Abstract
Many eukaryotes encode multiple isoforms of the cap-binding translation initiation factor (eIF4E). Leishmanias and other trypanosomatids encode four paralogs of this protein, but none can complement the eIF4E function in a yeast mutant. A low conservation is observed between the four paralogs, suggesting they assist these organisms survive a multitude of conditions encountered throughout the life cycle. Earlier attempts to decipher their function led to identification of LeishIF4E-4 as the canonical translation initiation factor. LeishIF4E-1 appears to function during thermal stress, via a mechanism not yet understood. LeishIF4E-3 hardly binds cap-4 and is, therefore, less likely to serve as a typical initiation factor. Although it interacts with an eIF4G homolog, LeishIF4G-4, the two polypeptides do not co-migrate on sucrose gradients. While LeishIF4E-3 enters large particles that increase in size during nutritional stress, LeishIF4G-4 is found only in the top fractions. Confocal microscopy localized LeishIF4E-3 (but not LeishIF4G-4) within nutritional stress-induced granules. Accordingly, interaction between the two proteins reduced upon starvation. We therefore propose that under normal conditions, LeishIF4G-4 sequesters LeishIF4E-3 in the cytoplasm. During a nutritional stress, LeishIF4E-3 is modified and released from LeishIF4G-4 to enter stress granules, where inactive mRNAs are stored. Binding of LeishIF4G-4 to LeishIF4E-3 requires a short peptide within the LeishIF4G-4 N-terminus, which bears no similarity to the consensus 4E-binding peptide, YXXXXLΦ. Mutational analysis combined with structure prediction indicates that this interaction is based on an obligatory, conserved α helix in LeishIF4G-4. These features further highlight the uniqueness of LeishIF4E-3 and how it interacts with its binding partners.
Introduction
Cap-dependent translation in eukaryotes begins with the assembly of a cap-binding complex, termed eIF4F, on the 5′ cap of the mRNA. eIF4F subsequently anchors the translation pre-initiation complex (PIC) that is responsible for recruiting and targeting the small ribosomal subunit to the initiation codon. The PIC comprises several different polypeptides, including subunits of eIF2 and eIF3, complexes that recruit tRNAmet and the small subunit of the ribosome, respectively. eIF4E, anchoring PIC to the mRNA 5′ cap, also interacts with eIF4G, a scaffold protein that is characterized by a typical domain known as the “middle domain of eukaryotic initiation factor 4G” (MIF4G).Citation1 The interaction between these two proteins is required for efficient translation of most cellular mRNAs. In higher eukaryotes, eIF4G binding to eIF4E is mediated through a conserved YXXXXLΦ peptide motif in eIF4G, where X is any amino acid and Φ is a hydrophobic residue.Citation2 This conserved peptide is at the N-terminus of eIF4G, as common in other eIF4E-binding proteins.
Many eukaryotes encode multiple eIF4E and eIF4G homologs that serve different biological functions. Three mammalian m7GTP-binding eIF4E homologs, varying in their ability to bind eIF4G and presenting different tissue specificities, have been identified. Only one of these paralogs serves as the translation initiation factor, with the others behaving as translational inhibitors.Citation3 In Caenorhabditis elegans there are five eIF4E homologs. As each possesses different affinity to m7GTP and the trimethyl guanosine (TMG) cap,Citation4-Citation6 preferences in binding to certain mRNA groups are observed. Thus, the different C. elegans eIF4E paralogs serve different functions during general, tissue-specific or stress-related translation.Citation7,Citation8 Multiple eIF4G homologs have also been reported. Human cells encode two eIF4G proteins, eIF4GI and eIF4GII, which are active in cap-dependent translation,Citation9 while a variant of this protein, DAP5, promotes cap-independent translation of specific genes.Citation10
Another group of organisms that encode multiple paralogs of eIF4E and eIF4G are the trypanosomatids, a family of parasitic protozoa that include organisms with a digenetic life cycle, such as the leishmanias and trypanosomes. Leishmania parasites reside within the intestinal tract of sand fly vectors as flagellated promastigotes. Upon transmission to a mammalian host, the parasites enter into macrophages, where they become obligatory intracellular organisms that exist within the phagolysosomal compartment. In addition to the typical temperature and pH stresses encountered upon transmission from the invertebrate vector to the mammalian host,Citation11 these parasites also experience nutritional stress, especially in the sand fly vector, since plant sugars, a common component of the sand fly diet, are provided on an irregular basis.Citation12
Trypanosomatids represent an ancient branching of the eukaryotic lineage and possesses several unusual molecular features, such as polycistronic transcription of protein-coding genes, and a lack of conventional mechanisms for transcriptional activation.Citation13,Citation14 The polycistronic transcripts are processed by trans-splicing and polyadenyaltionCitation15 to yield monocistronic mRNAs, each carrying a unique hypermethylated cap structure termed cap-4.Citation16 Cap-4 seems to play a key role in mRNA maturation,Citation17 although its involvement in translation clearly requires specific adaptations of cap-binding proteins and their complexes.
The genomes of several Leishmania and Trypanosoma species encode four eIF4E paralogsCitation18-Citation20 and six MIF4G domain proteins.Citation20-Citation22 LeishIF4E-4 and LeishIF4G-3 were identified as part of a typical eIF4F complex in promastigotes.Citation21 This complex is, however, inactivated upon exposure of the parasites to mammalian-like temperatures.Citation22 Apart from LeishIF4E-4, the biological functions of the other three paralogs remain mostly obscure. This is especially emphasized by the inability of any of the four paralogs to complement the missing eIF4E in a yeast mutant.Citation19 The only LeishIF4E paralog that fails to bind cap-4 and binds poorly to m7GTP in vivo is LeishIF4E-3, making it an unlikely candidate for serving as a canonical translation initiation factor. Moreover, we show here that the recently demonstrated pairing of LeishIF4E-3 with a MIF4G domain proteinCitation23 is not related to the assembly of a translation PIC. Nonetheless, LeishIF4E-3 likely assumes an important role, as silencing experiments have shown that its Trypanosoma brucei ortholog, TbIF4E-3, is essential; in contrast, the other homologs can complement each other.Citation23 Furthermore, TbIF4E-3 was detected in stress granules that form in response to heat shock.Citation24 Here, to better describe LeishIF4E-3 function, we have examined its binding partners under different stress conditions, and propose that LeishIF4E-3 acts during nutritional stress, when mRNAs are stored in an inactive state in stress granules until recovery.
Results
Characterization of LeishIF4E-3 and LeishIF4G-4 complexes
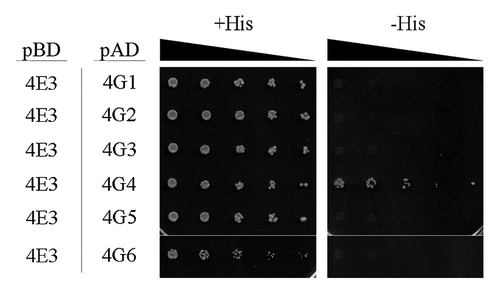
LeishIF4E-3 (LmjF28.2500) is one of the four eIF4E paralogs that were identified in the Leishmania genome. Its ortholog from T. brucei, TbIF4E-3 (Tb11.01.3630), was formerly shown to interact with TbIF4G-4 (Tb11.01.2330), and to a lesser extent, with TbIF4G-3 (Tb927.8.4820).Citation23 Yeast two-hybrid assays showed that LeishIF4E-3 could bind LeishIF4G-4, and excluded any interaction with other MIF4G domain proteins in Leishmania ().
Figure 1. LeishIF4E-3 interacts with LeishIF4G-4. Yeast two-hybrid assays were performed by co-transfection of wild typeYRG2 cells with plasmids encoding the Gal-4 Activation Domain (AD) fused to LeishIF4G-1 to -6 (LmjF30.1150, LmjF15.1320, LmjF16.1600, LmjF36.6060, LmjF10.1080 and LmjF15.0060, denoted 4G1 to 6, respectively) and the Gal-4 Binding Domain (BD) fused to LeishIF4E-3 (denoted 4E3). The cells were cultured under permissive (+His) and restrictive (-His) conditions and spotted in 3-fold dilutions. Expression of exogenous proteins in the transfected yeast strains is shown in Figure S1.
To verify that the interaction between LeishIF4E-3 and LeishIF4G-4 also occurs in vivo, each protein was independently fused to a C-terminal tag and expressed in Leishmania amazonensis. Analysis of the proteins that were captured by reciprocal coprecipitation (pull-down) assays showed that LeishIF4E-3 and LeishIF4G-4 interacted with each other ( and B). Binding specificity was verified by a control pull-down using tagged firefly luciferase, which failed to interact with either LeishIF4E-3 or LeishIF4G-4 (Fig. S3). Furthermore, the interaction between LeishIF4E-3 and LeishIF4G-4 was direct, since it was maintained also in the presence of RNaseA and RNaseT1 (Fig. S3). Other polypeptides that were captured by each of the tagged proteins were identified by mass spectrometry, in two independent experiments. These proteins were sorted, based on their detection in the individual LeishIF4E-3 and LeishIF4G-4 complexes (Table S1). lists only those proteins involved in translation, highlighting the overlapping and the unique components of each complex. Their co-precipitation only suggests that they are part of the pulled-down complex, and does not indicate on direct interactions with the tagged protein. Highly abundant elongation factors, i.e., LeishEF1-α and LeishEF2, as well as LeishIF4A-1, were found in both complexes. However, these are often co-precipitated with a variety of translation factors, or proteins involved in RNA metabolism, thus their presence was regarded with caution and did not lead to key conclusions. The initiation factors LeishIF2-α and seven subunits of LeishIF3 (a, c, d, e, i, k and l), as well as LeishEF1-β, were detected only in complexes that were pulled down with LeishIF4E-3. LeishPABP-2, whose role in translation is yet unclear,Citation25 was solely identified in the LeishIF4G-4 complex. Since no interaction between the two proteins was reported by yeast two-hybrid assays (Fig. S4), it could be mediated by RNA.
Figure 2. Reciprocal pull-down of LeishIF4E-3 and LeishIF4G-4. Transgenic L. amazonensis cells expressing SBP-tagged LeishIF4G-4 (A) or TAP-tagged LeishIF4E-3 (B) were grown under normal conditions and further subjected to nutritional starvation for 6 h. Following washes and cell disruption, protein complexes were affinity-purified over streptavidin-sepharose, separated by SDS-PAGE and immunoblotted with antibodies against LeishIF4G-4 or LeishIF4E-3. The lanes were loaded with 1% [supernatant (S), flow through (F)] or with 20% [wash (W), elution (E)] of the total experimental sample. (C) A list of proteins related to translation identified by mass spectrometry in the pulled-down complexes of tagged LeishIF4E-3 and LeishIF4G-4. The complexes were extracted from promastigotes grown under normal conditions. The results represent data from two independent experiments. The complete list of proteins is provided in Table S1.
![Figure 2. Reciprocal pull-down of LeishIF4E-3 and LeishIF4G-4. Transgenic L. amazonensis cells expressing SBP-tagged LeishIF4G-4 (A) or TAP-tagged LeishIF4E-3 (B) were grown under normal conditions and further subjected to nutritional starvation for 6 h. Following washes and cell disruption, protein complexes were affinity-purified over streptavidin-sepharose, separated by SDS-PAGE and immunoblotted with antibodies against LeishIF4G-4 or LeishIF4E-3. The lanes were loaded with 1% [supernatant (S), flow through (F)] or with 20% [wash (W), elution (E)] of the total experimental sample. (C) A list of proteins related to translation identified by mass spectrometry in the pulled-down complexes of tagged LeishIF4E-3 and LeishIF4G-4. The complexes were extracted from promastigotes grown under normal conditions. The results represent data from two independent experiments. The complete list of proteins is provided in Table S1.](/cms/asset/1379653f-fce0-4095-ae0e-174eaa1f272e/krnb_a_10922709_f0002.gif)
Changes in LeishIF4E-3 and LeishIF4G-4 expression in response to thermal and nutritional stress
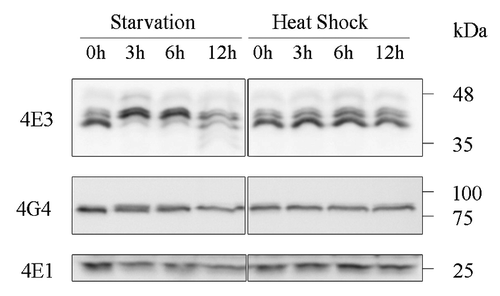
During their life cycle, Leishmania parasites encounter a broad range of environmental conditions, and many of them induce typical stress responses. It has been noted that components of most core metabolic pathways in these organisms are constitutively expressed, allowing for a quick switch to consumption of any new carbon source made available.Citation12 This expression profile is in line with the constant need to overcome the nutritional starvation encountered in the sand fly vector as a function of feeding capacity, and, to some extent, in the mammalian host. To date, the effects of nutritional stress on these parasites have received less attention, as compared with the more common thermal and pH stresses encountered upon transmission into the mammalian host.Citation11 We, therefore, compared the effects of the two types of stress on LeishIF4E-3 and LeishIF4G-4 expression, as well as on their ability to form large complexes. Western analysis of cells exposed to physiological heat shock (33°C) or to nutritional stress revealed that although steady-state levels of each protein were not initially altered under either conditions, nutritional stress, but not heat shock, caused a change in the pattern of migration of LeishIF4E-3 in SDS-PAGE beginning 3 h after the onset of stress (). The higher mass band is a hyperphosphorylated form of the protein, since dephosphorylation of whole cell extracts caused LeishIF4E-3 to migrate faster on SDS-PAGE (Fig. S5). Total protein levels were reduced only after 12 h of starvation, possibly due to the onset of apoptotic processes. To test whether the putative modification of LeishIF4E-3 could promote binding of the protein to the cap structure, extracts of stressed cells were passed over immobilized m7GTP. Such analysis showed that the relatively weak binding of LeishIF4E-3 to m7GTP did not increase following starvation (data not shown). Under the same stress conditions, the expression and migration patterns of LeishIF4G-4 were not affected ().
Figure 3. LeishIF4E-3 changes its pattern of expression only during nutritional stress. Wild type L. amazonensis promastigotes were subjected to nutritional starvation or heat shock (33°C) for 3, 6 or 12 h. Whole cell extracts were separated by SDS-PAGE and subjected to western analysis using specific antibodies against LeishIF4E-1, LeishIF4E-3 or LeishIF4G-4. LeishIF4E-1, which remains unchanged throughout differentiation, served as the loading control.
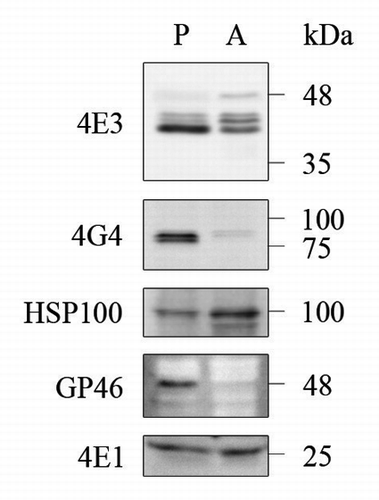
Prolonged exposure to stress in the form of heat shock or acidification of the growth media leads most Leishmania species, including L. amazonensis, to differentiate into axenic amastigotes. In many aspects, these cells resemble intracellular amastigotes, and as such are used to follow developmental patterns of gene expression during stage differentiation.Citation22,Citation26 Although expression of LeishIF4E-3 in axenic amastigotes that were subject to prolonged stress was only slightly reduced, the protein appeared to undergo post-translational modifications, unlike what was seen when the organism encountered heat shock for a shorter duration (). In contrast to LeishIF4E-3, expression of LeishIF4G-4 was hardly detected in axenic amastigotes ().
Figure 4. Differential expression of LeishIF4E-3 and LeishIFG-4 in promastigotes and axenic amastigotes. Whole cell extracts of wild type L. amazonensis late-log phase promastigotes (P) and axenic amastigotes, 9 d after differentiation was initiated (A), were separated by SDS-PAGE and subjected to western analysis using specific antibodies against GP46, HSP100, LeishIF4E-1, LeishIF4E-3 or LeishIF4G-4. LeishIF4E-1, which remains unchanged throughout differentiation, served as the loading control.
The interaction between LeishIF4E-3 and LeishIF4G-4 is impaired under nutritional stress
To test the effect of nutritional stress on binding between LeishIF4E-3 and LeishIF4G-4, reciprocal pull-down assays were performed on cells that were starved for 6 h. The ability of the tagged LeishIF4E-3 to capture LeishIF4G-4 in starved cells was reduced 7.5-fold, as compared with normal conditions (). However, the reciprocal binding of LeishIF4E-3 by the tagged LeishIF4G-4 did not change significantly following stress (). This apparent difference could originate from altered expression levels of the tagged proteins.
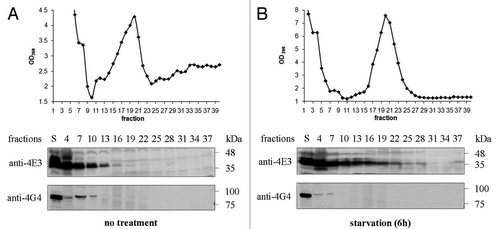
Co-migration between endogenous LeishIF4E-3 and LeishIF4G-4 was tested by fractionation of wild type L. amazonensis cell extracts over sucrose gradients. Under normal conditions, LeishIF4E-3 and LeishIF4G-4 showed only a limited degree of co-migration. While LeishIF4G-4 was detected only at the top of the gradient, LeishIF4E-3 entered into the heavier fractions of the gradient (), as previously reported.Citation19 However, following nutritional starvation, LeishIF4G-4 receded to the very top of the gradient, whereas LeishIF4E-3 migrated yet deeper into the heavier fractions (). This observation further emphasizes that interaction between LeishIF4G-4 and LeishIF4E-3 is reduced under stress.
Figure 5. LeishIF4E-3 and LeishIF4G-4 migration in sucrose gradients. Extracts of wild type L. amazonensis cells were fractionated over 10–40% sucrose gradients. The OD260 of the different gradient fractions is shown in the upper panels. Proteins were immunoblotted with antibodies specific for LeishIF4E-3 or LeishIF4G-4. Samples were obtained from the supernatants (S) or from TCA-precipitated gradient fractions. The experiment was performed on promastigotes grown under normal conditions (A) or subjected to nutritional starvation for 6 h (B).
LeishIF4E-3 enters stress granules during starvation
To view LeishIF4E-3 under normal and stress conditions, LeishIF4E-3 was examined by confocal microscopy using specific antibodies. Under normal conditions, LeishIF4E-3 was evenly dispersed throughout the cytoplasm of wild type cells (Fig. S6), and heat shock treatment did not change this pattern of staining (; Fig. S6). It should be noted that earlier work reported that exposure of T. brucei cells to an extreme heat shock of 41°C led to the appearance of stress granules containing TbIF4E-3.Citation24 However, the thermal stress conditions employed in the present study addressing Leishmania were much milder (33–37°C), in accordance with the actual temperatures that the parasites experience in skin lesions or within the viscera. In addition, this study addressed the effect of a nutritional stress at 25°C, a condition that may be encountered by the parasites within the insect vector. When Leishmania cells were depleted of nutrients, LeishIF4E-3 could be detected within cytoplasmic granules (; Fig. S6). Their accumulation became even more apparent after longer periods of stress. Nutritional stress is a hostile situation that requires specific adaptation, to promote survival of the parasites within the invertebrate vector. The granules formed appear to be part of the protective measures taken by the parasites to overcome starvation.
Figure 6. LeishIF4E-3 enters granules that do not contain LeishIF4G-4. Wild type L. amazonensis cells were subjected to heat shock or nutritional starvation for 3 and 6 h (presented in Fig. S6). The average number of granules per cell was counted and standard deviations were calculated for wild type cells experiencing heat shock (A) or nutrient starvation (B). LeishIF4G4-GFP-expressing L. amazonensis cells were subjected to heat shock or nutritional starvation for 3 and 6 h (C). LeishIF4E-3 was detected by a specific antibody and a secondary fluorescent antibody (DyLight). LeishIF4G-4 was visualized through its fusion with GFP. Nuclear and kinetoplast DNA were visualized by Hoechst staining. The average number of granules per cell was obtained as in A and B, for cells subjected to heat shock (D) or nutrient starvation (E).
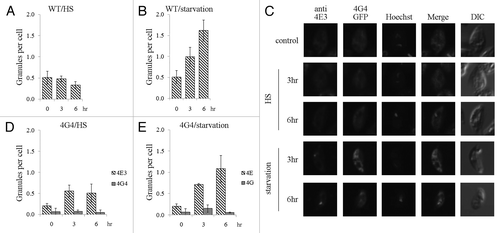
Co-localization of LeishIF4E-3 and LeishIF4G-4 was addressed in cells expressing the LeishIF4G-4-GFP fusion protein (Fig. S7). Under normal conditions, LeishIF4E-3 and LeishIF4G-4 are homogenously spread within the cytoplasm. Upon nutritional stress, however, while LeishIF4E-3 concentrated within granules, LeishIF4G-4 did not change its intracellular distribution (). The same held true in response to heat shock and during starvation ( and E).
The nature of the LeishIF4E-3-containing granules was examined by following the distribution of DHH1, a widely used marker of processing bodies (P-bodies),Citation27,Citation28 in cells expressing LeishDHH1 fused to GFP. The distribution of LeishIF4E-3 was monitored in the same cells by specific antibodies (Fig. S7). Confocal microscopy did not reveal co-localization of LeishDHH1- and LeishIF4E-3-containing granules, in response to thermal and nutritional stress (). LeishDHH1-containing granules mainly formed during heat shock and to a much lesser extent upon starvation ( and C).
Figure 7. LeishIF4E-3 enters stress granules during starvation. L. amazonensis cells expressing LeishDHH1-GFP (A) were subjected to heat shock or nutritional starvation for 3 and 6 h. LeishIF4E-3 was detected by a specific antibody and a secondary fluorescent antibody (DyLight). LeishDHH1 was visualized through its GFP fusion. Nuclear and kinetoplast DNA were visualized by Hoechst staining. The average number of granules per cell was counted and standard deviations were calculated for LeishIF4G4-GFP-expressing cells subjected to heat shock (B) and nutrient starvation (C). A similar analysis was performed for wild type cells experiencing nutritional stress, with or without cycloheximide (CHX) (D).
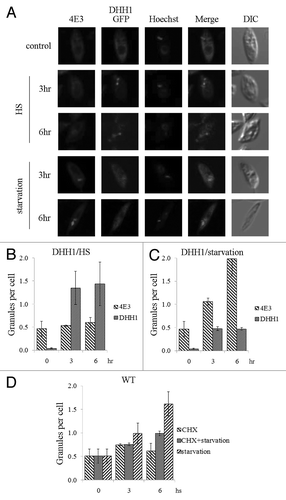
The formation of stress granules in trypanosomes, as in higher eukaryotes, depends on active translation and is, therefore, inhibited by cycloheximide.Citation24,Citation29 In wild type L. amazonensis cells, starvation induced the formation of LeishIF4E-3-containing granules. However, fewer granules were formed when nutritional stress was induced in the presence of cycloheximide and their appearance was delayed (), indicating that they were formed co-translationally.
The molecular basis for LeishIF4E-3 interaction with LeishIF4G-4
eIF4E-eIF4G interactions are typically mediated by a conserved YXXXXLΦ peptide.Citation2 However, LeishIF4G-4 does not contain this consensus motif. Hence, to identify the region responsible for binding of LeishIF4E-3, LeishIF4G-4 fragments were tested for their ability to bind LeishIF4E-3 in a yeast two-hybrid assay. This approach mapped the region responsible for the interaction between the two proteins to the N-terminus (residues 1–78) of LeishIF4G-4, rather than to the MIF4G domain (residues 79–335) or to the C-terminus (residues 336–765) of the protein (). Two series of deletions within the N-terminal 78 positions of LeishIF4G-4 narrowed the binding region first down to a short fragment comprising amino acids 17–31 ( and C) and then to the region spanning positions 22–31 ( and C).
Figure 8. Binding to LeishIF4E-3 is mediated by a short non-conserved peptide in the N-terminus of LeishIF4G-4. LeishIF4G-4 regions that interact with LeishIF4E-3 were identified using a yeast two-hybrid assay. (A, B) Wild type YRG2 cells were co-transfected with the yeast plasmids encoding AD and BD fusion proteins. The BD sequence was fused to the open reading frame of LeishIF4E-3. The AD sequence was fused to (A) the complete open reading frame of LeishIF4G-4, LeishIF4G-4(1–78), LeishIF4G-4(79–335), LeishIF4G-4(336–765), LeishIF4G-4-N (1–78) or to (B) the N-terminus of LeishIF4G-4, denoted LeishIF4G-4-N(1–78), and to LeishIF4G-4-N containing deletions Δ2–16, Δ17–31, Δ32–46, Δ63–78, Δ17–21, Δ22–26 and Δ27–31. The ability (+) or inability (-) of the yeast strains to growth on restrictive medium are marked in the Interaction column. (C) The LeishIF4G-4 peptide fragment that was mapped to be essential for binding of LeishIF4E-3 is shown in bold. The yeast growth on plates is shown in Figure S2 and expression of the exogenous proteins in the transfected yeast strains is shown in Figure S1.
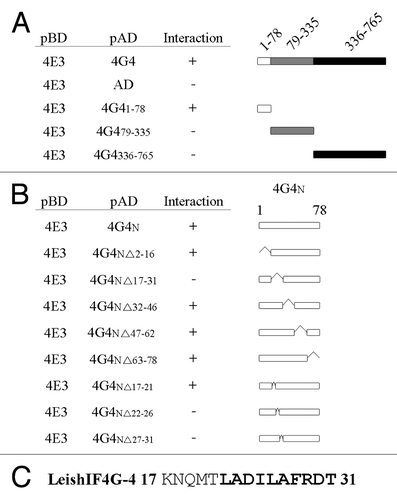
Multiple sequence alignment revealed that these residues are conserved among different trypanosomatid species (except for A23, which is conserved only within the Leishmania genus), suggesting that they serve a similar function in other trypanosomatids (). However, substitution of each amino acid in the region shown to be essential for binding LeishIF4E-3 with alanine did not disturb this activity (), thus excluding the requirement for a sequence motif. Secondary structure prediction using the PsiPred server revealed that the essential residues form an α helix () and that this structure was not destabilized by point substitutions to alanine. However, the L26P substitution, predicted to break the α helix structure, completely eliminated interaction between the two polypeptides as was exemplified both by yeast two-hybrid assays () and by in vivo pull-down assays in Leishmania cells expressing an SBP-tagged LeishIF4G-4 carrying the L26P mutation (). Thus, the interaction between LeishIF4E-3 and LeishIF4G-4 is mainly based on the secondary structure of the binding peptide in the latter.
Figure 9. LeishIF4E-3 binding depends on the structure of the conserved peptide rather than on its sequence. (A) Multiple sequence alignment of the LeishIF4E-3 binding region in LeishIF4G-4 derived from different trypanosomatid species [L. major (LmjF36.6060), L. infantum (LinJ.36.6320), L. braziliensis (LbrM.35.6370), L. tarentolae (LtaP36.6220), T. brucei (Tb11.01.2330) and T. cruzi (Tc00.1047053510285.100)]. The amino acids of the fragment that was shown to be essential for binding are shown in bold. (B) Point mutation analysis of LeishIF4G-4 amino acids 22–31, using yeast two-hybrid assays. Wild type YRG2 cells were co-transfected with plasmids encoding the AD and BD fusion proteins. The AD sequence was fused to the complete open reading frame of LeishIF4G-4 and to its N-terminus LeishIF4G-4-N (1–78), as well as to LeishIF4G-4-N (1–78) carrying the following mutations: L22A, D24A, I25A, L26A, F28A, R29A, D30A and T31A. The BD sequence was fused to wild type LeishIF4E-3 and to the LeishIF4E-3(W187A) mutant. The ability (+) or inability (-) of the yeast strains to growth on restrictive medium are marked in the Interaction column. (C) Secondary structure prediction of the LeishIF4G-4 region found to be essential for binding of LeishIF4E-3, as generated by the PsiPred server. Alpha helices (H) are shown as purple cylinders, and the confidence of the prediction is shown above (higher and darker blue columns represent higher confidence). Predictions are shown for the wild type sequence, as well as for the L26A and L26P mutants. (D) Mutational analysis of L26 in LeishIF4G-4 using a yeast two-hybrid assay. The assays were performed as described in B. The AD sequence was fused to the LeishIF4G-4-N (1–78) fragment carrying the L26A and L26P mutations. The BD sequence was fused to LeishIF4E-3. The yeast growth on plates is shown in Figure S2 and expression of the exogenous proteins in the transfected yeast strains is shown in Figure S1. (E) Transgenic L. amazonensis cells expressing SBP-tagged LeishIF4G-4 or LeishIF4G-4(L26P) were grown under normal conditions. Following washes and cell disruption, protein complexes were affinity-purified over streptavidin-sepharose, separated by SDS-PAGE and immunoblotted with antibodies against LeishIF4G-4 or LeishIF4E-3. The lanes were loaded with 1% [supernatant (S), flow through (F)] or with 20% [wash (W), elution (E)] of the total experimental sample.
![Figure 9. LeishIF4E-3 binding depends on the structure of the conserved peptide rather than on its sequence. (A) Multiple sequence alignment of the LeishIF4E-3 binding region in LeishIF4G-4 derived from different trypanosomatid species [L. major (LmjF36.6060), L. infantum (LinJ.36.6320), L. braziliensis (LbrM.35.6370), L. tarentolae (LtaP36.6220), T. brucei (Tb11.01.2330) and T. cruzi (Tc00.1047053510285.100)]. The amino acids of the fragment that was shown to be essential for binding are shown in bold. (B) Point mutation analysis of LeishIF4G-4 amino acids 22–31, using yeast two-hybrid assays. Wild type YRG2 cells were co-transfected with plasmids encoding the AD and BD fusion proteins. The AD sequence was fused to the complete open reading frame of LeishIF4G-4 and to its N-terminus LeishIF4G-4-N (1–78), as well as to LeishIF4G-4-N (1–78) carrying the following mutations: L22A, D24A, I25A, L26A, F28A, R29A, D30A and T31A. The BD sequence was fused to wild type LeishIF4E-3 and to the LeishIF4E-3(W187A) mutant. The ability (+) or inability (-) of the yeast strains to growth on restrictive medium are marked in the Interaction column. (C) Secondary structure prediction of the LeishIF4G-4 region found to be essential for binding of LeishIF4E-3, as generated by the PsiPred server. Alpha helices (H) are shown as purple cylinders, and the confidence of the prediction is shown above (higher and darker blue columns represent higher confidence). Predictions are shown for the wild type sequence, as well as for the L26A and L26P mutants. (D) Mutational analysis of L26 in LeishIF4G-4 using a yeast two-hybrid assay. The assays were performed as described in B. The AD sequence was fused to the LeishIF4G-4-N (1–78) fragment carrying the L26A and L26P mutations. The BD sequence was fused to LeishIF4E-3. The yeast growth on plates is shown in Figure S2 and expression of the exogenous proteins in the transfected yeast strains is shown in Figure S1. (E) Transgenic L. amazonensis cells expressing SBP-tagged LeishIF4G-4 or LeishIF4G-4(L26P) were grown under normal conditions. Following washes and cell disruption, protein complexes were affinity-purified over streptavidin-sepharose, separated by SDS-PAGE and immunoblotted with antibodies against LeishIF4G-4 or LeishIF4E-3. The lanes were loaded with 1% [supernatant (S), flow through (F)] or with 20% [wash (W), elution (E)] of the total experimental sample.](/cms/asset/a4ab007d-4a8a-441a-98d2-0a322f902aba/krnb_a_10922709_f0009.gif)
One of the murine eIF4E residues that is in direct contact with eIF4G and essential for interaction between the two proteins, is W73.Citation30,Citation31 The parallel residue in LeishIF4E-3, W187,Citation19,Citation23 appeared to be essential for binding to LeishIF4G-4, as its substitution to alanine prevented such interaction (). This appoints to a partial conservation of the structural basis for the interaction between LeishIF4E-3 and LeishIF4G-4 with the homologous proteins in higher eukaryotes.
Discussion
Trypanosomatids express four different isoforms of the cap-binding protein, eIF4E, which show a high degree of variability among them. Gene duplication in these organisms, in many cases, assists their ability to survive in extreme environments, which are encountered during their life cycle. Deciphering the exact role of each eIF4E homolog may, therefore, promote our understanding of the molecular mechanisms that developed to allow survival under different conditions. LeishIF4E-3 is the most abundant eIF4E homolog in L. major and T. brucei.Citation20,Citation23 Previous silencing studies of the T. brucei ortholog, TbIF4E-3, showed that it is the only trypanosomatid eIF4E that is essential, when individually silenced, in both life stages. In view of its efficient binding to TbIF4G-4, TbIF4E-3 was reasonably proposed to serve as a functional translation factor.Citation23
The interaction between eIF4E and eIF4G is central to the formation of the PIC and as such, the two factors should co-migrate on sucrose gradients with 43S and 48S particles, as was shown for LeishIF4E-4 and LeishIF4G-3.Citation21 Indeed, the interaction between LeishIF4E-3 and LeishIF4G-4 was verified in this study using yeast two-hybrid and reciprocal pull-down assays. However, despite the strong interaction between them, the two proteins failed to co-migrate on sucrose gradients. LeishIF4E-3 was found in nuclease-resistant 80S particles (ref. Citation19 and this study), whereas its partner, LeishIF4G-4, never entered any large complexes and was instead exclusively restricted to the light fractions at the top of the gradient without exception, thereby excluding the participation of LeishIF4G-4 in the heavier LeishIF4E-3 complexes. Exposure of the parasite cells to different stresses, similar to those encountered throughout the life cycle, indicated that the LeishIF4E-3 complex was mainly affected by nutritional stress, which promoted the entry of LeishlF4E-3 and not LeishiF4G-4 into the heavier fractions of the gradient. Heat shock (33–35°C), however, did not elicit a similar effect. This observation was supported by the results of the pull-down analysis, showing that the interaction between LeishIF4E-3 and LeishIF4G-4 is reduced in response to starvation. Leishmania parasites may encounter a nutritional stress in the insect vector, in cases that the sand fly fails to provide sufficient plant juices, or perhaps during metacyclogenesis, when the parasites migrate from the hindgut to the front mouthparts, prior to feeding on mammals.
Confocal microscopy showed that upon nutritional stress, LeishIF4E-3 enters cytosolic granules. As these do not contain LeishDHH1, it is unlikely that the two proteins co-localize in the same RNA granules. Since DHH1 is a typical marker of P-bodies, the LeishIF4E-3-containing granules most likely cannot be classified as such. Accordingly, previous experiments involving immuno-precipitation of Trypanosoma cruzi DHH1 did not reveal the presence of TcIF4E-3 or TcIF4G-4 in TcDHH1-containing granules.Citation32 Indeed, TbIF4G-4 has never been found to associate with any high molecular mass particles. This study also shows that LeishIF4G-4 is not present in LeishIF4E-3 containing granules.
TbIF4E-3 was previously reported to be a component of stress granules, although its contribution to granule biogenesis was not addressed.Citation24 We observed here that the formation of LeishIF4E-3 containing granules was inhibited by cycloheximide, a compound that traps ribosomes on the mRNA as polysomes. Such inhibition typically occurs during the formation of stress granules in higher eukaroytes, suggesting that they are formed co-translationally.Citation29,Citation33 In addition, mass spectrometry analysis showed that seven subunits of LeishIF3 could be captured by LeishIF4E-3 but none was pulled-down by LeishIF4G-4. Subunits of eIF3 from higher eukaryotes are likewise found in stress granulesCitation34 and were, furthermore, shown to be required for granule formation.Citation35,Citation36
We propose that LeishIF4E-3 can be found in two states. Under normal conditions, LeishIF4E-3 associates with LeishIF4G-4 in the cytoplasm, possibly in an inactive state, whereas during nutritional stress, LeishIF4E-3 dissociates from its binding partner to enter stress granules. This effect is not observed during thermal stress. However, it is well known that different conditions may affect the nature of stress granules and their contents in yeast,Citation37,Citation38 mammalsCitation39-Citation42 and trypanosomes.Citation24,Citation27 One point that remains unclear in the model proposed here is whether and how LeishIF4E-3 interacts with the cap structure within the granules. This issue requires attention, since LeishIF4E-3 does not bind well to the cap structure under normal conditionsCitation19,Citation21 and the possibility that its binding is induced by nutritional stress was excluded (data not shown). However, if LeishIF4E-3 indeed accompanies mRNAs into storage granules, it is reasonable to expect that it will not bind strongly to these mRNAs, excluding its ability to compete with the general translation factors.
Entry into stress granules may occasionally occur in a modification-dependent manner.Citation33 Western analysis with antibodies against LeishIF4E-3 revealed several bands close in size that were affected by incubation with λ-phosphtase, suggesting that it is a phosphoprotein. Furthermore, it was previously shown that the T. brucei ortholog, TbIF4E-3, becomes phosphorylated in bloodstream form parasites.Citation43 Here we show that the slower migrating band of LeishIF4E-3 is enriched during starvation and in axenic amastigotes. This larger form of LeishIF4E-3 did not bind to LeishIF4G-4, as indicated by western analysis of pulled-down polypeptides.
The molecular mechanism of binding between LeishIF4E-3 and LeishIF4G-4 was also addressed in this study. The typical eIF4E binding peptide known in higher eukaryotes consists of a YXXXXLΦ sequence element. In higher eukaryotes, this peptide was shown to be required for the binding of eIF4E by eIF4G, as well as by 4E-BP.Citation2,Citation30 A partially conserved eIF4E binding element has been formerly described in LeishIF4G-3.Citation21 However, the other five eIF4G homologs in this organism, including LeishIF4G-4, do not contain YXXXXLΦ, or a similar motif. We further show that the binding between LeishIF4G-4 and LeishIF4E-3 is mediated through a structural element that involves an α-helix and does not depend on the peptide sequence. It should be noted that although the T. brucei ortholog of LeishIF4E-3 contains a YXXXXLX sequence (156 YRTKFLP 162), it is located within the MIF4G domain of the protein (amino acids 87–300) and not in its N-terminus, as expected. Furthermore, this region is not conserved in the Leishmania ortholog. This element in T. brucei is, therefore, unlikely to promote binding to TbIF4E-3. Moreover, the region in LeishIF4G-4 that was shown to be responsible for the binding of LeishIF4E-3 is conserved in TbIF4G-4, suggesting that the structural basis for the interaction is similar in the two species.
In conclusion, we propose that LeishIF4G-4 does not function as a typical translation factor but rather regulates the function of LeishIF4E-3, possibly sequestering a large proportion of the latter protein and releasing it under starvation conditions, to enter stress granules. LeishIF4G-4 itself is not found at these sites. Further research is needed to decipher the exact role of LeishIF4E-3 during nutritional stress. As TbIF4E-3 is the only essential eIF4E homolog in T. brucei that cannot be replaced by any of the other paralogs,Citation23 its function appears to be unique. In view of the above and its altered mode of interaction with the MIF4G domain protein, LeishIF4E-3 can be considered as a promising target for the development of novel drugs against trypanosomatids.
Materials and Methods
Organisms
Leishmania amazonensis promastigotes were cultured in Medium 199 (pH 7) supplemented with 10% fetal calf serum (FCS), 5 µg/mL hemin, 0.1 mM adenine, 40 mM Hepes, pH 7.4, 4 mM L-glutamine, 100 U/mL penicillin and 100 µg/mL streptomycin at 25°C. Host-free differentiation and maintenance of L. amazonensis amastigotes were performed in Schneider’s medium (pH 5.5) supplemented with 20% FCS, 4 mM L-glutamine, 60 U/mL penicillin and 60 µg/mL streptomycin at 33°C. The cells were transferred to the acidic medium (pH 5.5) and grown at 25°C for up to one week and then transferred to 33°C.Citation44,Citation45
In vivo pull-down analysis
LeishIF4G-4, LeishIF4G-4(L26P) and firefly luciferase were expressed as fusion proteins bearing a Streptavidin Binding Peptide (SBP) tag,Citation22 while LeishIF4E-3 was fused with the TAP tag (which includes both Protein A and SBP tags).Citation46. DNA encoding the two tags was cloned in frame to appear at the C-terminus of each protein, resulting in the generation of plasmids pSBP-LeishIF4G-4, pSBP-LeishIF4G-4(L26P), pSBP-Luciferase and pTAP-LeishIF4E-3 that were previously described.Citation22
For pull-down assays, transgenic cells (0.6–1 × 109) were harvested, washed, resuspended in binding buffer (BB) [35 mM Hepes, pH 7.5, 10 mM MgCl2, 100 mM KCl, 1 mM DTT, 2 mM iodoacetamide, protease inhibitor cocktail (Sigma)] and disrupted by sonication. Supernatants were agitated with streptavidin-Sepharose beads (GE Healthcare) for 2 h at 4°C. Control pull-down assays were performed in the presence of RNaseA (100 µg/ml) and RNaseT1 (100 U/ml), which were added to the supernatants during incubation with streptavidin-Sepharose beads. In all experiments the beads were further washed with BB containing 0.1% NP-40 and the proteins were eluted with BB supplemented with 2 mM biotin.
Protein fractions were resolved over 8%, 12% or 15% SDS-PAGE and immunoblotted using specific antibodies against LeishIF4E-3, LeishIF4G-4 or SBP. Quantification of the eluted and immunostained proteins was performed using ImageJ software. The fraction of eluted LeishIF4E-3 or LeishIF4G-4 was calculated as a percent of the total protein in question in the supernatant. This value was then normalized, relative to the percentage of eluted tagged protein.
Mass spectrometry analysis
Proteins that were pulled-down with tagged LeishIF4E-3 or LeishIF4G-4 from L. amazonensis cell lines (3–4 × 109 cells) were precipitated by TCA and further resolved on 10% SDS-PAGE. Gels were stained by Coomassie blue and the stained lanes were split into three or four segments that were each subjected to LC-MS/MS analysis on an Orbitrap (Thermo) mass spectrometer. Proteins were identified by BLAST analysis against the L. major database using Sequest 3.31 software (Smoler Protein Research Center, Technion). Control experiments were performed on non-transfected wild type cells, and the identified proteins were eliminated from the complete list (see Table S1).
Monitoring protein expression levels
Promastigotes in the late log growth phase, stressed or not, and axenic amastigotes grown for at least 8 d after differentiation, were used in this experiment. The cells were harvested, washed in PBS, resuspended in BB and lysed by addition of 1% Triton X-100. The protein concentration in the whole cell extracts was determined by the BCA assay (Thermo) and 50 µg of protein was resolved on 8,%, 10%, 12% or 15% SDS-PAGE. Steady-state expression of target proteins was determined by western analysis using specific antibodies against LeishIF4E-3, LeishIF4G-4, GP46 or HSP100. Protein loads were verified by blotting using anti-serum against LeishIF4E-1, previously shown to be equally expressed in all Leishmania life stages.Citation22
Sucrose gradients and polysome analysis
L. amazonensis promastigote cells (0.5–1 × 109 per gradient), non-treated or starved by incubation in PBS for 6 h, were incubated for 5 min in 5 ml of medium containing 100 µg/ml cycloheximide. After centrifugation, the cells were washed twice in PBS containing 100 µg/ml cycloheximide and once with lysis buffer [15 mM Tris-HCl, pH 8, 0.3 M KCl, 5 mM MgCl2, 0.5 mM DTT, 100 µg/ml cycloheximide, 1 mg/ml heparin, 2 mM iodoacetamide and protease inhibitor cocktail (Sigma)]. The cell pellet was resuspended in lysis buffer and lysed upon addition of Triton X-100 to a final concentration of 1% and incubated on ice for 10 min. The cell lysates were centrifuged at 12,000 × g at 4°C for 15 min. The cell supernatants were loaded on an 11 ml 10–40% sucrose step gradient prepared in polysome buffer (20 mM TRIS-HCl, pH 8, 140 mM KCl, 5 mM MgCl2, 0.5 mM DTT, 100 µg/ml cycloheximide, 0.5 mg/ml heparin, 2 mM iodoacetamide, protease inhibitor cocktail (Sigma) and varying concentrations of sucrose) and centrifuged for 160 min at 35,000 rpm in a Beckman SW40 rotor. Fractions (300 µl) were collected from the top, and the optical density at 260 nm was monitored. Proteins were recovered by TCA precipitation, separated by 8% or 15% SDS-PAGE, and further subjected to immunoblotting using antibodies against LeishIF4E-3 or LeishIF4G-4.
Yeast two-hybrid assays
A yeast two-hybrid assay was performed using the commercial GAL4-based Two-Hybrid Phagemid Vector Kit (Stratagene) following the manufacturer’s instructions. The LeishIF4E-3 ORF was cloned into the GAL4-binding domain vector (pBD) using EcoRI sites on both sides (the list of primers used in this study is given in Table S2). The ORFs of LeishIF4G-1 through -6, LeishIF4G-4(1–78), LeishIF4G-4(79–335) and LeishIF4G-4(336–765) were cloned into the GAL4 activation domain vector (pAD) at the BamHI and XbaI sites. Deletions and point mutation were introduced using the site-directed mutagenesis methodology, resulting in the generation of plasmids pBD-LeishIF4E-3(W187A), pAD-LeishIF4G-4(1–78) containing the following mutations: Δ2–16, Δ17–31, Δ32–46, Δ47–62, Δ63–78, Δ17–21, Δ22–26, Δ27–31, L22A, D24A, I25A, L26A, L26P, F28A, R29A, D30A and T31A. All constructs were confirmed by DNA sequencing. The YRG-2 (MATa ura352 his3–200 ade2–101 lys2–801 trp1–901 leu2–3 112 gal4–542 gal80–538 LYS2::UASGAL1-TATA GAL1-HIS3 URA3::UASGAL4 17mers(x3) TATACYC1-lacZ) yeast strain was co-transformed with one pAD and one pBD construct. Yeast transformants were cultured in Liquid SD-2 (-Trp/-Leu) medium at 30°C overnight, diluted to a final concentration of O.D600 = 0.15 and grown to O.D600 = 0.5. Yeast was spotted onto SD-2 (-Trp/-Leu) and SD-3 (-Trp/-Leu/-His) plates.
Proteins were extracted from log phase yeast culture (OD600 = 5) that were harvested, washed with 20% TCA and resuspended in 200 µl 20% TCA. Glass beads were then added and the cells were vortexed for 5 min. Following removal of the glass beads, proteins were centrifuged at 13,000 g for 10 min. The pellet was resuspended in SDS-PAGE sample buffer, boiled for 2 min and centrifuged for an additional 2 min at 13,000 g. The resulting supernatants (OD600 = 1.0) were separated by SDS–PAGE (12%) and the gels were blotted and probed with specific antibodies against the AD or BD domains (Santa Cruz).
Confocal microscopy
GFP fusions were prepared by cloning the ORFs of LeishIF4G-4 and LeishDHH1 into the pX-GFP Leishmania expression vector (kindly provided by S. M. Beverley, Washington University, St. Louis, MO) at the BamHI and XbaI sites for LeishIF4G-4 and at the BamHI and EcoRV sites for LeishDHH1.
Wild type or transgenic Leishmania cells (~2 × 107) were fixed in 4% paraformaldehyde in phosphate-buffered saline (PBS) for 30 min. The cells were washed three times in PBS and then incubated in PBS containing 0.3% bovine serum albumin and 0.3% Triton X-100 for 1 h. After 3 additional washes in PBS, the cells were incubated with the primary antibody (anti-LeishIF4E-3, 1:50) in PBS for 1 h, washed tree times and incubated with a secondary antibody (1:100) conjugated to DyLight fluorophore (KPL) and Hoechst (1:1000) in PBS for another hour. Following three final washes in PBS, an anti-bleaching substance [1,4-diazabicyclo(2.2.2)octane – DABCO] was added, a coverslip was applied and the preparation was sealed with nail polish. Confocal images were taken on an Olympus FV1000 laser-scanning confocal microscope.
The average number of granules per cell was calculated. The number of granules was counted in at least 100 cells for each of two independent slides for each treatment tested. Averages and standard deviations were calculated for each treatment.
Protein dephosphorylation
Wild type late log L. amazonensis promastigotes (~8 × 106 per aliquot) were washed in cold PBS and resuspended in λ-phosphatase buffer (NEB) supplied with MnCl2 (NEB) and a cocktail of protease inhibitors (Sigma). The cells were lysed by the addition of Triton X-100 (1% final concentration). λ-phosphatase was added (10 units, NEB), with and without phosphatase inhibitors (50 mM Sodium Fluoride and 10 mM Sodium Orthovanadate) and the reactions were incubated at 30°C for 40 min. The proteins were resolved on 15% SDS-PAGE and immuno-blotted with antibodies against LeishIF4E-3.
Additional material
Download Zip (3 MB)Acknowledgments
This work was supported by the Israel Science Foundation, (ISF, grant No 395/09) and by the US-Israel Binational Foundation (BSF, grant number 2007287). We are grateful to Charles Jaffe from the Hebrew University of Jerusalem and Joachim Clos from the Bernhard Nocht Institute for Tropical Diseases, Hamburg, Germany for providing specific antibodies against GP46 and Hsp100, respectively. We acknowledge Steve Beverley from Washington University, St. Louis, MO for providing the pX-GFP expression vector.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- Marcotrigiano J, Lomakin IB, Sonenberg N, Pestova TV, Hellen CU, Burley SK. A conserved HEAT domain within eIF4G directs assembly of the translation initiation machinery. Mol Cell 2001; 7:193 - 203; http://dx.doi.org/10.1016/S1097-2765(01)00167-8; PMID: 11172724
- Mader S, Lee H, Pause A, Sonenberg N. The translation initiation factor eIF-4E binds to a common motif shared by the translation factor eIF-4 gamma and the translational repressors 4E-binding proteins. Mol Cell Biol 1995; 15:4990 - 7; PMID: 7651417
- Joshi B, Cameron A, Jagus R. Characterization of mammalian eIF4E-family members. Eur J Biochem 2004; 271:2189 - 203; http://dx.doi.org/10.1111/j.1432-1033.2004.04149.x; PMID: 15153109
- Keiper BD, Lamphear BJ, Deshpande AM, Jankowska-Anyszka M, Aamodt EJ, Blumenthal T, et al. Functional characterization of five eIF4E isoforms in Caenorhabditis elegans.. J Biol Chem 2000; 275:10590 - 6; http://dx.doi.org/10.1074/jbc.275.14.10590; PMID: 10744754
- Miyoshi H, Dwyer DS, Keiper BD, Jankowska-Anyszka M, Darzynkiewicz E, Rhoads RE. Discrimination between mono- and trimethylated cap structures by two isoforms of Caenorhabditis elegans eIF4E. EMBO J 2002; 21:4680 - 90; http://dx.doi.org/10.1093/emboj/cdf473; PMID: 12198170
- Jankowska-Anyszka M, Lamphear BJ, Aamodt EJ, Harrington T, Darzynkiewicz E, Stolarski R, et al. Multiple isoforms of eukaryotic protein synthesis initiation factor 4E in Caenorhabditis elegans can distinguish between mono- and trimethylated mRNA cap structures. J Biol Chem 1998; 273:10538 - 42; http://dx.doi.org/10.1074/jbc.273.17.10538; PMID: 9553113
- Amiri A, Keiper BD, Kawasaki I, Fan Y, Kohara Y, Rhoads RE, et al. An isoform of eIF4E is a component of germ granules and is required for spermatogenesis in C. elegans.. Development 2001; 128:3899 - 912; PMID: 11641215
- Dinkova TD, Keiper BD, Korneeva NL, Aamodt EJ, Rhoads RE. Translation of a small subset of Caenorhabditis elegans mRNAs is dependent on a specific eukaryotic translation initiation factor 4E isoform. Mol Cell Biol 2005; 25:100 - 13; http://dx.doi.org/10.1128/MCB.25.1.100-113.2005; PMID: 15601834
- Sonenberg N, Hinnebusch AG. Regulation of translation initiation in eukaryotes: mechanisms and biological targets. Cell 2009; 136:731 - 45; http://dx.doi.org/10.1016/j.cell.2009.01.042; PMID: 19239892
- Liberman N, Marash L, Kimchi A. The translation initiation factor DAP5 is a regulator of cell survival during mitosis. Cell Cycle 2009; 8:204 - 9; http://dx.doi.org/10.4161/cc.8.2.7384; PMID: 19158497
- Zilberstein D, Shapira M. The role of pH and temperature in the development of Leishmania parasites. Annu Rev Microbiol 1994; 48:449 - 70; http://dx.doi.org/10.1146/annurev.mi.48.100194.002313; PMID: 7826014
- Saunders EC, DE Souza DP, Naderer T, Sernee MF, Ralton JE, Doyle MA, et al. Central carbon metabolism of Leishmania parasites. Parasitology 2010; 137:1303 - 13; http://dx.doi.org/10.1017/S0031182010000077; PMID: 20158936
- Clayton CE. Life without transcriptional control? From fly to man and back again. EMBO J 2002; 21:1881 - 8; http://dx.doi.org/10.1093/emboj/21.8.1881; PMID: 11953307
- De Gaudenzi JG, Noé G, Campo VA, Frasch AC, Cassola A. Gene expression regulation in trypanosomatids. Essays Biochem 2011; 51:31 - 46; PMID: 22023440
- Michaeli S. Trans-splicing in trypanosomes: machinery and its impact on the parasite transcriptome. Future Microbiol 2011; 6:459 - 74; http://dx.doi.org/10.2217/fmb.11.20; PMID: 21526946
- Bangs JD, Crain PF, Hashizume T, McCloskey JA, Boothroyd JC. Mass spectrometry of mRNA cap 4 from trypanosomatids reveals two novel nucleosides. J Biol Chem 1992; 267:9805 - 15; PMID: 1349605
- Mandelboim M, Estraño CL, Tschudi C, Ullu E, Michaeli S. On the role of exon and intron sequences in trans-splicing utilization and cap 4 modification of the trypanosomatid Leptomonas collosoma SL RNA. J Biol Chem 2002; 277:35210 - 8; http://dx.doi.org/10.1074/jbc.M201910200; PMID: 12121975
- Yoffe Y, Zuberek J, Lewdorowicz M, Zeira Z, Keasar C, Orr-Dahan I, et al. Cap-binding activity of an eIF4E homolog from Leishmania.. RNA 2004; 10:1764 - 75; http://dx.doi.org/10.1261/rna.7520404; PMID: 15388875
- Yoffe Y, Zuberek J, Lerer A, Lewdorowicz M, Stepinski J, Altmann M, et al. Binding specificities and potential roles of isoforms of eukaryotic initiation factor 4E in Leishmania.. Eukaryot Cell 2006; 5:1969 - 79; http://dx.doi.org/10.1128/EC.00230-06; PMID: 17041189
- Dhalia R, Reis CR, Freire ER, Rocha PO, Katz R, Muniz JR, et al. Translation initiation in Leishmania major: characterisation of multiple eIF4F subunit homologues. Mol Biochem Parasitol 2005; 140:23 - 41; http://dx.doi.org/10.1016/j.molbiopara.2004.12.001; PMID: 15694484
- Yoffe Y, Léger M, Zinoviev A, Zuberek J, Darzynkiewicz E, Wagner G, et al. Evolutionary changes in the Leishmania eIF4F complex involve variations in the eIF4E-eIF4G interactions. Nucleic Acids Res 2009; 37:3243 - 53; http://dx.doi.org/10.1093/nar/gkp190; PMID: 19321500
- Zinoviev A, Léger M, Wagner G, Shapira M. A novel 4E-interacting protein in Leishmania is involved in stage-specific translation pathways. Nucleic Acids Res 2011; 39:8404 - 15; http://dx.doi.org/10.1093/nar/gkr555; PMID: 21764780
- Freire ER, Dhalia R, Moura DM, da Costa Lima TD, Lima RP, Reis CR, et al. The four trypanosomatid eIF4E homologues fall into two separate groups, with distinct features in primary sequence and biological properties. Mol Biochem Parasitol 2011; 176:25 - 36; http://dx.doi.org/10.1016/j.molbiopara.2010.11.011; PMID: 21111007
- Kramer S, Queiroz R, Ellis L, Webb H, Hoheisel JD, Clayton C, et al. Heat shock causes a decrease in polysomes and the appearance of stress granules in trypanosomes independently of eIF2(alpha) phosphorylation at Thr169. J Cell Sci 2008; 121:3002 - 14; http://dx.doi.org/10.1242/jcs.031823; PMID: 18713834
- da Costa Lima TD, Moura DM, Reis CR, Vasconcelos JR, Ellis L, Carrington M, et al. Functional characterization of three leishmania poly(a) binding protein homologues with distinct binding properties to RNA and protein partners. Eukaryot Cell 2010; 9:1484 - 94; http://dx.doi.org/10.1128/EC.00148-10; PMID: 20675580
- Rosenzweig D, Smith D, Opperdoes F, Stern S, Olafson RW, Zilberstein D. Retooling Leishmania metabolism: from sand fly gut to human macrophage. FASEB J 2008; 22:590 - 602; http://dx.doi.org/10.1096/fj.07-9254com; PMID: 17884972
- Cassola A, De Gaudenzi JG, Frasch AC. Recruitment of mRNAs to cytoplasmic ribonucleoprotein granules in trypanosomes. Mol Microbiol 2007; 65:655 - 70; http://dx.doi.org/10.1111/j.1365-2958.2007.05833.x; PMID: 17635187
- Holetz FB, Correa A, Avila AR, Nakamura CV, Krieger MA, Goldenberg S. Evidence of P-body-like structures in Trypanosoma cruzi.. Biochem Biophys Res Commun 2007; 356:1062 - 7; http://dx.doi.org/10.1016/j.bbrc.2007.03.104; PMID: 17399688
- Anderson P, Kedersha N. RNA granules: post-transcriptional and epigenetic modulators of gene expression. Nat Rev Mol Cell Biol 2009; 10:430 - 6; http://dx.doi.org/10.1038/nrm2694; PMID: 19461665
- Marcotrigiano J, Gingras AC, Sonenberg N, Burley SK. Cap-dependent translation initiation in eukaryotes is regulated by a molecular mimic of eIF4G. Mol Cell 1999; 3:707 - 16; http://dx.doi.org/10.1016/S1097-2765(01)80003-4; PMID: 10394359
- Gross JD, Moerke NJ, von der Haar T, Lugovskoy AA, Sachs AB, McCarthy JE, et al. Ribosome loading onto the mRNA cap is driven by conformational coupling between eIF4G and eIF4E. Cell 2003; 115:739 - 50; http://dx.doi.org/10.1016/S0092-8674(03)00975-9; PMID: 14675538
- Holetz FB, Alves LR, Probst CM, Dallagiovanna B, Marchini FK, Manque P, et al. Protein and mRNA content of TcDHH1-containing mRNPs in Trypanosoma cruzi.. FEBS J 2010; 277:3415 - 26; http://dx.doi.org/10.1111/j.1742-4658.2010.07747.x; PMID: 20629747
- Buchan JR, Parker R. Eukaryotic stress granules: the ins and outs of translation. Mol Cell 2009; 36:932 - 41; http://dx.doi.org/10.1016/j.molcel.2009.11.020; PMID: 20064460
- Kedersha N, Chen S, Gilks N, Li W, Miller IJ, Stahl J, et al. Evidence that ternary complex (eIF2-GTP-tRNA(i)(Met))-deficient preinitiation complexes are core constituents of mammalian stress granules. Mol Biol Cell 2002; 13:195 - 210; http://dx.doi.org/10.1091/mbc.01-05-0221; PMID: 11809833
- Ohn T, Kedersha N, Hickman T, Tisdale S, Anderson P. A functional RNAi screen links O-GlcNAc modification of ribosomal proteins to stress granule and processing body assembly. Nat Cell Biol 2008; 10:1224 - 31; http://dx.doi.org/10.1038/ncb1783; PMID: 18794846
- Mokas S, Mills JR, Garreau C, Fournier MJ, Robert F, Arya P, et al. Uncoupling stress granule assembly and translation initiation inhibition. Mol Biol Cell 2009; 20:2673 - 83; http://dx.doi.org/10.1091/mbc.E08-10-1061; PMID: 19369421
- Buchan JR, Muhlrad D, Parker R. P bodies promote stress granule assembly in Saccharomyces cerevisiae.. J Cell Biol 2008; 183:441 - 55; http://dx.doi.org/10.1083/jcb.200807043; PMID: 18981231
- Grousl T, Ivanov P, Frýdlová I, Vasicová P, Janda F, Vojtová J, et al. Robust heat shock induces eIF2alpha-phosphorylation-independent assembly of stress granules containing eIF3 and 40S ribosomal subunits in budding yeast, Saccharomyces cerevisiae.. J Cell Sci 2009; 122:2078 - 88; http://dx.doi.org/10.1242/jcs.045104; PMID: 19470581
- Kedersha NL, Gupta M, Li W, Miller I, Anderson P. RNA-binding proteins TIA-1 and TIAR link the phosphorylation of eIF-2 alpha to the assembly of mammalian stress granules. J Cell Biol 1999; 147:1431 - 42; http://dx.doi.org/10.1083/jcb.147.7.1431; PMID: 10613902
- Stoecklin G, Stubbs T, Kedersha N, Wax S, Rigby WF, Blackwell TK, et al. MK2-induced tristetraprolin:14-3-3 complexes prevent stress granule association and ARE-mRNA decay. EMBO J 2004; 23:1313 - 24; http://dx.doi.org/10.1038/sj.emboj.7600163; PMID: 15014438
- Serman A, Le Roy F, Aigueperse C, Kress M, Dautry F, Weil D. GW body disassembly triggered by siRNAs independently of their silencing activity. Nucleic Acids Res 2007; 35:4715 - 27; http://dx.doi.org/10.1093/nar/gkm491; PMID: 17604308
- Thomas MG, Martinez Tosar LJ, Desbats MA, Leishman CC, Boccaccio GL. Mammalian Staufen 1 is recruited to stress granules and impairs their assembly. J Cell Sci 2009; 122:563 - 73; http://dx.doi.org/10.1242/jcs.038208; PMID: 19193871
- Nett IR, Martin DM, Miranda-Saavedra D, Lamont D, Barber JD, Mehlert A, et al. The phosphoproteome of bloodstream form Trypanosoma brucei, causative agent of African sleeping sickness. Mol Cell Proteomics 2009; 8:1527 - 38; http://dx.doi.org/10.1074/mcp.M800556-MCP200; PMID: 19346560
- Cysne-Finkelstein L, Temporal RM, Alves FA, Leon LL. Leishmania amazonensis: long-term cultivation of axenic amastigotes is associated to metacyclogenesis of promastigotes. Exp Parasitol 1998; 89:58 - 62; http://dx.doi.org/10.1006/expr.1998.4276; PMID: 9603489
- Hodgkinson VH, Soong L, Duboise SM, McMahon-Pratt D. Leishmania amazonensis: cultivation and characterization of axenic amastigote-like organisms. Exp Parasitol 1996; 83:94 - 105; http://dx.doi.org/10.1006/expr.1996.0053; PMID: 8654556
- Aphasizhev R, Aphasizheva I, Nelson RE, Gao G, Simpson AM, Kang X, et al. Isolation of a U-insertion/deletion editing complex from Leishmania tarentolae mitochondria. EMBO J 2003; 22:913 - 24; http://dx.doi.org/10.1093/emboj/cdg083; PMID: 12574127