degree of homology between the multiple sib genes, there is specificity in the regulation.
The Sibs are highly homologous in sequence, so how could there be such specificity in regulating Ibs production? Despite the homology among the sib gene sequences, there are two highly variable regions. Using a series of chimeric and truncated Sib constructs, Han et al. concluded that these two variable regions were responsible for dictating the specificity in the regulation.Citation16 These regions are referred to as TRD1 (target recognition domain) and TRD2 (). TRD1 pairs within the coding region of the ibs mRNA; TRD2 is directly antisense to the ribosome binding site. By performing in vitro structure probing, they demonstrated that both TRD1 and TRD2 interact with ibsC mRNA. Specifically, TRD1 of SibC contains a YUNR motif (U-turn) that interacts with a YUNR in ibsC.Citation1,Citation16,Citation17 TRD2, however, does not interact through a U-turn motif but instead interacts with its target through a single-stranded region flanked by two loops. Through their mutational analyses, Han showed that interactions with either TRD1 or TRD2 independently could halt IbsC-mediated toxicity.Citation16
Since each Sib sRNA is encoded directly antisense to its ibs target, they could potentially form extensive base pairing interactions (greater than 60 nucleotides). However, this appears not to be the case. In vitro structure probing of the SibC and ibsC RNAs reveal that the RNAs interact over a much smaller region and do not form a complete RNA duplex when incubated at 37°C; formation of a complete duplex was only seen when the in vitro RNAs were subjected to a denaturation step and then allowed to anneal.Citation16 Although the initial interactions may extend into the surrounding sequences, there appear to be limits as to how extensive the interactions may be. How this is controlled is not quite clear.
The Sib sRNAs have been detected under all growth conditions examined thus far; this combined with their very strong promoter elements suggests that they may be transcribed constitutively.Citation6 However, ibs-specific mRNAs have not been detected from the chromosome of a wild type E. coli strain. They have been detected upon either deletion of the cognate Sib promoter (preventing expression of the antisense sRNA) or in a strain deleted for the gene rnc. The rnc gene encodes the double stranded ribonuclease, RNase III, which has been associated with cleavage of type I toxin-antitoxin complexes.Citation1 In a Δrnc strain, both ibsC mRNA and SibC can be detected (Fozo E, unpublished observations). Thus, it would appear that in a wild type strain, SibC expression, subsequent formation of SibC-ibsC RNA duplex, followed by cleavage by RNase III, keep ibsC mRNA levels low.
Are the ibs genes ever expressed then? This question has yet to be answered. As stated, detection of the ibs transcripts in wild type E. coli has not been observed. Furthermore, mapping of the transcription start site was reported only upon deletion of the corresponding antisense sib gene or when cloned onto a multicopy plasmid (Fozo E, unpublished observations).Citation16 These observations, combined with the fact that the sib genes may be transcribed constitutively, suggest that ibs expression is tightly regulated. This may be why there are multiple copies of the locus within a bacteria’s chromosome: to ensure adequate production of “total” Ibs proteins (see below for further discussion).
The data clearly shows that the Ibs-Sib loci are type I toxin-antitoxin systems, but what is their biological function? Most studies of Ibs protein function have relied upon their overproduction from multicopy plasmids. When overproduced, the Ibs proteins caused membrane depolarization in the majority of cells within 20 min; this damage correlated with a massive decrease in colony forming units.Citation6 Additionally, this overproduction led to large changes in gene expression. Of note was induction of the psp and cpx operons: both operons are induced upon membrane damage.Citation18-Citation20 These data suggest that the small, hydrophobic proteins target the cellular membrane and high enough levels will cause membrane damage.
To better understand how low levels of IbsC effect E. coli, we utilized a strain in which the sibC promoter (ΔPsibC) was deleted. This leaves the ibsC coding region and promoter region intact, and leads to detectable expression of the ibsC mRNA.Citation6 Although there were no major growth effects when comparing the ΔPsibC strain to the parental strain, there were elevated levels of psp transcripts, suggesting membrane damage had occurred. Along with this, proton motive force dissipation was higher in the mutant population of cells vs. a wild type population [7.5% vs. 1.0% respectively, as measured by the uptake of the dye DiBAC4(3); Fontaine and Fozo, unpublished observations]. Thus, even low levels of IbsC can cause membrane damage.
A biochemical approach also revealed some interesting observations in regards to critical residues for Ibs function. Generation of a large panel of Ibs mutant proteins revealed that multiple amino acid substitutions could be made yet toxicity was still maintained.Citation21 This occurs even though the proteins are only 18 or 19 amino acids long. However, residues within the predicted transmembrane domain were critical for toxicity, suggesting that these amino acids are responsible for membrane localization or could be involved in protein-protein interactions. Similarly, a mutagenesis screen to identify critical residues for Fst-induced toxicity revealed a similar pattern: mutations in the conserved transmembrane domain significantly reduced toxicity (see article by Weaver, within this issue).Citation22
Divergent Type I Toxin-Antitoxin Systems
As stated previously, the known plasmid encoded type I loci are arranged genetically such that the antitoxin overlaps either the 5′ or 3′ UTR of the toxin mRNA. Yet, the Ibs-Sib locus found within bacterial chromosomes does not follow this arrangement. Besides this locus, other newly described chromosomal loci also have “nontraditional” genetic arrangements. In these cases, the antitoxins are transcribed divergently from the toxin genes and have 18–21 nucleotides of perfect complementarity to the toxin. The divergent families include Tis-IstR (described by Wagner E and Unoson C, in a previous issue), ShoB-OhsC and the Zor-Orz loci.
Translational Control at Multiple Levels: ShoB-OhsC
The type I locus ShoB-OhsC was discovered in a cloning-based strategy to identify novel sRNAs in E. coli.Citation15 In this screen, the sRNA RyfC (now referred to as OhsC) was identified in a 694 bp intergenic region between a gene of unknown function, yfhL, and a gene involved in fatty acid biosynthesis, acpS. Through northern analysis along with 5′ and 3′ RACE, the authors confirmed that OhsC was encoded in this region and 60 nt in length. Using the gene sequence in a BLAST search, a hit (19 nts identical) was found on the opposite strand of DNA and divergent from the ohsC gene (). Upon closer examination of this sequence, the authors noted potential promoter elements and an open reading frame that could encode a 26 amino acid protein.Citation15 Northern analysis confirmed that this gene, ryfB (now known as shoB), was transcribed, and two transcripts approximately 280 and 320 nt were observed. Through 5′ RACE the authors were able to demonstrate that shoB possessed a 180 nt 5′ UTR, and it is in this long UTR where the region of complementarity to OhsC is found ().
Given the complementarity between OhsC and shoB and that shoB could encode a small, hydrophobic protein, we hypothesized that they comprised a new type I toxin-antitoxin locus.Citation6 To test this hypothesis, we overproduced shoB, and indeed, it was lethal to E. coli. Co-expression of OhsC could alleviate this toxicity, suggesting it is an antitoxin.Citation6
As mentioned, there are multiple 5′ ends of the shoB mRNA.Citation15 To further confirm that OhsC can directly repress expression of shoB, we generated translational reporter fusions to the full-length shoB mRNA, and to the second longest shoB mRNA that had been mapped.Citation15 No translational activity was measured using the full-length shoB mRNA (see below for details). We did detect translational activity using the shorter gene fusion, and in the presence of OhsC, translation of this reporter was reduced 2–4 fold.Citation6 However, the region of pairing of OhsC to shoB is far from the coding region and translational start site of shoB. So how does OhsC repress expression? The full-length shoB mRNA is predicted to fold into a tight secondary structure that obscures the ribosome binding site and the start codon (). This is similar to what has been reported for the tisB mRNA (reviewed in a previous issue Wagner E and Unoson C).Citation23,Citation24 In the case of tisB, the ribosome binds to a stand-by site in the 5′ UTR, upstream of the real tisB translation initiation site.Citation23,Citation24 When the structure opens, the ribosome moves to the real site, translating tisB. A similar scenario could be occurring with shoB, with binding of OhsC either blocking ribosome docking in the 5′ UTR or impacting the secondary structure in that region. Alternatively, translation of shoB may occur as has been observed for the plasmid-encoded hok toxin, where translation of an open reading frame, mok, within its 5′UTR is required for hok translation.Citation25 OhsC binding may prevent translation of an internal open reading frame within the long 5′ UTR of shoB. Experimental evidence is needed to confirm how and when shoB is translated and how its translation may occur.
As mentioned, multiple 5′ ends of the shoB mRNA were mapped. However, when the full-length mRNA (corresponding to the start of transcription) was fused to a translational reporter gene, the fusion had no activity.Citation6 This suggests that the full-length shoB mRNA is not translated, and is likely due to its predicted secondary structure that obscures the ribosome binding site (). Thus, this secondary structure could hinder translation independently of the presence of OhsC. Indeed, fusions generated to a shorter shoB transcript did yield translational activity. Combined, the data suggest that full-length shoB mRNA must be processed in order to be translated; it is interesting to note that the chromosomally encoded tisB toxin and the plasmid-encoded hok toxin must also be processed for translation to occur.Citation1
Expression of the shoB mRNA and OhsC sRNA differ from each other depending upon growth condition.Citation6,Citation15 For example, OhsC, the antitoxin, is highly expressed in stationary phase of growth in minimal media. Conversely, shoB expression is low under this condition. ShoB expression is high however, during exponential phase of growth in LB, whereas OhsC is quite low. This variation in their expression patterns could indicate when ShoB is expressed in E. coli. Additional work to confirm this is needed.
The biological role of ShoB in bacteria is not well understood; functional studies are limited only to overproduction of the toxin from a plasmid in E. coli MG1655. Like all type I toxins, the protein is very hydrophobic, and it is not surprising that overproduction led to membrane damage. Experiments measuring membrane depolarization of bacterial cells showed that within 5 min of overproduction 98% of the bacteria have membrane damage.Citation6 However, it is not known if endogenous expression levels would lead to membrane damage.
Additionally, overproduction induced expression of numerous genes in E. coli. When comparing overexpression of ShoB to three other type I toxins (IbsC, LdrD and TisB), a common set of genes were induced. These included genes involved in stress responses, such as heat shock and superoxide, as well as several genes involved in sugar transport. ShoB, but not the other toxins, induced the expression of additional sugar transport genes.Citation6 Induction of the stress response genes correlates well with the severe toxicity of these proteins. However, why sugar transport genes may be induced is not clear, but could signify an interesting response to membrane damage, or perhaps ShoB is involved in metabolism or macromolecule synthesis. One caveat regarding this data: we observed quite a bit of variation in these experiments. This may be due to uneven expression of the shoB mRNA due to its secondary structure and/or the use of multicopy plasmids in these experiments. Future work will hopefully clarify this variation.
Newest Divergent Family: Zor-Orz
The most recently discovered divergent type I locus, the zor-orz family, was identified in a bioinformatic search. This search was designed to discover new type I loci, and used characteristics of known type I toxins to generate search parameters. These parameters included tandemly duplicated, small, hydrophobic proteins.Citation7 The search identified a previously annotated gene in the enterohemorrhagic bacterium E. coli O157:H7 str. EDL933, z3289. Encoded in tandem of z3289 is the highly homologous gene z3290. These genes encode hydrophobic, 29 amino acid proteins that differ only by a single amino acid. Overproduction of either protein was toxic to E. coli.Citation7 The gene z3289 is now referred to as zorO (Z-protein often repeated) and z3290 is now referred to as zorP (Wen J and Fozo E, in preparation). Initial attempts to identify an antisense sRNA to either gene were not successful. As the known type I antitoxins are highly structured, RNA folding predictions of the regions flanking the genes were used to identify areas with a high likelihood of secondary structure.Citation7 Sequences of strong putative secondary structures were identified divergent of each protein-encoding gene. Analysis of these regions revealed -35 and -10 binding sites for RNA polymerase. Northern analysis confirmed transcription of these divergently transcribed RNAs, which are now referred to as Orz (Overexpression Reduces Z-protein toxicity; Wen J and Fozo E, in preparation). The sRNA OrzO is encoded divergent of zorO and OrzP is encoded divergent of zorP ().
Given that the Orz sRNAs are divergent of the zor genes, could they possibly serve as antitoxins? Similar to IstR and OhsC, each Orz sRNA shares 18 nt complementarity to its cognate zor mRNA (, data not shown). Like the Sib sRNAs, there is a high degree of homology between OrzO and OrzP which may lead to cross talk in the regulation of the toxin genes.Citation6,Citation7,Citation16 However, overexpression of OrzO but not OrzP could repress ZorO-induced toxicity (Wen J and Fozo E, in preparation). Ongoing experiments are in progress to determine how this specificity may occur.
Like IstR and OhsC, the region of Orz complementarity occurs in the long (180 nt) 5′ UTR of the zor mRNA (). As was seen with shoB, a translational fusion to the full-length zorO mRNA shows virtually no activity (Won C and Fozo E, unpublished observations). Analogous to tisB and shoB, the 5′ UTR of the zor mRNAs are predicted to be folded in tight secondary structures that obscure the ribosome binding site and start codon (). It could be that a stand-by ribosome binding site or translation of a small open reading frame in the 5′ UTR is needed for translation.Citation23-Citation25 Ongoing experiments are aimed to clarify these two possibilities.
Beyond direct repression of the zor gene expression by the Orz sRNAs, there appear to be other layers of regulatory control. The zor mRNA levels are sensitive to carbon source and growth phase, which may reflect when they are translated. Also, primer extension analysis revealed that each zor-orz pair might share overlapping promoter elements. Such a genetic arrangement is highly unusual and may lead to competition for RNA polymerase and/or other transcription factors. Current studies are in progress to decipher how this arrangement impacts expression of the toxins and their antitoxins.
The biological function of the Zor proteins is not known. There may be some clues based upon their distribution across species (see below) as well as the variation in mRNA expression dependent upon growth conditions. Functional studies utilizing a variety of deletion strains are ongoing to elucidate why bacteria possess these gene pairs.
Three Families from E. coli: “Wild” vs. Laboratory Strains
Although the three families described within were all discovered in E. coli, there are some differences in their distribution across bacterial species. One main difference between these families is whether or not they are duplicated within a single bacterial strain. When examining the total number of type I toxin-antitoxin loci across 774 sequenced bacterial strains, those bacteria that contained the Ibs-Sib locus could have anywhere from one to seven copies of the gene pair.Citation7 The ShoB-OhsC locus, however, is found only 1 time per genome, while the Zor-Orz locus may be present as either a single or duplicated gene pair ().Citation7
Table 1. Distribution of selected type I toxin loci across selected speciesa.
Why are there multiple copies of some loci but not all? In the case of the Ibs-Sib, it is not known, but there are some experimental observations that might provide clues for their duplication. As mentioned, it is difficult to detect ibs mRNA expression, and the combined data suggests that the toxin is expressed at very low levels. Yet the ibs possess nearly canonical ribosome binding sites and there has been little, if any, degeneration of the either the ribosome binding site or the coding sequence across species. This suggests that the sequences are being maintained; but their expression is low. One possibility could be that each Ibs protein has a different function. However, given their incredible homology, small size and because multiple amino acid substitutions can be tolerated within the protein, they likely have the same target, although each individual Ibs protein may interact with that target differently.Citation21
Multiple ibs genes may be maintained because each gene is not expressed equivalently by the bacterium. Variation in gene expression patterns would allow the bacterium to fine tune Ibs production. Differences in promoters, mRNA stability, translational control, etc., are several parameters that would influence production of an individual gene. Thus, careful evaluation of the transcriptional and translational control of each gene, both in terms of how quickly each is expressed under a given condition and the total amount of production from an individual ibs gene is necessary to confirm this possibility.
Alternatively, the multiple copies of the ibs genes may serve to ensure sufficient Ibs protein production. This hypothesis assumes that the Ibs proteins function redundantly. It also assumes that the cell may require Ibs protein production to be maintained at a specific level while avoiding the toxicity associated with Ibs overproduction. If ibs expression were tightly controlled, having multiple copies would increase the total protein production in the cell.
Do multiple copies of the Ibs-Sib locus or Zor-Orz locus correlate with pathogenesis? Of the three loci discussed herein, the Ibs-Sib locus is the most broadly distributed across species; not only is it found in E. coli, Shigella and Salmonella species, it is also found in other γ-proteobacter species including Citrobacter koseri and various Hemophilus species, including H. influenzae, an agent associated with ear and respiratory infections, and H. somnus, a respiratory pathogen of ruminants.Citation7 Recently, homologs have been identified in the ε-proteobacter species Helicobacter pylori, a causative agent of ulcers.Citation26 Attempts to link the number of copies of this locus to the environmental niche or pathogenicity of the organism have not proven fruitful. For example, H. somnus 2336, a respiratory pathogen of cattle, E. coli O157:H7 str. Sakai, an intestinal pathogen of humans and intestinal colonizer of cattle, Shigella boydii, an intestinal pathogen, and E. coli SE11, a human commensal, each have 7 copies. Many other intestinal colonizers and pathogens, like Salmonella species, have only 2 copies (). While it is possible that there are trends in the copy number and niche/virulence of the organism, without a more complete investigation of the function of the locus, we are unable to make any conclusions.
In regard to the Zor-Orz locus, like the other divergently encoded type I toxin-antitoxin loci, its distribution is far more limited than other systems.Citation7 It is found in E. coli and closely related E. fergusonii and Shigella strains. Interestingly, in E. coli strains, its distribution is varied. It is found in commensal species, such as E. coli SE11, pathogenic species such as E. coli O157:H7 EDL933 and in environmental isolates such as E. coli TW09276.Citation7,Citation27 “Wild” E. coli strains, i.e., those that are commensal, pathogens or environmental isolates, can possess either 1 or 2 copies of the locus (). Laboratory strains, which typically can no longer naturally colonize humans, including E. coli MG1655, do not possess the locus at all. In fact, the intergenic region in MG1655 where the zor-orz locus is located (in between two hypothetical genes yehI and yehL) is only approximately 0.3 Kb, whereas it is approximately 1.1 Kb in E. coli O157:H7 EDL933. It may be that this locus was energetically costly in the laboratory and deleted as the genome was streamlined. Initial studies indicated that this locus did not appear to be mobile and it is very limited in its distribution.Citation7 Performing a long-term evolutionary experiment to see if this locus is dispensable from a “wild” strain during laboratory cultivation should help unravel why this locus is present or absent. This experiment will also lend itself to exploring the possible function of the zor-orz genes: are they necessary for survival in the host or environment?
The ShoB-OhsC locus, like Zor-Orz, is limited in its distribution to mainly E. coli and Shigella species.Citation7 To date, no examples of a divergently encoded type I toxin-antitoxin pair have been described outside of E. coli and its close relatives. This limited distribution suggests that the divergent toxin-antitoxin pairs are relative newcomers to genomes. For ShoB-OhsC, there is only a single copy of the pair found in commensals, laboratory strains and pathogens. Such a distribution suggests that these genes may be important for function in conditions common to the intestine and the laboratory. The fact that this locus is not duplicated may indicate that ShoB protein levels are able to reach levels high enough for function and that perhaps repression of ShoB expression is not nearly as tightly regulated as Ibs production. Again, conclusive evidence supporting this is not yet available.
Although there has been an increase in the number of different type I loci families identified across bacterial species, the function for a single gene copy of many of these still remains unknown. However, clues about when they may function may be obtained by studying their distribution across species. For example, while the Ibs-Sib and ShoB-OhsC locus are in laboratory strains as well as “wild” E. coli, the Zor-Orz pairs are missing from laboratory strains. This would imply that the Ibs-Sib and ShoB-OhsC pairs are being maintained regardless of environmental niche, while the Zor-Orz locus may have a specific function in conditions common only in the animal host. Using such information, we can specifically design functional and environmental experiments to test such theories and to hopefully begin to unravel why bacteria possess so many toxic genes.
Acknowledgments
I would like to thank my collaborators for helpful discussion regarding regulation and possible function of type I loci. I also thank J. Wen and J. Wells for comments regarding this manuscript.
References
- Fozo EM, Hemm MR, Storz G. Small toxic proteins and the antisense RNAs that repress them. Microbiol Mol Biol Rev 2008; 72:579 - 89; http://dx.doi.org/10.1128/MMBR.00025-08; PMID: 19052321
- Gerdes K, Larsen JE, Molin S. Stable inheritance of plasmid R1 requires two different loci. J Bacteriol 1985; 161:292 - 8; PMID: 2981804
- Gerdes K, Rasmussen PB, Molin S. Unique type of plasmid maintenance function: postsegregational killing of plasmid-free cells. Proc Natl Acad Sci USA 1986; 83:3116 - 20; http://dx.doi.org/10.1073/pnas.83.10.3116; PMID: 3517851
- Weaver KE, Jensen KD, Colwell A, Sriram SI. Functional analysis of the Enterococcus faecalis plasmid pAD1-encoded stability determinant par.. Mol Microbiol 1996; 20:53 - 63; http://dx.doi.org/10.1111/j.1365-2958.1996.tb02488.x; PMID: 8861204
- Weaver KE, Tritle DJ. Identification and characterization of an Enterococcus faecalis plasmid pAD1-encoded stability determinant which produces two small RNA molecules necessary for its function. Plasmid 1994; 32:168 - 81; http://dx.doi.org/10.1006/plas.1994.1053; PMID: 7531349
- Fozo EM, Kawano M, Fontaine F, Kaya Y, Mendieta KS, Jones KL, et al. Repression of small toxic protein synthesis by the Sib and OhsC small RNAs. Mol Microbiol 2008; 70:1076 - 93; http://dx.doi.org/10.1111/j.1365-2958.2008.06394.x; PMID: 18710431
- Fozo EM, Makarova KS, Shabalina SA, Yutin N, Koonin EV, Storz G. Abundance of type I toxin-antitoxin systems in bacteria: searches for new candidates and discovery of novel families. Nucleic Acids Res 2010; 38:3743 - 59; http://dx.doi.org/10.1093/nar/gkq054; PMID: 20156992
- Vogel J, Argaman L, Wagner EG, Altuvia S. The small RNA IstR inhibits synthesis of an SOS-induced toxic peptide. Curr Biol 2004; 14:2271 - 6; http://dx.doi.org/10.1016/j.cub.2004.12.003; PMID: 15620655
- Rudd KE. Novel intergenic repeats of Escherichia coli K-12. Res Microbiol 1999; 150:653 - 64; http://dx.doi.org/10.1016/S0923-2508(99)00126-6; PMID: 10673004
- Argaman L, Hershberg R, Vogel J, Bejerano G, Wagner EG, Margalit H, et al. Novel small RNA-encoding genes in the intergenic regions of Escherichia coli.. Curr Biol 2001; 11:941 - 50; http://dx.doi.org/10.1016/S0960-9822(01)00270-6; PMID: 11448770
- Hershberg R, Altuvia S, Margalit H. A survey of small RNA-encoding genes in Escherichia coli.. Nucleic Acids Res 2003; 31:1813 - 20; http://dx.doi.org/10.1093/nar/gkg297; PMID: 12654996
- Rivas E, Klein RJ, Jones TA, Eddy SR. Computational identification of noncoding RNAs in E. coli by comparative genomics. Curr Biol 2001; 11:1369 - 73; http://dx.doi.org/10.1016/S0960-9822(01)00401-8; PMID: 11553332
- Wassarman KM, Repoila F, Rosenow C, Storz G, Gottesman S. Identification of novel small RNAs using comparative genomics and microarrays. Genes Dev 2001; 15:1637 - 51; http://dx.doi.org/10.1101/gad.901001; PMID: 11445539
- Waters LS, Storz G. Regulatory RNAs in bacteria. Cell 2009; 136:615 - 28; http://dx.doi.org/10.1016/j.cell.2009.01.043; PMID: 19239884
- Kawano M, Reynolds AA, Miranda-Rios J, Storz G. Detection of 5′- and 3′-UTR-derived small RNAs and cis-encoded antisense RNAs in Escherichia coli.. Nucleic Acids Res 2005; 33:1040 - 50; http://dx.doi.org/10.1093/nar/gki256; PMID: 15718303
- Han K, Kim KS, Bak G, Park H, Lee Y. Recognition and discrimination of target mRNAs by Sib RNAs, a cis-encoded sRNA family. Nucleic Acids Res 2010; 38:5851 - 66; http://dx.doi.org/10.1093/nar/gkq292; PMID: 20453032
- Franch T, Gerdes K. U-turns and regulatory RNAs. Curr Opin Microbiol 2000; 3:159 - 64; http://dx.doi.org/10.1016/S1369-5274(00)00069-2; PMID: 10744992
- Darwin AJ. The phage-shock-protein response. Mol Microbiol 2005; 57:621 - 8; http://dx.doi.org/10.1111/j.1365-2958.2005.04694.x; PMID: 16045608
- Kobayashi R, Suzuki T, Yoshida M. Escherichia coli phage-shock protein A (PspA) binds to membrane phospholipids and repairs proton leakage of the damaged membranes. Mol Microbiol 2007; 66:100 - 9; http://dx.doi.org/10.1111/j.1365-2958.2007.05893.x; PMID: 17725563
- Vogt SL, Raivio TL. Just scratching the surface: an expanding view of the Cpx envelope stress response. FEMS Microbiol Lett 2012; 326:2 - 11; http://dx.doi.org/10.1111/j.1574-6968.2011.02406.x; PMID: 22092948
- Mok WW, Patel NH, Li Y. Decoding toxicity: deducing the sequence requirements of IbsC, a type I toxin in Escherichia coli.. J Biol Chem 2010; 285:41627 - 36; http://dx.doi.org/10.1074/jbc.M110.149179; PMID: 20980267
- Weaver KE, Reddy SG, Brinkman CL, Patel S, Bayles KW, Endres JL. Identification and characterization of a family of toxin-antitoxin systems related to the Enterococcus faecalis plasmid pAD1 par addiction module. Microbiology 2009; 155:2930 - 40; http://dx.doi.org/10.1099/mic.0.030932-0; PMID: 19542006
- Darfeuille F, Unoson C, Vogel J, Wagner EG. An antisense RNA inhibits translation by competing with standby ribosomes. Mol Cell 2007; 26:381 - 92; http://dx.doi.org/10.1016/j.molcel.2007.04.003; PMID: 17499044
- Unoson C, Wagner EG. Dealing with stable structures at ribosome binding sites: bacterial translation and ribosome standby. RNA Biol 2007; 4:113 - 7; http://dx.doi.org/10.4161/rna.4.3.5350; PMID: 18094628
- Thisted T, Gerdes K. Mechanism of post-segregational killing by the hok/sok system of plasmid R1. Sok antisense RNA regulates hok gene expression indirectly through the overlapping mok gene. J Mol Biol 1992; 223:41 - 54; http://dx.doi.org/10.1016/0022-2836(92)90714-U; PMID: 1370544
- Sharma CM, Hoffmann S, Darfeuille F, Reignier J, Findeiss S, Sittka A, et al. The primary transcriptome of the major human pathogen Helicobacter pylori.. Nature 2010; 464:250 - 5; http://dx.doi.org/10.1038/nature08756; PMID: 20164839
- Luo C, Walk ST, Gordon DM, Feldgarden M, Tiedje JM, Konstantinidis KT. Genome sequencing of environmental Escherichia coli expands understanding of the ecology and speciation of the model bacterial species. Proc Natl Acad Sci USA 2011; 108:7200 - 5; http://dx.doi.org/10.1073/pnas.1015622108; PMID: 21482770
- Zuker M. Mfold web server for nucleic acid folding and hybridization prediction. Nucleic Acids Res 2003; 31:3406 - 15; http://dx.doi.org/10.1093/nar/gkg595; PMID: 12824337