Abstract
Toxin-antitoxin (TA) systems are categorized into three classes based on the type of antitoxin. In type I TA systems, the antitoxin is a small antisense RNA that inhibits translation of small toxic proteins by binding to the corresponding mRNAs. Those type I TA systems were originally identified as plasmid stabilization modules rendering a post-segregational killing (PSK) effect on the host cells. The type I TA loci also exist on the Escherichia coli chromosome but their biological functions are less clear. Genetic organization and regulatory elements of hok/sok and ldr/rdl families are very similar and the toxins are predicted to contain a transmembrane domain, but otherwise share no detectable sequence similarity. This review will give an overview of the type I TA modules of E. coli K-12, especially hok/sok, ldr/rdl and SOS-inducible symE/symR systems, which are regulated by divergently overlapping cis-encoded antisense RNAs.
Introduction
In the last ten years, small regulatory RNAs (sRNAs) have become the focus of a broad range of studies due to their potential involvement in various levels of biological systems.Citation1,Citation2 These sRNAs play an important role for regulating gene expression via a base-pairing mechanism with target mRNAs.Citation3,Citation4 In E. coli, most of the sRNAs bind to an RNA chaperon, Hfq, that stimulates duplex formation by two complementary RNAs. Hfq also stabilizes certain sRNAs and mRNAs.Citation5 Some sRNAs, like microRNAs discovered in eukaryotes and viruses, have been characterized to control gene expression via trans-acting means. Such sRNAs are partially complementary to their target RNAs; therefore, they often have multiple targets.Citation6 In contrast to the trans-encoded sRNAs encoded on the bacterial chromosome, cis-encoded sRNAs are found mainly in plasmids, phages and transposons. They are encoded in the same DNA locus and are therefore completely complementary to their targets.Citation7,Citation8 Originally cis-encoded sRNAs were identified as regulatory RNAs that control initiation of plasmid replication and thereby plasmid copy number by changing the secondary structure of the RNA to which the antisense RNA was complementary (RNA I of ColEI and pMB1).Citation9 Copy number control antisense RNAs can also act via transcriptional attenuation, inhibition of translation and promotion of RNA degradation. One of the cis-acting antisense RNAs encoded by plasmids, Sok antitoxin RNA, represses the synthesis of a small, hydrophobic protein (Hok) that kills the host cell by damaging the bacterial cell membrane.Citation10 Because the protein toxin leads to cell death only of cells in which the plasmid is lost, these toxin-antitoxin (TA) modules have been termed post-segregational-killing (PSK) systemsCitation11 or addiction modules.Citation12
In addition to the hok/sok systems for plasmid addiction, a number of chromosomally encoded type I TA modules have been recently identified and characterized.Citation13-Citation17 There are three types of TA systems, classified as type I, II and III, which are distinguished by the nature of the antitoxins and the composition of TA gene systems.Citation18,Citation19 This review will focus on the type I TA system, whose toxin translation is inhibited by antisense sRNA antitoxin (antitoxin of type II TA system is a small, unstable protein; type III antitoxin is an RNA that inhibits toxin activity), especially hok/sok, ldr/rdl and symE/symR systems in E. coli with an emphasis on their common aspects and merit to the host cells, and point out future issues in the field.
Plasmid-Encoded Type I TA Modules
The first TA systems were identified on plasmids. The hok/sok system of the E. coli plasmid R1 was originally discovered in a screen for a locus that mediates efficient plasmid stabilization by killing plasmid-free cells,Citation11,Citation20 and perhaps it is one of the most characterized systems at a molecular regulatory level among all TA systems so far.Citation10 hok/sok homologs are also found in other low-copy plasmids. The F plasmid carries two hok/sok homologus loci, flm and srnB.Citation21-Citation24 There is one hok/sok homologous locus (pndA/pndB) on plasmid R483.Citation25,Citation26 These loci code for three small genes that in hok/sok have been denoted hok (host killing), sok (suppression of killing) and mok (modulation of killing).Citation27 The hok gene encodes a highly toxic transmembrane protein of 52 amino acids (aa) that irreversibly damages the cell membrane, and is thus lethal to host cells.Citation28,Citation29 The mok reading frame overlaps extensively with hok, and is required for expression and regulation of hok translation.Citation30,Citation31 The sok gene specifies a small cis-acting antisense RNA of 64 nucleotides (nt) that is complementary to the hok mRNA leader region.Citation32,Citation33 Sok RNA is quite labile (half-life of ~30 sec), but is constitutively expressed from a relatively strong promoter. In contrast, hok mRNA is very stable (half-life of ~20 min)Citation34 and is constitutively expressed from a relatively weak promoter.Citation32,Citation33 Genetic analyses showed that Sok RNA inhibits translation of the mok reading frame and that translation of hok is coupled to the translation of mok.Citation31 Consequently, Sok RNA indirectly inhibits translation of hok by preventing mok translation.Citation35 Because Sok RNA is very unstable and is quickly degraded when the R1 plasmid is lost from the cell, the more stable hok mRNA is translated and cells increase plasmid maintenance by killing plasmid-free cells. This plasmid stabilization system is as a result of a phenotype called post-segregational killing (PSK).Citation10
Through the painstaking molecular studies by the Gerdes group, the detailed mechanisms leading to activation of hok translation in plasmid-free cells are well understood. The full-length hok mRNA which is translationally inactive and prevents binding of Sok RNA, is accumulated in plasmid-carrying cells.Citation34,Citation36,Citation37 This primary mRNA is then activated by 3′ end processing by the RNase II (rnb gene product) and polyribonucleotide nucleotidyltransferase (PNPase: pnp gene product), leading to generation of the truncated mRNA.Citation38-Citation40 After refolding, this mRNA contains the Sok RNA target hairpin structure and therefore is active with respect to both translation and Sok RNA binding. In plasmid-carrying cells, however, the Sok RNA rapidly binds to the truncated hok mRNA and thereby inhibits its translation by preventing ribosome entry. This hok mRNA-Sok RNA duplex is cleaved by RNase III, thus truncated hok mRNA is not translated.Citation36 In plasmid-free cells, the Sok RNA is rapidly degraded due to its instability. Then, the continued 3′ processing of full-length hok mRNA leads to accumulation of the stable truncated mRNA, resulting in Hok protein synthesis and selective killing of the plasmid-free cells.
Chromosome-Encoded Type I TA Modules
hok/sok gene family
hok/sok loci are also found on the chromosomes of enterobacteria.Citation10,Citation13 The chromosome of E. coli K-12 contains six hok/sok homologous loci (A, B, C, D, E and X) (). All of the hok/sok genes, with the exception of the hokB/sokB locus, appear to have degenerated with mutations and transposon insertions. Three hok/sok loci (hokA/sokA, hokC/sokC and hokE/sokE) are inactivated by insertion sequence (IS) elements; however, about half of the 72 wild type E. coli strains of the ECOR collection encode a hokA/sokA and hokC/sokC systems without an IS element.Citation13 The hokD gene, formerly known as relF, is encoded by the third gene of the relBEF operon. This gene does not contain upstream regulatory elements and lacks the sok gene. Thus, the hokD locus probably constitutes an evolutionary relic of an ancient hok-homolog. It was previously reported that the hokE locus does not encode the sok antisense RNA gene, but a cloning-based screen for sRNAs identified sokE expression.Citation41 This screen also isolated cDNA that corresponds to novel Sok RNA (SokX) expressed from an intergenic region between ygcB and cysH genes at 68.2 min on the genetic map ().Citation41 It is not known whether all of the Sok RNAs act as antisense RNA. Given that the sokE and sokX regions are predicted not to express hok transcripts,Citation13,Citation41 it is possible that the SokE and SokX RNAs play roles independently of a role as a regulator of a hok/sok locus. The hokB/sokB locus seems to contain all the regulatory elements as previously described for the hok system of plasmid R1 (). However, all the hok/sok homologs on the chromosome have lost PSK activity, probably through inactivation by IS elements, point mutations and a genetic rearrangement. The chromosome-encoded mRNA is insufficiently translated in vitro, further explaining the absence of the PSK phenotype.Citation13 Therefore the biological function of the chromosomally encoded hok/sok loci is not understood. It is possible that induction of a chromosomal hok gene may be activated by as-yet unknown signals and is required in natural conditions rather than laboratory environments.Citation16
Figure 1. Localization of three different type I TA loci on the E. coli K-12 genome. hok/sok (pink), ldr/rdl (blue) and symE/symR (green) loci are shown. Asterisks show genes that are clearly degenerated or relics.
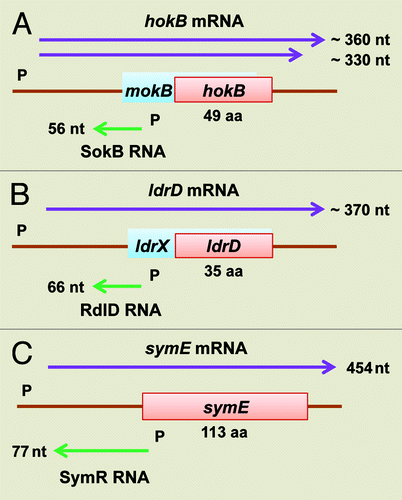
Figure 2. Genetic organization of hokB/sokB, ldrD/rdlD, and symE/symR type I TA modules of E. coli K-12. (A) The hokB/sokB locus is located between cybB and trg at 32.1 min (). This system contains all of the regulatory elements as described for hok/sok system in plasmid R1, such as fbi (foldback inhibition) element, tac (translational activation) element, ucb (upstream complementary box) promoter sequences, Shine-Dalgarno sequences and an overlapping reading frame mokB (mediation of killing). The mokB reading frame is out-of-frame with hokB and terminates 38 nt upstream of hokB. (B) The ldrD/rdlD locus is located between bcsG and yhjV at 79.7 min (). A second open reading frame ldrX, noted by Gerdes and Wagner,Citation16 that overlaps with ldrD as in-frame, thus they share the same translational termination codon. It is predicted that RdlD RNA regulates ldrD translation by regulating ldrX translation. (C) The symE/symR locus is located between restriction-modification related genes mcrB and hsdS at 98.7 min (). The sym E promoter has a LexA binding site and is strongly induced by DNA damaging agents. SymR is encoded opposite the 5′ untranslated region (UTR) of symE, and base pairing can extend over the Shine-Dalgarno sequence as well as the initiation codon of symE.
ldr/rdl gene family
The E. coli K-12 chromosome encodes another family of type I TA modules. Four copies of long direct repeat (LDR) sequences were detected upon completion of the E. coli genomic sequence. Three of the repeats (LDR-A, -B, -C), each approximately 500 bp in length, are located as tandem repeats at 27.4 min on the genetic map.Citation42,Citation43 Another single fourth copy (LDR-D), 450 bp in length and nearly identical to LDR-A, -B and -C, is located at 79.7 min.Citation44 It is interesting that the LDR sequences are symmetrically positioned on the chromosome (), but the reasons for this are unknown. In previous studies, another four statistically significant LDR sequences were identified with more than 187 bp matched to LDR-A near the LDR loci. However, these are probably remnant sequences due to their loss of sense and antisense genes.Citation45 There are several similarities between LDRs (ldr/rdl modules) and hok/sok modules: (1) they both encode a small toxic protein (LdrD: 35 aa; HokB: 49 aa) whose overexpression leads to rapid host cell killing; (2) they both produce a highly stable mRNAs (ldr and hok) that are repressed by small unstable antisense RNAs (Rdl and Sok); (3) both classes of mRNAs have stable secondary structures (ldrD mRNA: 374 nt in length, ∆G = –177.24 kcal mol−1; hok mRNA: 433 nt in length, ∆G = –197.02 kcal mol−1); (4) both mRNAs encode open reading frames (ldrX and mok) that overlap the toxin genes;Citation16 and (5) in many enteric bacteria, both loci are present on the chromosome as multiple copies.Citation15,Citation46 Despite their highly similar genetic organization and regulatory mode, there are no sequence similarities between the two systems at the protein or DNA sequence levels. Moreover, the ldr/rdl module has no PSK activity and does not show PSK-like effect in LDR-locus deleted cells.Citation14 LDR-homologous sequences have not yet been reported in known plasmid sequences. These results suggest that the LDRs are genetic elements that are not used for stabilizing plasmid inheritance.
symE/symR gene family
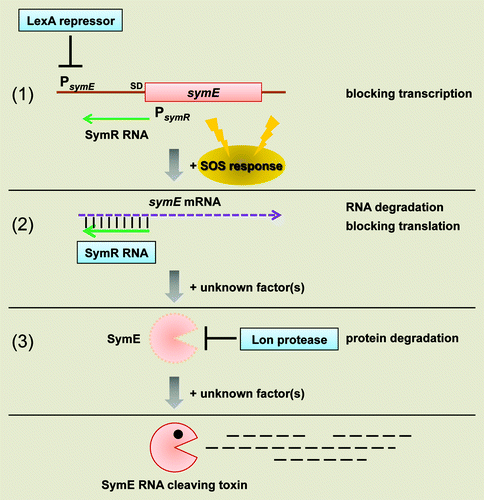
In a cloning-based screen for E. coli sRNAs, several cis-encoded antisense RNAs were identified.Citation41 One such 77 nucleotide RNA was denoted symR (symbiotic RNA) based on its genomic position opposite to the 5′ end of the symE (SOS-induced yjiW gene with similarity to MazE) mRNA ().Citation17 The SymR promoter is embedded in the symE coding sequence and the SymR antisense RNA is transcribed from three nucleotides behind the start codon of symE (). The SymE synthesis is tightly repressed at multiple levels; by the LexA repressor at the level of transcription, by the SymR RNA at the level of mRNA stability and translation, and by the Lon protease at the level of protein stability (). This multilayer control system is probably an effective way to keep endogenous SymE toxin level low enough not to damage the cell until it is required. In contrast to the previously mentioned antitoxin RNAs and proteins, which are rapidly degraded by ribonuclease and protease activity respectively, SymR antitoxin RNA is quite stable and, surprisingly, the SymE toxin is degraded by the Lon protease.
Figure 3. Model for SymE synthesis. SOS-induced symE gene is repressed at three levels by (1) the LexA repressor (transcriptionally), (2) the SymR antisense RNA (post-transcriptionally and/or translationally) and (3) the Lon protease (post-translationally). Other as-yet unknown factors such as ribonucleases and chaperon proteins could be involved in the modulation of SymE synthesis. Endogenous levels of the SymE protein might play a role in degrading particular RNA damaged concomitantly with DNA.
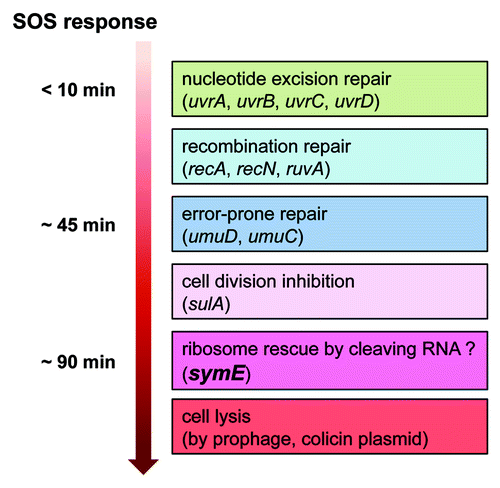
After SOS response induced by the DNA damaging agent mitomycin C, SymE synthesis is very slow; detection occurred 30 min after induction with a peak at 90 min. One interpretation of this is that the timing of SymE activity is dependent on the initial activity of DNA repair proteins ().Citation17 This late expression of symE after the SOS response could be controlled by SymR, the Lon protease and other potential unknown factors. Without SOS-induction, i.e., in normally growing cells, SymE levels were slightly elevated when one of the symR or lon genes was disrupted, similar to levels observed in a lexA repressor mutant strain. However, SymE levels were significantly elevated in the double mutant and were induced even further upon deactivation of the LexA repressor by mitomycin C treatment. This indicates that the contributions of LexA, SymR and Lon to SymE repression are additive. RNase III is not required for repression. In addition, Hfq RNA chaperon protein is not necessary for SymR regulation of SymE synthesis, whereas most trans-encoded antisense RNAs in E. coli require Hfq for binding between sRNA and the target mRNAs.Citation5
Figure 4. Model for timing of SymE synthesis during the SOS response. The SOS genetic network consists of more than 40 genes in E. coli that carry out diverse functions in response to DNA damage, including nucleotide excision repair, homologous recombination, translesion DNA replication, and cell division arrest. The network is controlled by the LexA repressor that downregulates itself and the expression of the other SOS genes, but the peak timing of the induced protein levels seems to be different. SymE protein synthesis may occur at the late stage of the SOS response but before cell lysis. It is suggested that SymE promotion of RNA cleavage may be important for ribosome rescue by recycling of RNAs damaged under SOS-inducing conditions.
Characteristics of the Small Toxic Proteins
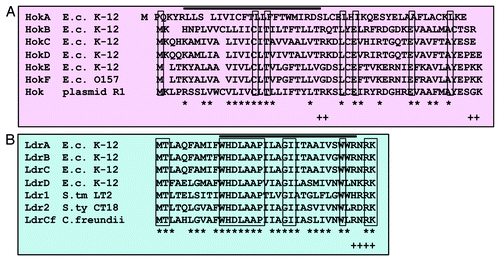
The Hok and Ldr toxin protein family members have some common features. They are very hydrophobic and contain one α-helical transmembrane domain and positively charged amino acids flanked by the domain ().Citation47 All the toxins in the type I TA systems of E. coli characterized so far have a transmembrane domain and are predicted to be localized in inner membrane and/or interact with other proteins in the cell membrane or periplasm. It is proposed that an oligomeric form of the toxins, similar to phage holin proteins, creates pore-like structures and permeabilizes the membrane to impair ATP synthesis; consequently replication, transcription and translation may be inhibited.Citation18
Figure 5. Multiple amino acid sequence alignments. (A) Hok proteins from E. coli K-12, E. coli O157, and plasmid R1. (B) Ldr proteins from E. coli K-12, Salmonell typhimurium LT2, Salmonella typhi CT18, and Citrobacter freundii. Identical amino acids are boxed, and similar amino acids are indicated by an asterisk at the bottom. The similarity of amino acids was determined by the following rules: L = I = M = V = f = W = A, K = R = H, D = E = Q = N, G = A = S, t = V, A = V and f = Y = H = W. + shows C-terminal positive charged residues. The black line above the aligned amino acids indicates a putative trans-membrane α-helical domain predicted by a computer program (SOSUI: http://bp.nuap.nagoya-u.ac.jp/sosui/).
Overexpression of Hok protein from a multi-copy plasmid has been shown to lead to loss of the cell membrane potential, arrest of respiration, efflux/influx of small molecules and change of morphology to so-called “ghost-cells,” which inhibit cell growth and reduce colony-forming ability. Therefore, Hok proteins are likely to kill the cells by mediating irreversible damage to the host cell membrane.Citation48
Ectopic expression of Ldr protein causes rapid growth inhibition, loss of cell viability, inhibition of global translation and nucleoid condensation. The condensation of nucleoid structure is a quick reaction that is observed within two minutes of induction of ldrD. This speed, together with the failure to detect the ldrD gene product, makes it seem unlikely that this nucleoid condensation is caused by the accumulation of LdrD on the chromosome. LdrD might interact with an unknown target that is important for maintaining normal nucleoid structure and cell growth. The physiological function of LdrD related to this phenotype is at present unknown; however, microarray analysis suggests that overexpression of ldrD leads to physiological alteration in purine metabolism and decreases cAMP levels in the cell.Citation14 Thus, the identification of the specific molecular target(s) of the small protein will be an important next subject of investigation.
SymE overexpression also affects cell growth and global protein synthesis. Although SymE belongs to type I TA systems, it is not a hydrophobic protein and does not show functional homology to other type I toxin proteins. SymE actually promotes RNA degradation of mRNAs and noncoding RNAs but not SymR RNA. This resembles the function of type II toxins such as MazF, which can cleave mRNA independent of the ribosome (). However, SymE has homology to the AbrB-fold superfamily proteins such as MazE, which act as transcriptional factors and antitoxins in various type II TA modules. Analyses of amino acid conservation and operon organization of the SymE-like gene family imply that SymE has evolved into an RNA cleavage protein with toxin-like properties from a transcription factor or antitoxin.Citation17 It is interesting to note that many SymE family genes locate adjacent to an antitoxin-like gene encoding a protein antitoxin. In case symE homologs solely exist on the genome, SymR-like antisense RNA might antagonize SymE toxicity; this was inferred from an observation that nucleotide sequences around initiation codon of SymE-like genes are relatively conserved among the families.Citation17
Figure 6. Summary of the type I and II toxins. The targets, types of activity, and cellular processes that are affected by the endogenous toxin expression levels need to be examined in more detail. Asterisk denotes paired antitoxin gene.
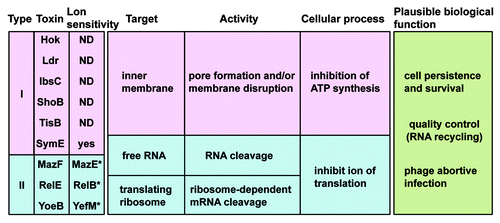
The biological roles of the Hok, Ldr and SymE proteins expressed from the chromosome are unclear. None of the chromosome-encoded hok/sok and ldr/rdl systems has PSK activity, and antitoxin-deficient strains do not show growth inhibition and killing effects to the cells. Thus, it has been proposed that they are beneficial to cell survival by being part of the global cellular response to environmental stress such as amino acid and/or carbon source starvation, rather than being cell-killing modules (not toxins actually). In the case of SymE, which is induced in response to DNA damage or other factors causing SOS response, it has been suggested that the RNA cleavage property of SymE may be important for recycling of RNAs damaged under these conditions.Citation17 Additional experiments are required to reveal what conditions determine activity and to clarify functional roles in the cell.
Diversity, Evolution And Merit Of The Type I TA Family
In E. coli K-12, multiple and polymorphic TA modules are present on the chromosome, encoding five hok/sok and four ldr/rdl (LDR) loci but only one copy of symE/symR. Those homologs of type I toxins are also found in other enteric bacteria. The hok genes are found in a broader spectrum of enterobacteria, whereas ldr and symE genes are only conserved in closely related enteric bacteria. The hok gene loci are repeated multiple times (e.g., E. coli O157 H7 EDL933 has 13 copies) and are almost randomly scattered on the chromosome.Citation15 Meanwhile, multiple ldr genes are located as a tandem repeat sequence. In contrast to type II TA systems, type I systems seem unlikely to move via horizontal gene transfer but rather have evolved by lineage-specific gene duplication.Citation46,Citation49 It is also interesting to compare corresponding genetic loci of the toxin genes between bacterial species to speculate on their evolution. For example, one of the ldr genes in Salmonella exists between tsx and yajD genes at 9.1 min on the genetic map. In contrast, a REP (repetitive extragenic palindromic)-like sequence is present between tsx and yajD genes in E. coli. This may imply that REP-like sequences could be used as a target sequence for LDR-insertion or that they exist as remnant sequences after excision of an LDR sequence. Interestingly, symE genes tend to associate with mobile or selfish elements such as transposons, restriction-modification (RM) modules and pathogenicity islands.Citation50,Citation51 The symE/symR module in E. coli K-12 is located between mcrB and hsdS RM-related genes. It is also found that another TA module, a relBE system in the Gram-positive bacterium Streptococcus mutants, is associated with the RM system gene hsdS.Citation52 These observations may suggest that the symE/symR module is distributed from one chromosome to another by utilizing selective advantages of mobile elements.
A clear understanding of the importance of type I TA modules has yet to be obtained despite more than 25 y of devoted study. Each TA cassette seems not seem to be essential because it can be deleted easily from the chromosome.Citation14,Citation17 So, what is the biological significance to bacteria of containing multiple TA systems in their genome, though the gene products are highly toxic to the cell when overexpressed? It is speculated that chromosomally encoded TA systems act as integral regulators of cellular activity as cell survival/persistence elements during starvation or antibiotic exposition, quality control elements by RNA recycling, beyond PSK-like functions in stabilizing mobile genetic elements.Citation18,Citation53,Citation54 I favor the model that they have a benefit as a defense system against invasion factors like bacteriophages. Since bacteriophage infection can lead to rapid cessation of the host cell transcription,Citation55 the unstable antitoxin RNAs can be depleted much faster than the stable toxin-encoding mRNAs and consequently, the toxin protein is synthesized. In addition, it is reported that the hok/sok modules could reduce the efficiency of T4 plating and decrease the plaque size when carried on a high-copy plasmid.Citation56 The toxin is actually not bactericidal to the cell but may play a role in interference with phage propagation by modulating the membrane or preventing mature particle formation. The SOS-induced SymE protein might also contribute to preventing the spread of phage infection if it targets preferentially phage mRNA to inhibit its translation. In addition, any toxin that reduces host metabolism upon phage or infected-cell attack to the natural bacteria cell could cause abortive phage infection or immune response respectively, so this effect could be common for TA systems. It is worth mentioning the evidence that free-living prokaryotes including pathogenic bacteria, which grow very slowly, contain abundant TA loci whereas obligate host-associated intracellular organisms have no TA loci.Citation57 Consequently, the TA systems seem like non-redundant systems and their diversity and multiplicity may be explained by a natural consequence of pathogen-host conflict to increase the diversity and hence fitness to respond to various forms of stimuli.Citation58
Concluding Remarks
Our knowledge of TA systems is still limited but there is increasing evidence that a number of TA modules are harbored in a diverse range of bacteria and archaea.Citation59 Future investigation of the TA systems will provide insights into fascinating questions as why such dispensable multiple units are tolerated in evolution, as well as when and how TA loci are activated to play roles in natural bacterial cell populations. Two approaches should be useful to obtain one more piece of the whole picture; (1) detection of endogenously expressed toxin proteins in a variety of conditions through fusion to a reporter molecule at single-cell resolution by using fluorescence microscopy, since it seems that neither all cells relieve from tight repression of toxin gene expression nor do they show a strong phenotypic effect to be observed by a population handling study, (2) investigation of differences between a wild-type stain and its mutant strain where all known TA modules are deleted or disrupted under conditions in which they may act, for example, phage infection, sudden depletion of nutrient factors, harmful environment and mixed culture with other types of cells. Recently, the biological function(s) of type II TA loci in E. coli K-12 have been investigated using these fundamental approaches, and a common function of type II TA loci in bacterial persistence was proposed.Citation60
In addition, despite the variation in the entire sequences and modes of action of the toxins across type I TA families, these toxin-antitoxin gene pairs seem like a conserved family consisting of a minimum unit of regulatory circuit composed of different but related genes encoded in the same locus. These so called symbiotic relationship systems probably require complementary features to ensure their retention under evolutionary pressure; an intact toxin gene is necessary to maintain a functional antitoxin gene on the genome and an antitoxin gene is indispensable for maintaining cellular activity in the survival of the fittest. Thus, the TA systems might have some advantages in survival, and are sophisticated and diverse enough to adapt to various environmental circumstances. Therefore, it can be expected that revealing the biological role even from one particular TA module might give hints revealing the significance of existence on the genome and common properties of all toxin-antitoxin systems. Continual research introducing novel ideas will give answers to those pressing questions.
Acknowledgments
The author is very much grateful to Gisela Storz for her comments on the manuscript and stimulating discussions to the symE/symR project, and Kenn Gerdes for his improvement on the manuscript and pioneering studies to the hok/sok system. The author also thanks Mori Hirotada for his encouragement to the LDR project and pleasant Sake meeting.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- Mattick JS, Makunin IV. Small regulatory RNAs in mammals. Hum Mol Genet 2005; 14:Spec No 1 R121 - 32; http://dx.doi.org/10.1093/hmg/ddi101; PMID: 15809264
- Storz G, Altuvia S, Wassarman KM. An abundance of RNA regulators. Annu Rev Biochem 2005; 74:199 - 217; http://dx.doi.org/10.1146/annurev.biochem.74.082803.133136; PMID: 15952886
- Storz G, Gottesman S. Versatile roles of small RNA regulators in bacteria. The RNA world 3rd ed 2006; 567-94.
- Beisel CL, Storz G. Base pairing small RNAs and their roles in global regulatory networks. FEMS Microbiol Rev 2010; 34:866 - 82; PMID: 20662934
- Aiba H. Mechanism of RNA silencing by Hfq-binding small RNAs. Curr Opin Microbiol 2007; 10:134 - 9; http://dx.doi.org/10.1016/j.mib.2007.03.010; PMID: 17383928
- Gottesman S, Storz G. Bacterial small RNA regulators: versatile roles and rapidly evolving variations. Cold Spring Harb Perspect Biol 2011; 3:a003798; http://dx.doi.org/10.1101/cshperspect.a003798; PMID: 20980440
- Wagner EG, Altuvia S, Romby P. Antisense RNAs in bacteria and their genetic elements. Adv Genet 2002; 46:361 - 98; http://dx.doi.org/10.1016/S0065-2660(02)46013-0; PMID: 11931231
- Brantl S. Regulatory mechanisms employed by cis-encoded antisense RNAs. Curr Opin Microbiol 2007; 10:102 - 9; http://dx.doi.org/10.1016/j.mib.2007.03.012; PMID: 17387036
- Tomizawa J, Itoh T, Selzer G, Som T. Inhibition of ColE1 RNA primer formation by a plasmid-specified small RNA. Proc Natl Acad Sci U S A 1981; 78:1421 - 5; http://dx.doi.org/10.1073/pnas.78.3.1421; PMID: 6165011
- Gerdes K, Gultyaev AP, Franch T, Pedersen K, Mikkelsen ND. Antisense RNA-regulated programmed cell death. Annu Rev Genet 1997; 31:1 - 31; http://dx.doi.org/10.1146/annurev.genet.31.1.1; PMID: 9442888
- Gerdes K, Rasmussen PB, Molin S. Unique type of plasmid maintenance function: postsegregational killing of plasmid-free cells. Proc Natl Acad Sci U S A 1986; 83:3116 - 20; http://dx.doi.org/10.1073/pnas.83.10.3116; PMID: 3517851
- Yarmolinsky MB. Programmed cell death in bacterial populations. Science 1995; 267:836 - 7; http://dx.doi.org/10.1126/science.7846528; PMID: 7846528
- Pedersen K, Gerdes K. Multiple hok genes on the chromosome of Escherichia coli. Mol Microbiol 1999; 32:1090 - 102; http://dx.doi.org/10.1046/j.1365-2958.1999.01431.x; PMID: 10361310
- Kawano M, Oshima T, Kasai H, Mori H. Molecular characterization of long direct repeat (LDR) sequences expressing a stable mRNA encoding for a 35-amino-acid cell-killing peptide and a cis-encoded small antisense RNA in Escherichia coli. Mol Microbiol 2002; 45:333 - 49; http://dx.doi.org/10.1046/j.1365-2958.2002.03042.x; PMID: 12123448
- Faridani OR, Nikravesh A, Pandey DP, Gerdes K, Good L. Competitive inhibition of natural antisense Sok-RNA interactions activates Hok-mediated cell killing in Escherichia coli. Nucleic Acids Res 2006; 34:5915 - 22; http://dx.doi.org/10.1093/nar/gkl750; PMID: 17065468
- Gerdes K, Wagner EG. RNA antitoxins. Curr Opin Microbiol 2007; 10:117 - 24; http://dx.doi.org/10.1016/j.mib.2007.03.003; PMID: 17376733
- Kawano M, Aravind L, Storz G. An antisense RNA controls synthesis of an SOS-induced toxin evolved from an antitoxin. Mol Microbiol 2007; 64:738 - 54; http://dx.doi.org/10.1111/j.1365-2958.2007.05688.x; PMID: 17462020
- Yamaguchi Y, Park JH, Inouye M. Toxin-antitoxin systems in bacteria and archaea. Annu Rev Genet 2011; 45:61 - 79; http://dx.doi.org/10.1146/annurev-genet-110410-132412; PMID: 22060041
- Hayes F, Van Melderen L. Toxins-antitoxins: diversity, evolution and function. Crit Rev Biochem Mol Biol 2011; 46:386 - 408; http://dx.doi.org/10.3109/10409238.2011.600437; PMID: 21819231
- Gerdes K, Larsen JE, Molin S. Stable inheritance of plasmid R1 requires two different loci. J Bacteriol 1985; 161:292 - 8; PMID: 2981804
- Loh SM, Cram DS, Skurray RA. Nucleotide sequence and transcriptional analysis of a third function (Flm) involved in F-plasmid maintenance. Gene 1988; 66:259 - 68; http://dx.doi.org/10.1016/0378-1119(88)90362-9; PMID: 3049248
- Golub EI, Panzer HA. The F factor of Escherichia coli carries a locus of stable plasmid inheritance stm, similar to the parB locus of plasmid RI. Mol Gen Genet 1988; 214:353 - 7; http://dx.doi.org/10.1007/BF00337735; PMID: 3070354
- Onishi Y. F factor promotes turnover of stable RNA in escherichia coli. Science 1975; 187:257 - 8; http://dx.doi.org/10.1126/science.1089310; PMID: 1089310
- Ohnishi Y, Iguma H, Ono T, Nagaishi H, Clark AJ. Genetic mapping of the F plasmid gene that promotes degradation of stable ribonucleic acid in Escherichia coli. J Bacteriol 1977; 132:784 - 9; PMID: 336605
- Ohnishi Y, Akimoto S. I-like R plasmids promote degradation of stable ribonucleic acid in Escherichia coli. J Bacteriol 1980; 144:833 - 5; PMID: 6159347
- Nielsen AK, Thorsted P, Thisted T, Wagner EG, Gerdes K. The rifampicin-inducible genes srnB from F and pnd from R483 are regulated by antisense RNAs and mediate plasmid maintenance by killing of plasmid-free segregants. Mol Microbiol 1991; 5:1961 - 73; http://dx.doi.org/10.1111/j.1365-2958.1991.tb00818.x; PMID: 1722558
- Gerdes K, Poulsen LK, Thisted T, Nielsen AK, Martinussen J, Andreasen PH. The hok killer gene family in gram-negative bacteria. New Biol 1990; 2:946 - 56; PMID: 2101633
- Gerdes K, Bech FW, Jørgensen ST, Løbner-Olesen A, Rasmussen PB, Atlung T, et al. Mechanism of postsegregational killing by the hok gene product of the parB system of plasmid R1 and its homology with the relF gene product of the E. coli relB operon. EMBO J 1986; 5:2023 - 9; PMID: 3019679
- Gerdes K, Rasmussen PB, Molin S. Unique type of plasmid maintenance function: postsegregational killing of plasmid-free cells. Proc Natl Acad Sci U S A 1986; 83:3116 - 20; http://dx.doi.org/10.1073/pnas.83.10.3116; PMID: 3517851
- Loh SM, Cram DS, Skurray RA. Nucleotide sequence and transcriptional analysis of a third function (Flm) involved in F-plasmid maintenance. Gene 1988; 66:259 - 68; http://dx.doi.org/10.1016/0378-1119(88)90362-9; PMID: 3049248
- Thisted T, Gerdes K. Mechanism of post-segregational killing by the hok/sok system of plasmid R1. Sok antisense RNA regulates hok gene expression indirectly through the overlapping mok gene. J Mol Biol 1992; 223:41 - 54; http://dx.doi.org/10.1016/0022-2836(92)90714-U; PMID: 1370544
- Gerdes K, Helin K, Christensen OW, Løbner-Olesen A. Translational control and differential RNA decay are key elements regulating postsegregational expression of the killer protein encoded by the parB locus of plasmid R1. J Mol Biol 1988; 203:119 - 29; http://dx.doi.org/10.1016/0022-2836(88)90096-4; PMID: 2460630
- Gerdes K, Thisted T, Martinussen J. Mechanism of post-segregational killing by the hok/sok system of plasmid R1: sok antisense RNA regulates formation of a hok mRNA species correlated with killing of plasmid-free cells. Mol Microbiol 1990; 4:1807 - 18; http://dx.doi.org/10.1111/j.1365-2958.1990.tb02029.x; PMID: 1707122
- Franch T, Gultyaev AP, Gerdes K. Programmed cell death by hok/sok of plasmid R1: processing at the hok mRNA 3′-end triggers structural rearrangements that allow translation and antisense RNA binding. J Mol Biol 1997; 273:38 - 51; http://dx.doi.org/10.1006/jmbi.1997.1294; PMID: 9367744
- Thisted T, Gerdes K. Mechanism of post-segregational killing by the hok/sok system of plasmid R1. Sok antisense RNA regulates hok gene expression indirectly through the overlapping mok gene. J Mol Biol 1992; 223:41 - 54; http://dx.doi.org/10.1016/0022-2836(92)90714-U; PMID: 1370544
- Gerdes K, Nielsen A, Thorsted P, Wagner EG. Mechanism of killer gene activation. Antisense RNA-dependent RNase III cleavage ensures rapid turn-over of the stable hok, srnB and pndA effector messenger RNAs. J Mol Biol 1992; 226:637 - 49; http://dx.doi.org/10.1016/0022-2836(92)90621-P; PMID: 1380562
- Thisted T, Nielsen AK, Gerdes K. Mechanism of post-segregational killing: translation of Hok, SrnB and Pnd mRNAs of plasmids R1, F and R483 is activated by 3′-end processing. EMBO J 1994; 13:1950 - 9; PMID: 8168492
- Cohen SN. Surprises at the 3′ end of prokaryotic RNA. Cell 1995; 80:829 - 32; http://dx.doi.org/10.1016/0092-8674(95)90284-8; PMID: 7535193
- Donovan WP, Kushner SR. Polynucleotide phosphorylase and ribonuclease II are required for cell viability and mRNA turnover in Escherichia coli K-12. Proc Natl Acad Sci U S A 1986; 83:120 - 4; http://dx.doi.org/10.1073/pnas.83.1.120; PMID: 2417233
- McLaren RS, Newbury SF, Dance GS, Causton HC, Higgins CF. mRNA degradation by processive 3′-5′ exoribonucleases in vitro and the implications for prokaryotic mRNA decay in vivo. J Mol Biol 1991; 221:81 - 95; PMID: 1920421
- Kawano M, Reynolds AA, Miranda-Rios J, Storz G. Detection of 5′- and 3′-UTR-derived small RNAs and cis-encoded antisense RNAs in Escherichia coli. Nucleic Acids Res 2005; 33:1040 - 50; http://dx.doi.org/10.1093/nar/gki256; PMID: 15718303
- Oshima T, Aiba H, Baba T, Fujita K, Hayashi K, Honjo A, et al. A 718-kb DNA sequence of the Escherichia coli K-12 genome corresponding to the 12.7-28.0 min region on the linkage map (supplement). DNA Res 1996; 3:211 - 23; http://dx.doi.org/10.1093/dnares/3.3.211; PMID: 8905239
- Yoshida T, Obata N, Oosawa K. Color-coding reveals tandem repeats in the Escherichia coli genome. J Mol Biol 2000; 298:343 - 9; http://dx.doi.org/10.1006/jmbi.2000.3667; PMID: 10772854
- Blattner FR, Plunkett G 3rd, Bloch CA, Perna NT, Burland V, Riley M, et al. The complete genome sequence of Escherichia coli K-12. Science 1997; 277:1453 - 62; http://dx.doi.org/10.1126/science.277.5331.1453; PMID: 9278503
- Kawano M, Kanaya S, Oshima T, Masuda Y, Ara T, Mori H. Distribution of repetitive sequences on the leading and lagging strands of the Escherichia coli genome: comparative study of Long Direct Repeat (LDR) sequences. DNA Res 2002; 9:1 - 10; http://dx.doi.org/10.1093/dnares/9.1.1; PMID: 11939563
- Fozo EM, Makarova KS, Shabalina SA, Yutin N, Koonin EV, Storz G. Abundance of type I toxin-antitoxin systems in bacteria: searches for new candidates and discovery of novel families. Nucleic Acids Res 2010; 38:3743 - 59; http://dx.doi.org/10.1093/nar/gkq054; PMID: 20156992
- Fozo EM, Hemm MR, Storz G. Small toxic proteins and the antisense RNAs that repress them. Microbiol Mol Biol Rev 2008; 72:579 - 89; http://dx.doi.org/10.1128/MMBR.00025-08; PMID: 19052321
- Gerdes K, Bech FW, Jørgensen ST, Løbner-Olesen A, Rasmussen PB, Atlung T, et al. Mechanism of postsegregational killing by the hok gene product of the parB system of plasmid R1 and its homology with the relF gene product of the E. coli relB operon. EMBO J 1986; 5:2023 - 9; PMID: 3019679
- Van Melderen L. Toxin-antitoxin systems: why so many, what for?. Curr Opin Microbiol 2010; 13:781 - 5; http://dx.doi.org/10.1016/j.mib.2010.10.006; PMID: 21041110
- Kobayashi I, Nobusato A, Kobayashi-Takahashi N, Uchiyama I. Shaping the genome--restriction-modification systems as mobile genetic elements. Curr Opin Genet Dev 1999; 9:649 - 56; http://dx.doi.org/10.1016/S0959-437X(99)00026-X; PMID: 10607611
- Schmidt H, Hensel M. Pathogenicity islands in bacterial pathogenesis. Clin Microbiol Rev 2004; 17:14 - 56; http://dx.doi.org/10.1128/CMR.17.1.14-56.2004; PMID: 14726454
- Lemos JA, Brown TA Jr., Abranches J, Burne RA. Characteristics of Streptococcus mutans strains lacking the MazEF and RelBE toxin-antitoxin modules. FEMS Microbiol Lett 2005; 253:251 - 7; http://dx.doi.org/10.1016/j.femsle.2005.09.045; PMID: 16243456
- Fozo EM, Hemm MR, Storz G. Small toxic proteins and the antisense RNAs that repress them. Microbiol Mol Biol Rev 2008; 72:579 - 89; http://dx.doi.org/10.1128/MMBR.00025-08; PMID: 19052321
- Hayes F, Van Melderen L. Toxins-antitoxins: diversity, evolution and function. Crit Rev Biochem Mol Biol 2011; 46:386 - 408; http://dx.doi.org/10.3109/10409238.2011.600437; PMID: 21819231
- Pineda M, Gregory BD, Szczypinski B, Baxter KR, Hochschild A, Miller ES, et al. A family of anti-sigma70 proteins in T4-type phages and bacteria that are similar to AsiA, a Transcription inhibitor and co-activator of bacteriophage T4. J Mol Biol 2004; 344:1183 - 97; http://dx.doi.org/10.1016/j.jmb.2004.10.003; PMID: 15561138
- Pecota DC, Wood TK. Exclusion of T4 phage by the hok/sok killer locus from plasmid R1. J Bacteriol 1996; 178:2044 - 50; PMID: 8606182
- Pandey DP, Gerdes K. Toxin-antitoxin loci are highly abundant in free-living but lost from host-associated prokaryotes. Nucleic Acids Res 2005; 33:966 - 76; http://dx.doi.org/10.1093/nar/gki201; PMID: 15718296
- Magnuson RD. Hypothetical functions of toxin-antitoxin systems. J Bacteriol 2007; 189:6089 - 92; http://dx.doi.org/10.1128/JB.00958-07; PMID: 17616596
- Shao Y, Harrison EM, Bi D, Tai C, He X, Ou HY, et al. TADB: a web-based resource for Type 2 toxin-antitoxin loci in bacteria and archaea. Nucleic Acids Res 2011; 39:Database issue D606 - 11; http://dx.doi.org/10.1093/nar/gkq908; PMID: 20929871
- Maisonneuve E, Shakespeare LJ, Jørgensen MG, Gerdes K. Bacterial persistence by RNA endonucleases. Proc Natl Acad Sci U S A 2011; 108:13206 - 11; http://dx.doi.org/10.1073/pnas.1100186108; PMID: 21788497