Abstract
Long non-coding RNAs (lncRNAs) have been found to perform various functions in a wide variety of important biological processes. To make easier interpretation of lncRNA functionality and conduct deep mining on these transcribed sequences, it is convenient to classify lncRNAs into different groups. Here, we summarize classification methods of lncRNAs according to their four major features, namely, genomic location and context, effect exerted on DNA sequences, mechanism of functioning and their targeting mechanism. In combination with the presently available function annotations, we explore potential relationships between different classification categories, and generalize and compare biological features of different lncRNAs within each category. Finally, we present our view on potential further studies. We believe that the classifications of lncRNAs as indicated above are of fundamental importance for lncRNA studies, helpful for further investigation of specific lncRNAs, for formulation of new hypothesis based on different features of lncRNA and for exploration of the underlying lncRNA functional mechanisms.
Introduction
In contrast to a small proportion of the mammalian genome (e.g., human, mouse) that are transcribed into mRNAs, the vast majority of the genome is transcribed into what was previously regarded as “dark matter”—non-coding RNAs (ncRNAs) that do not encode information about proteins.Citation1-Citation5 Among these ncRNAs, long ncRNAs (lncRNAs) represent the most prevalent and functionally diverse class.Citation6-Citation8 There is no definition of lncRNA that is based on biological argumentation and widely accepted in the community. The most commonly used definition is based on the threshold of 200 nucleotides (nt) of the RNA length.Citation6,Citation7,Citation9 It conventionally divides ncRNAs into lncRNAs that have more than 200 nt in length and the remaining ones that are considered “small” RNAs. Small ncRNAs include many different RNAs, such as microRNAs (miRNAs), small nucleolar RNAs (snoRNAs), piwiRNAs (piRNAs) (Taft et al., 2010). Undoubtedly, the definition of lncRNA merely based on length is arbitrary. One attempt to distinguish lncRNAs from small ncRNAs, based more on the biological argumentation is proposed by Amaral et al. defining lncRNAs as those ncRNAs that function either as primary or spliced transcripts, independent of extant known classes of small ncRNAs.Citation10 Therefore, there are some lncRNAs that do not exceed the arbitrary threshold in length (such as BC1and snaR, which are less than or close to 200 nt but included in lncRNAdbCitation10).
LncRNAs are observed in a large diversity of species, including animals,Citation11,Citation12 plants,Citation13 yeast,Citation14 prokaryotesCitation15 and even viruses.Citation16 However, lncRNAs are poorly conserved among different species when compared with the well-studied RNAs (such as mRNAs, miRNAs, snoRNAs),Citation4,Citation17,Citation18 invoking uncertainty about whether a given lncRNA is functional at all due to poor interspecies conservation, or it conveys functional species-specific characteristics. In addition, lncRNAs are usually low expressed,Citation4,Citation19,Citation20 making them to look like more as transcriptional noise. Despite this, a lot of evidence has accumulated showing that lncRNAs play a significant role in a wide variety of important biological processes,Citation21,Citation22 including transcription,Citation23,Citation24 splicing,Citation25,Citation26 translation,Citation27,Citation28 protein localization,Citation29,Citation30 cellular structure integrity,Citation31,Citation32 imprinting,Citation33-Citation35 cell cycleCitation36,Citation37 and apoptosis,Citation16,Citation38 stem cell pluripotencyCitation39 and reprogrammingCitation40 and heat shock response.Citation41,Citation42 It has been suggested that lncRNAs may regulate cancer progressionCitation43 and development of many other human diseases.Citation44 Moreover, a considerable number of lncRNAs are 3′ polyadenylated, 5′ capped, multi-exonicCitation2,Citation4,Citation20 and exhibit transcriptional activation activity similar to that of mRNAs.Citation4,Citation45,Citation46 As a consequence, all these functions and biological features of lncRNAs make them interesting and important research topic.
Recent advances in experimental and computational technologies make it feasible to conduct deep mining on more and more transcribed sequences.Citation2,Citation20,Citation47-Citation49 At present, there are 73,370 lncRNA entries from 1,239 organisms according to NONCODE v3.0Citation50 (a database of literature documented lncRNAs). Conversely, among all these lncRNAs, only a small proportion (less than 200 according to LncRNAdb,Citation10 a database of lncRNA annotation) has been functionally annotated. To better understand their functional significance, it helps to classify lncRNAs into different groups that are useful for exploring their underlying mechanisms of actions, for formulating new hypotheses and for providing insights in differences of such major classes of lncRNAs. Here, we summarize classification methods of lncRNAs according to their different features as discussed in what follows, including their (1) genome location and context, (2) exerted effect on DNA sequences, (3) mechanism of functioning and (4) targeting mechanism. Finally, we provide our perspectives on potential further studies.
Genomic Location and Context
Intergenic lncRNAs and intronic lncRNAs
There is a large number of non-coding regions (accounting for 98–99% in the human genome) interspersed between coding regions.Citation51,Citation52 Since lncRNAs are located and transcribed from different genomic locations, those transcribed from intergenic regions are named intergenic lncRNAs () and, in contrast, those transcribed entirely from introns of protein-coding genes are named intronic lncRNAs ().
Figure 1. Genomic location and context of lncRNAs. Protein-coding genes and their exons are represented by blue color, while lncRNAs and their exons are represented by red color. Panels are mainly based on lncRNA location annotation of GENCODE.Citation4 (A) Intergenic lncRNA, transcribed intergenically from both strands. (B) Intronic lncRNA, transcribed entirely from introns of protein-coding genes. (C) Sense lncRNA, transcribed from the sense strand of protein-coding genes and contain exons from protein-coding genes, overlapping with part of protein-coding genes or covering the entire sequence of a protein-coding gene through an intron. (D) Antisense lncRNA, transcribed from the antisense strand of protein-coding genes, overlapping with exonic or intronic regions or covering the entire protein-coding sequence through an intron.
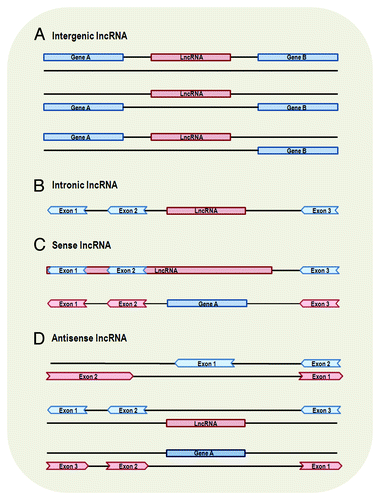
It is suggested that intergenic lncRNAs and intronic lncRNAs are most likely regulated through different transcription activation mechanismsCitation53 and may have different poly(A) modifications and manifest activities in different cellular locations.Citation48 However, only a small portion of intronic lncRNAs has been explored regarding their function. In contrast, there is a large number of long intergenic non-coding RNAs (lincRNAs) that function through different types of mechanisms: cis or trans transcriptional regulation (described below), translational control, splicing regulation, other post-transcriptional regulation, etc. (see ). Also, lincRNAs have been extensively studied about their expression feature and conservation among species.
Table 1. Functional mechanisms and genomic locations of lncRNAs
It is found that lincRNAs are transcriptionally activated similarly to mRNAs,Citation45,Citation46,Citation54,Citation55 as they are more conserved than intronsCitation45,Citation46,Citation55 and antisense transcripts,Citation45 more tissue-specifically expressed than protein-coding genesCitation4,Citation20,Citation45 and more stable than intronic lncRNAsCitation56. “K4-K36” domain (with histone H3K4 trimethylation at their 5′ end and histone H3K36 trimethylation in the body of the gene), an indicator of active transcription in protein-coding genes, is found to prevalently exist in transcriptionally active lincRNAs.Citation45,Citation46,Citation54,Citation55 Approximately 70% of lincRNAs with “K4-K36” domain show evidence of RNA transcription, which is similar to the proportion (~72%) of protein-coding genes.Citation46 Most importantly, nearly 70% of the transcription active domains (K4-K36 domain) of lincRNAs in human are conserved in the orthologous region of mouse, which is comparable to the corresponding proportion (80%) of protein-coding genes.Citation46 In addition, lincRNAs are found to be conserved across multiple vertebrate species.Citation20 The above evidences (active transcription, a degree of domain conservation, tissue-specific expression, stability) strongly indicate the functional importance of lincRNAs. In reality, lincRNAs are found to perform important functions in many cellular processes, from embryonic stem cell pluripotency to cell proliferation and cancer progression.Citation38,Citation46,Citation53,Citation54
Sense and antisense lncRNAs
Sense lncRNAs are transcribed from the sense strand of protein-coding genes, containing exons from protein-coding genes. They may overlap with part of protein-coding genes, or cover the entire sequence of a protein-coding gene (). Antisense lncRNAs, to the contrary, are transcribed from the antisense strand of protein-coding genes. According to GENCODE (a database of manually curated lncRNAs) annotation,Citation4 antisense lncRNAs may appear in three scenarios: (1) transcripts from the antisense strand of protein-coding genes overlap an exon of a sense gene through lncRNAs’ exons, (2) transcripts from the intron of a sense gene do not have exon-exon overlap with this sense gene and (3) transcripts cover the entire sequence of a sense gene through an intron (). Sense and antisense lncRNAs are proved to be genuine transcripts by strand-specific assay or sequencing,Citation45,Citation47 qRT-PCR validationCitation45 and by sequencing 5′ and 3′ ends of full-length cDNACitation2 and, thus, they do not represent truncated CDSs or transcriptional noise. Most of the lncRNAs that come from protein-coding genes or the antisense of the protein-coding genes can be obtained using CAGE (cap-analysis gene expression) and oligo-dT guided reverse transcription, suggesting that they also possess mRNA-like features of 3′ polyadenylation and 5′ capping.Citation2 Moreover, sense and antisense lncRNAs can also be multi-exonic.Citation45,Citation57
Many antisense lncRNAs function through different types of mechanisms (similar to lincRNAs) (). It has been found that as many as 87% coding transcripts have antisense partners in the mouse genomeCitation49 and ~32% of the human lncRNAs are antisense to coding genes,Citation4 suggesting that antisense regulation is likely to be commonly utilized. However, in comparison with lincRNAs and antisense lncRNAs, sense lncRNAs have been less explored for their functions (). It is suggestedCitation4 that most lncRNAs tend not to have protein-coding potential. Intriguingly, some sense lncRNAs are special in the sense that they can function as both RNA and protein-coding gene. For instance, SRA (steroid receptor RNA activator) can translate into protein, and the RNA sequence can also act as a scaffold for several co-activator and repressor proteins to form complexes that regulate gene transcription;Citation58 ENOD40 (early nodulin 40) can translate into proteinsCitation59 and is also needed for correct subcellular localization of RNP particles in legume plants.Citation30 These findings have challenged our understanding of gene classification and, in the meantime, broadened the known roles of lncRNA. Such special relationship between the sense lncRNAs and protein-coding genes may provide novel insights into the evolution of gene function.
Present studies are mainly focused on lincRNAs and antisense lncRNAs (especially lincRNAs), though less is known about intronic lncRNAs and sense lncRNAs. Some biological features of lncRNA are quite prominent, such as the high conservation of lincRNAs among mammals.Citation45,Citation46,Citation55 Also, another evidence, albeit not extensive, may indicate the special feature of the sense lncRNAs’ coding potential.Citation58,Citation59 Additionally, as antisense lncRNAs and sense lncRNAs are correlated differently with coding genes, they are most likely to exert different effects on gene locus or mRNAs. Therefore, genomic location and context can be used for classification of lncRNA, though a classification of lncRNA using exclusively genomic localization and context may not be fully adequate.
Effects Exerted on DNA Sequences
It has been found that lncRNAs are predominately localized in nucleus and chromatin,Citation4 suggesting that lncRNAs may have a significant impact on DNA sequences. Also, a large proportion of lncRNAs are involved in transcriptional regulation (~42% of the 182 assessed entries according to lncRNAdb; ). Therefore, it is meaningful to classify lncRNAs based on their effects exerted on DNA sequences: cis-lncRNAs (cis-acting lncRNAs) that regulate the expression of genes in close genomic proximity and trans-lncRNAs (trans-acting lncRNAs) that regulate the expression of distant genes ().
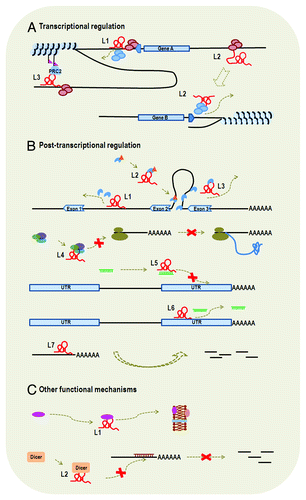
Figure 2. Functional mechanisms of lncRNAs. LncRNA is represented by a letter “L” and a number appended. (A) Transcriptional regulation: We listed examples of cis-lncRNA (L1 and L3) and trans-lncRNA (L2). L1 is transcribed from the promoter region of gene A and its binding to promoter of gene A blocks the binding of transcription factors, thus affecting transcription initiation of gene A. L3 functions to modify chromatin protein in its vicinity through recruiting the complex of PRC2. L2 influences transcription of gene B from a distant region through interaction with transcription factor or RNA polymerase. Therefore, L1 and L2 also function through transcriptional interference, whereas L3 functions through chromatin modification. (B) Post-transcriptional regulation: L1, L2 and L3 all influence gene splicing. Specifically, L1 binds to intronic area to inhibit binding of splicing factor, L2 functions to modulate the pool of modified (such as phosphorylation) splicing factor and L3 binds to splicing factor to block spliceosomal complex formation. L4 interacts with translational factors to inhibit translation. L5 and L6 are two examples of ceRNAs, which interact directly or indirectly with miRNAs. L5 binds to miRNA and, thus, inhibits the binding of miRNA to the 3′ UTR of target mRNA. L6 binds to the 3′ UTR of target mRNA, which also blocks the binding of miRNA to the target gene. L7 serves as natural antisense inhibitor to promote degradation of mRNA. (C) Other functional mechanisms. L1 is involved in protein transportation and L2 binds to Dicer to influence RNA interference.
Cis-lncRNAs
It seems plausible that cis-lncRNAs function through transcriptional interference or chromatin modification, ranging from yeast [e.g., SRG1 (regulatory gene 1) RNAsCitation60,Citation61] to plants [e.g., COLDAIR (cold assisted intronic noncoding RNA) Citation62] to mammals [e.g., DHFR (dihydrofolate reductase) upstream transcripts,Citation23,Citation63 Xist (X inactive-specific transcript)Citation64] (detailed below). Regarding the mechanism of transcriptional interference, lncRNAs may influence the transcription activity of target genes through promoter binding to block PIC (preinitiation complex) formation,Citation23 or by interacting with transcription factorsCitation23,Citation60,Citation61 (). Such cis-lncRNAs may be transcribed from genes’ promoter regions. For instance, DHFR upstream transcripts, ~0.8–7.3 kb lncRNAs from the promoter region of DHFR, can form stable triplex structures with the promoter of DHFRCitation23,Citation63 and interact with TFIIB to efficiently dissociate PIC.Citation23 Likewise, SRG1 RNAs, ~0.4–1.9 kb lncRNAs from the promoter region of SER3 (Ser3p), are found to cover the promoter of SER3 coding gene to prevent transcription factor binding to the promoter and, thus, repress the expression of SER3.Citation60,Citation61 Also, the transcription of lncRNAs in promoter regions may induce chromatin remodeling and, thus, activate the downstream protein-coding genes’ transcription, such as fbp1 promoter RNAs.Citation65
Cis-lncRNAs that function through chromatin modification often recruit chromatin modification complexes, e.g., PRC (polycomb repressive complex) or Rpd3S HDAC (Rpd3 small histone deacetylase complexes). The most studied chromatin modification complex is PRC and one well-known example is Xist (a 19 kb lncRNA in human), which binds to PRC2 to induce H3K27me3 modification and, thus, leads to transcriptional silencing of genes on the X chromosome.Citation64 Similar examples can be found in MEG3 (maternally expressed 3) (a ~1.6 kb lncRNA)Citation66 and COLDAIR (a ~1.1 kb lncRNA).Citation62 Also, there are other chromatin modification complexes that are recruited by lncRNAs. GAL10-ncRNA (Gal10p-noncoding RNA) (a ~4 kb lncRNA) has been reported to recruit the Rpd3S HDAC complex, resulting in a decrease in some histone 3 acetylation to repress the expression of GAL1.Citation14 Although the above-mentioned examples of PRC and Rpd3S HDAC are all implicated in negative regulation of gene expression, interaction of lncRNA with chromatin modification complexes is also involved in positive regulation. A case example is HOTTIP (HOXA transcript at the distal tip) (a ~3.8 kb lncRNA in human) that recruits a MLL chromatin modifying complex to maintain a domain of active chromatin over the 5′ end of HOXA (homeobox A cluster) gene cluster.Citation67
Trans-lncRNAs
Although it may be easier for lncRNAs to influence genes in their immediate vicinity probably based on sequence complimentary to the locus from which they are transcribed, lncRNAs can also function in trans-acting mode to target distant gene loci. For instance, HOTAIR (HOX antisense intergenic RNA), a ~2.2 kb lncRNA that is transcribed from the HOXC (homeobox C cluster) gene locus in chromosome 12, can be transported by the Suz-Twelve protein to regulate the homologous target sites at HOXD (homeobox D cluster) gene locus in chromosome 224. Also, HOTAIR are found to bind to many other genomic loci that tend to possess specific DNA motifs and influence gene expression by recruiting chromatin modification complexes.Citation68,Citation69
Therefore, unlike cis-lncRNAs, trans-lncRNAs may function independently of sequence complementary to target gene locus. In addition to chromatin modification complexes,Citation68,Citation69 they may bind to transcription elongation factorsCitation70,Citation71 or RNA polymerasesCitation41 to affect transcription. It is reported that 7SK RNA (a ~330 bp lncRNA) functions as a central scaffold to coordinate protein-protein interactions in 7SK snRNP (small nuclear ribonucleoproteins), which comprises transcription elongation factor—P-TEFb (positive transcription elongation factor b). This activity consequently leads to repression of transcription elongation at many gene loci.Citation70,Citation71 Another lncRNA, B2 SINE RNA, has been found to stably bind to polymerase II complex to block its activity during heat shock response.Citation41
Mechanisms of Functioning
While only a small number of lncRNAs has been well documented, it is believed that lncRNAs are involved in a wide variety of cellular molecular functions. According to their mechanisms of functioning, lncRNAs roughly fall into three groups that affect transcriptional regulation, post-transcriptional regulation or other functions ().
Transcriptional regulation
As mentioned above, there is a large number of lncRNAs that regulate gene transcription through transcriptional interference (e.g., DHFR upstream transcripts, SRG1 RNAs, 7SK snRNA, B2 SINE RNA) and chromatin remodeling (e.g., fbp1 (fructose-1,6-bisphosphatase-1) promoter RNAs, Xist, MEG3, GAL10-ncRNA, HOTAIR, HOTTIP and COLDAIR). Therefore, lncRNAs responsible for transcription regulation can be sub-divided according to the mechanism of their functioning: (1) transcriptional interference and (2) chromatin remodeling (). Besides, there are other related functional mechanisms, for example, the regulation effect: a set of lncRNAs transcribed from enhancers are termed eRNAs (enhancer RNAs) as they positively regulate genes’ transcription, such as ncRNA-a1 (activating long ncRNA 1),Citation54 Evf-2 (embryonic ventral forebrain-2) RNA,Citation72 Alpha-250/Alpha-280.Citation73
Post-transcriptional regulation
There are two common post-transcriptional regulation mechanisms that lncRNAs get involved in, namely, splicing regulation and translational control (). LncRNAs that influence mRNA splicing may function through binding toCitation74 or modulatingCitation26 splicing factors, or directly hybridizing with mRNA sequences to block splicing.Citation25,Citation28 MIAT (myocardial infarction associated transcript), a ~9–10 kb lncRNA, contains strong intron branch point sequences (UACUAAC repeats) and is able to bind to SF1 (splicing factor 1) to inhibit splicing and spliceosomal complex formation.Citation74 Malat1 (metastasis-associated lung adenocarcinoma transcript 1), a ~7 kb lncRNA, can bind to SR splicing factor [serine–arginine (SR)-rich splicing factor] and regulate its distribution in nuclear speckle domains.Citation26 Also, it is suggested that Malat1 may modulate the pools of phosphorylated SR and, thus, influence alternative splicing of pre-mRNAs.Citation26 Additionally, other splicing regulation mechanisms may exist. LUST (LUCA-15-specific transcript), a ~1.4–2.4 kb lncRNA, is the antisense transcript of RBM5 (RNA binding motif protein 5) and is hypothesized to regulate the expression of RBM5 splice variants through masking a sense-strand regulatory sequence.Citation25
LncRNAs that participate in translational control may function through binding to translation factorsCitation27,Citation75 or ribosome.Citation76,Citation77 There are two lncRNAs, BC1 (brain cytoplasmic RNA 1) and BC200 (200 nt brain cytoplasmic RNA), which can bind eIF4A (eukaryotic translation initiation factor 4A), PABP (poly(A)-binding protein) and other factors, to repress translation initiation by blocking assembly of the required complex.Citation27,Citation75 snaR (small NF90-associated RNAs), a cytoplasmic lncRNAs, can bind to ribosome, presumably influencing translation of mRNAs.Citation76 Gadd7 (growth arrested DNA-damage inducible gene 7), which is associated with active translation, is hypothesized to bind to ribosome.Citation77 It should be noted that in some cases translation and splicing are associated with each other. Zeb2 (zinc finger E-box-binding homeobox 2) translation requires retention of an intron. Zeb2NAT (Zeb2 natural antisense transcript) (a lncRNA that is more than 1.2 kb), which overlaps the 5′ splicing site of an intron, can inhibit splicing of the intron to allow translation of Zeb2.Citation28
Aside from splicing regulation and translational control, there are other post-transcriptional regulation mechanisms utilized by lncRNAs. The findings of siRNA (small interfering RNA) mechanismCitation78,Citation79 and competing endogenous RNAsCitation80-Citation83 have opened up new aspects of post-transcriptional regulation and recent studies suggest that lncRNAs are also implicated in these processes, displaying direct siRNA mechanismsCitation78,Citation79 or interacting with miRNAs.Citation80-Citation83
LncRNAs may function as natural antisense inhibitors to promote degradation of mRNACitation78,Citation79 (). It has been found that 21A, a ~300 bp lncRNA, which shows high sequence homology to CENP-F (centromere protein F) intronic portions, can reduce CENP-F expression at both mRNA and protein level through antisense inhibitor.Citation78 1/2-sbsRNA1 (half-STAU1-binding site RNA1), a ~0.7 kb lncRNA, has been found to bind to mRNAs’ 3′ UTR through Alu elements, and reduces mRNA abundance.Citation79
Moreover, there are many lncRNAs that interact directly or indirectly with miRNAs to stabilize target mRNAsCitation80-Citation83 (). These lncRNAs are called ceRNAs (competing endogenous RNAs).Citation80-Citation83 For instance, linc-MD1 (long intergenic ncRNA that is associated with muscle differentiation), a ~0.5 kb lncRNA, acts as sponge/target mimic of miR-133 and miR-135 to regulate the expression of two transcription factors: MAML1 (mastermind-like protein 1) and MEF2C (myocyte-specific enhancer factor 2C), which activate the expression of muscle-specific genes.Citation80 Similar examples can be found in IPS1 (induced by phosphate starvation 1) RNACitation84 and HULC (highly up-reglated in liver cancer) RNA.Citation85 Some pseudogenes can function as a sponge/target mimic for miRNAs to stabilize their homologous mRNAs, such as KRASP1 and PTENP1, which are pseudogenes of KRAS (V-Ki-ras2 Kirsten rat sarcoma viral oncogene homolog) and PTEN (phosphatase and tensin homolog), respectively.Citation83,Citation86 In addition, some antisense lncRNAs may bind to mRNA to mask the binding sites of miRNA and thus stabilize mRNA. BACE1AS (BACE1 antisense RNA), a ~2 kb lncRNA, has been reported to be activated in Alzheimer disease to form an RNA duplex with BACE1 (β-secretase 1) mRNA,Citation87 which may mask the binding site for miR-485-5p and, thus, prevent translational repression of BACE1 mRNA by miRNA.Citation88
Other mechanisms of lncRNA functioning
In addition to transcriptional regulation and post-transcriptional regulation, lncRNAs may function through other mechanisms (), such as protein localization,Citation29,Citation30 telomere replication,Citation89 RNA interference,Citation90 beyond transcription and translation regulation, etc. Considering the limitation of the currently available knowledge, it is difficult to categorize these lncRNAs into some more “stable” groups and, thus, we roughly place them into lncRNAs with “other functional mechanisms.” For instance, meiRNA, a ~0.5 kb lncRNA, is required for nuclear localization of Mei2.Citation29 ENOD40 RNA is required for correct subcellular localization of RNP particles in legume plants.Citation30 TERC (telomerase RNA component), which is part of telomerase reverse transcriptase, acts as template to extend telomere during DNA replication in eukaryote.Citation89 In ciliated protozoa, maternal RNA templates have been found to guide reproducible rearrangement during the transition from germline nucleus to somatic nucleus.Citation91 In addition, lncRNAs may be involved in RNA interference by regulation of Dicer1. It is suggested that rncs-1 (RNA noncoding and starvation upregulated) reduces Dicer-generated siRNA and affects levels of Dicer-regulated genes.Citation90
Targeting Mechanisms of lncRNAs
According to their mode of action, lncRNAs may also be classified based on their targeting mechanisms, mainly associated with the following categoriesCitation92: (1) signal: show cell type-specific expression and respond to diverse stimuli, such as Xist,Citation64 COLDAIRCitation62; (2) decoy: bind and titrate away a protein target, but does not exert any additional functions, such as DHFR upstream transcripts,Citation23 PANDA;Citation93 (3) guide: bind proteins and then direct the localization of ribonucleoprotein complex to specific targets, such as Xist,Citation64 HOTAIR;Citation24,Citation68 (4) scaffold: serve as central platforms to bring together multiple proteins to form ribonucleoprotein complexes, such as HOTAIR,Citation24,Citation68 7SL.Citation94,Citation95 Alternatively, lncRNAs can be grouped based on the types of interactions they make with their targets: RNA-RNA pairings, RNA-DNA hybrids, RNA structure mediated interactions and protein linkers.Citation96
However, a single targeting archetype in referring to mode of action may not be sufficient to fully describe one lncRNA since one lncRNA may contain multiple archetypes. There are many lncRNAs that are induced by endogenous and exogenous signals to express, and they also can possess the binding sites of chromatin modification complexes (such as PRC) to repress or activate expression of a set of genes, e.g., Xist,Citation64 Air,Citation97 COLDAIR,Citation62 HOTTIP,Citation67 HOTAIR,Citation24,Citation68 lincRNA-p21.Citation38 Therefore, these lncRNAs operate in a dual mode as both signal and guide. Some signal lncRNAs may also function as decoys that bind and titrate away a protein target, e.g., PANDA.Citation93 More complexly, some lncRNAs have more than two archetypes, such as HOTAIR that functions according to three archetypes: as anatomic signal, guiding the chromatin-modifying complexes to the target gene, and as a scaffold for PRC2 and LSD1.Citation24,Citation68
Albeit, there are multiple groups of lncRNAs based on different classification methods as mentioned above, different groups appear to be linked closely to one another. LncRNAs that function via transcriptional interference mechanism may target gene loci through RNA-DNA hybrids and operate as the archetypes of decoy, e.g., DHFR upstream transcripts;Citation23 lncRNAs that affect chromatin modification may target gene loci through RNA structure mediated interaction, which, thus, acts as the archetypes of signal, guide and scaffold, e.g., HOTAIR;Citation24,Citation68 ceRNAs that directly or indirectly regulate mRNA level target miRNAs or mRNAs through RNA-RNA pairing, may also operate as the archetype of decoy, for example, KRASP1 and PTENP1;Citation86 lncRNAs that regulate splicing may function through RNA-RNA pairing to influence their targets and operate as the archetype of decoy (e.g., Malat1Citation26).
Perspectives
Here, we summarized classification methods of lncRNAs according to their four major characteristics (genomic location and context, effects on DNA sequences, functional mechanisms and targeting mechanisms). Based on function annotations presently available, we explored potential relationships between different classification categories and investigated biological features of different lncRNAs within each category. Although lncRNA could be described in terms of other features, such classifications of lncRNAs as summarized here are of fundamental importance for lncRNA studies, helpful for further investigation of specific groups of lncRNA, for generation of new hypothesis based on different lncRNA groups and for exploration of lncRNA underlying functional mechanisms.
Classification of lncRNAs
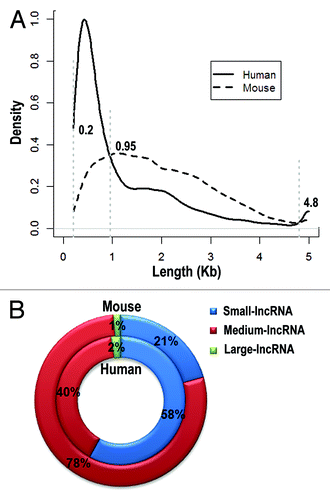
When studying lncRNAs, it is straightforward to investigate their functional features by classifying them into different groups. However, classification of lncRNAs is highly dependent on the current existing knowledge, thus requiring frequent validation of the classification system, exploring new classification systems and, when necessary, abandoning old ones. One possible example is the definition of lncRNA that is longer than 200 nt. We investigated the length of lncRNAs and compared their distribution between human and mouse (data from NONCODE V3.0Citation50). Clearly, lncRNAs can be further divided into different groups based on their length distribution (): small-lncRNA (200~950 nt), medium-lncRNA (950~4,800 nt), large-lncRNA (4,800 nt~). In human, the majority of lncRNAs are small-lncRNAs (58%), whereas the majority of lncRNAs in mouse are medium-lncRNAs (78%). In addition, human genome contains more small-lncRNAs and large-lncRNAs than mouse, but less medium-lncRNAs. However, the number of manually annotated lncRNAs by GENCODE released recently is less than half of NONCODE V3.0,Citation4,Citation50 suggesting the uncertainty of the comparative results. While the differences could be due to the annotation levels, if proved to reflect the genuine situation, such differences may imply distinct evolutionary process of lncRNAs between human and mouse and need further evolutionary analysis.
Figure 3. Length distribution of lncRNAs in human and mouse. LncRNAs are divided into three groups based on their length distribution: small-lncRNA (200~950 nt), medium-lncRNA (950~4,800 nt) and large-lncRNA (4,800 nt~). Density distributions of lncRNA length are shown in (A) and percentages of three lncRNA groups are depicted in (B).
Investigation of specific groups of lncRNAs
Classification of lncRNAs is useful for focusing on specific groups. Currently, lincRNAs that recruit chromatin modification complexes have attracted a lot of attention. It is reported that lincRNAs tend to bind to specific chromatin-modifying complexes,Citation55 so that lincRNA-associated chromatin modification may represent a specific regulation mechanism. Additionally, lincRNAs recruiting the same chromatin-modifying complex may diverge greatly between different species.Citation66 Therefore, lincRNAs may affect specific variety of target genes in different biological processes, such as PCAT-1, which regulates a variety of prostate cancer implicated genes through recruiting PRC2.Citation53 These findings shed lights on the functional significance of the lincRNA group (that functions through recruiting PRC2) and stimulate further work on other chromatin-modifying complexes. Notably, there is a large number of antisense lncRNAs, which function through recruiting chromatin modification complexes to regulate transcription activity according to available evidences (). It has been suggested that most of the PRC2 binding transcripts are antisense and sense lncRNAs rather than lincRNAs.Citation66 Therefore, it would be desirable for future studies to explore more chromatin complex binding features of the antisense and sense lncRNAs.
Regulation network of lncRNA and small ncRNA
The discovery of lncRNAs and their regulatory roles challenges the initially miRNA-centered regulatory networks, which may help comprehensively understand gene regulation by both small ncRNA and lncRNA and, accordingly, provide new insights into complex processes of gene regulation. As mentioned above, ceRNAs, a group of lncRNAs interacting with miRNAs, can impose an additional level on post-transcriptional regulation.Citation80-Citation88 In addition to those lncRNAs that act as sponge/target mimic of miRNAs,Citation80-Citation86 it should be noted that more than 50% of the sense and antisense transcript pairs may be composed of coding and antisense non-coding transcripts,Citation49 and the formed RNA duplex may also influence the interaction between miRNAs and their target mRNAs.Citation87,Citation88 According to our previous integrative analysis of miRNAs and mRNAs in NSCLC (non-small cell lung carcinoma),Citation98 we found that most of the target mRNAs do not vary significantly, in spite of the dramatic increase or decrease of their miRNAs, indicating that gene regulation network may be more sophisticated.
Considering their similar background in gene expression and function overlap between lncRNA and small ncRNA, it is attractive to investigate whether there is a certain evolutionary association between the two components. It is found that some lncRNAs contain miRNAs in their gene locus.Citation99-Citation101 H19 (gene comes from colon pH19), a ~2.3 kb lncRNA, contains mir-675 in its exon and also serves as a precursor of this miRNA in addition to its transcription regulation activity.Citation99 Also, lncRNA LOC554202 contains mir-31 in its intron, and both miR-31 and the host lncRNA are found to be lowly expressed in triple-negative breast cancer.Citation100 It has been found that five lncRNAs, namely, MEG3, MEG8 (maternally expressed 3), MEG9 (maternally expressed 3), antiPeg11 (antisense transcript to Peg11/Rtl1) and Rian (RNA imprinted and accumulated in nucleus), contain a lot of miRNAs and snoRNAs and function through transcriptional regulation; interestingly, these five lncRNAs and their endogenous small ncRNA may target the same gene,Citation101 but it remains unclear whether those five lncRNAs can serve as precursors of their corresponding miRNAs. In addition, it is suggested that some lncRNAs in human may be preferentially post-processed into snoRNAs.Citation4,Citation102 Taken together, future studies focused on this aspect may bring new unexpected insights into the evolutionary relationships between small ncRNA and lncRNA.
| Abbreviations: | ||
| lncRNAs | = | long non-coding RNAs |
| miRNAs | = | microRNAs |
| snoRNAs | = | small nucleolar RNAs |
| piRNAs | = | piwiRNAs |
| lincRNAs | = | long intergenic non-coding RNAs |
| CAGE | = | cap-analysis gene expression |
| SRA | = | steroid receptor RNA activator |
| ENOD40 | = | early nodulin 40 |
| SRG1 | = | SER3 regulatory gene 1 |
| COLDAIR | = | cold assisted intronic noncoding RNA |
| DHFR | = | dihydrofolate reductase |
| Xist | = | X inactive-specific transcript |
| SER3 | = | Ser3p |
| PRC | = | polycomb repressive complex |
| Rpd3S HDAC | = | Rpd3 small histone deacetylase complexes |
| MEG3/8/9 | = | maternally expressed 3/8/9 |
| GAL10-ncRNA | = | Gal10p-noncoding RNA |
| HOTTIP | = | HOXA transcript at the distal tip |
| HOXA | = | homeobox A cluster |
| HOTAIR | = | HOX antisense intergenic RNA |
| HOXC | = | homeobox C cluster |
| HOXD | = | homeobox D cluster |
| snRNP | = | small nuclear ribonucleoproteins |
| P-TEFb | = | positive transcription elongation factor b |
| B2 SINE RNA | = | B2 short interspersed element RNA |
| fbp1 | = | fructose-1,6-bisphosphatase-1 |
| eRNAs | = | ncRNA-a1, activating long ncRNA 1 |
| Evf-2 | = | embryonic ventral forebrain-2 |
| MIAT | = | myocardial infarction associated transcript |
| SF1 | = | splicing factor 1 |
| Malat1 | = | metastasis-associated lung adenocarcinoma transcript 1 |
| SR splicing factor | = | serine–arginine (SR)-rich splicing factor |
| LUST | = | LUCA-15-specific transcript |
| RBM5 | = | RNA binding motif protein 5 |
| BC1 | = | brain cytoplasmic RNA 1 |
| BC200 | = | 200nt brain cytoplasmic RNA |
| eIF4A | = | eukaryotic translation initiation factor 4A |
| PABP | = | poly(A)-binding protein |
| Gadd7 | = | growth arrested DNA-damage inducible gene 7 |
| snaR | = | small NF90-associated RNAs |
| Zeb2 | = | zinc finger E-box-binding homeobox 2 |
| Zeb2NAT | = | Zeb2 natural antisense transcript |
| siRNA | = | small interfering RNA |
| CENP-F | = | centromere protein F |
| 1/2-sbsRNA1 | = | half-STAU1-binding site RNA1 |
| ceRNAs | = | competing endogenous RNAs |
| linc-MD1 | = | long intergenic ncRNA that is associated with muscle differentiation |
| MAML1 | = | mastermind-like protein 1 |
| MEF2C | = | myocyte-specific enhancer factor 2C |
| IPS1 | = | induced by phosphate starvation 1 |
| HULC | = | highly up-reglated in liver cancer |
| KRAS | = | V-Ki-ras2 Kirsten rat sarcoma viral oncogene homolog |
| PTEN | = | phosphatase and tensin homolog |
| BACE1 | = | beta-secretase 1 |
| BACE1AS | = | BACE1 antisense RNA |
| Mei2 | = | meiosis protein 2 |
| TERC | = | telomerase RNA component |
| rncs-1 | = | RNA noncoding and starvation up-regulated |
| NSCLC | = | non-small cell lung carcinoma |
| H19 | = | gene comes from colon pH19 |
| Rian | = | RNA imprinted and accumulated in nucleus |
| antiPeg11 | = | antisense transcript to Peg11/Rtl1 |
Acknowledgments
We thank Hao Wu and Gang Wu for their valuable comments on this manuscript. This work was supported by grants from National Natural Science Foundation of China (Grant No. 31200978 to L.M.), King Abdullah University of Science and Technology research funds (to V.B.B.), the “100-Talent Program” of Chinese Academy of Sciences (Y1SLXb1365 to Z.Z.) and National Programs for High Technology Research and Development (863 Program; Grant No. 2012AA020409 to Z.Z.), the Ministry of Science and Technology of the People’s Republic of China.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- Okazaki Y, Furuno M, Kasukawa T, Adachi J, Bono H, Kondo S, et al, FANTOM Consortium, RIKEN Genome Exploration Research Group Phase I & II Team. Analysis of the mouse transcriptome based on functional annotation of 60,770 full-length cDNAs. Nature 2002; 420:563 - 73; http://dx.doi.org/10.1038/nature01266; PMID: 12466851
- Carninci P, Kasukawa T, Katayama S, Gough J, Frith MC, Maeda N, et al, FANTOM Consortium, RIKEN Genome Exploration Research Group and Genome Science Group (Genome Network Project Core Group). The transcriptional landscape of the mammalian genome. Science 2005; 309:1559 - 63; http://dx.doi.org/10.1126/science.1112014; PMID: 16141072
- Johnson JM, Edwards S, Shoemaker D, Schadt EE. Dark matter in the genome: evidence of widespread transcription detected by microarray tiling experiments. Trends Genet 2005; 21:93 - 102; http://dx.doi.org/10.1016/j.tig.2004.12.009; PMID: 15661355
- Derrien T, Johnson R, Bussotti G, Tanzer A, Djebali S, Tilgner H, et al. The GENCODE v7 catalog of human long noncoding RNAs: analysis of their gene structure, evolution, and expression. Genome Res 2012; 22:1775 - 89; http://dx.doi.org/10.1101/gr.132159.111; PMID: 22955988
- Bánfai B, Jia H, Khatun J, Wood E, Risk B, Gundling WE Jr., et al. Long noncoding RNAs are rarely translated in two human cell lines. Genome Res 2012; 22:1646 - 57; http://dx.doi.org/10.1101/gr.134767.111; PMID: 22955977
- Mercer TR, Dinger ME, Mattick JS. Long non-coding RNAs: insights into functions. Nat Rev Genet 2009; 10:155 - 9; http://dx.doi.org/10.1038/nrg2521; PMID: 19188922
- Wilusz JE, Sunwoo H, Spector DL. Long noncoding RNAs: functional surprises from the RNA world. Genes Dev 2009; 23:1494 - 504; http://dx.doi.org/10.1101/gad.1800909; PMID: 19571179
- Taft RJ, Pang KC, Mercer TR, Dinger M, Mattick JS. Non-coding RNAs: regulators of disease. J Pathol 2010; 220:126 - 39; http://dx.doi.org/10.1002/path.2638; PMID: 19882673
- Kapranov P, Cheng J, Dike S, Nix DA, Duttagupta R, Willingham AT, et al. RNA maps reveal new RNA classes and a possible function for pervasive transcription. Science 2007; 316:1484 - 8; http://dx.doi.org/10.1126/science.1138341; PMID: 17510325
- Amaral PP, Clark MB, Gascoigne DK, Dinger ME, Mattick JS. lncRNAdb: a reference database for long noncoding RNAs. Nucleic Acids Res 2011; 39:Database issue D146 - 51; http://dx.doi.org/10.1093/nar/gkq1138; PMID: 21112873
- Brown CJ, Hendrich BD, Rupert JL, Lafrenière RG, Xing Y, Lawrence J, et al. The human XIST gene: analysis of a 17 kb inactive X-specific RNA that contains conserved repeats and is highly localized within the nucleus. Cell 1992; 71:527 - 42; http://dx.doi.org/10.1016/0092-8674(92)90520-M; PMID: 1423611
- Clemson CM, McNeil JA, Willard HF, Lawrence JB. XIST RNA paints the inactive X chromosome at interphase: evidence for a novel RNA involved in nuclear/chromosome structure. J Cell Biol 1996; 132:259 - 75; http://dx.doi.org/10.1083/jcb.132.3.259; PMID: 8636206
- Swiezewski S, Liu F, Magusin A, Dean C. Cold-induced silencing by long antisense transcripts of an Arabidopsis Polycomb target. Nature 2009; 462:799 - 802; http://dx.doi.org/10.1038/nature08618; PMID: 20010688
- Houseley J, Rubbi L, Grunstein M, Tollervey D, Vogelauer M. A ncRNA modulates histone modification and mRNA induction in the yeast GAL gene cluster. Mol Cell 2008; 32:685 - 95; http://dx.doi.org/10.1016/j.molcel.2008.09.027; PMID: 19061643
- Bernstein HD, Zopf D, Freymann DM, Walter P. Functional substitution of the signal recognition particle 54-kDa subunit by its Escherichia coli homolog. Proc Natl Acad Sci U S A 1993; 90:5229 - 33; http://dx.doi.org/10.1073/pnas.90.11.5229; PMID: 8389475
- Reeves MB, Davies AA, McSharry BP, Wilkinson GW, Sinclair JH. Complex I binding by a virally encoded RNA regulates mitochondria-induced cell death. Science 2007; 316:1345 - 8; http://dx.doi.org/10.1126/science.1142984; PMID: 17540903
- Pang KC, Frith MC, Mattick JS. Rapid evolution of noncoding RNAs: lack of conservation does not mean lack of function. Trends Genet 2006; 22:1 - 5; http://dx.doi.org/10.1016/j.tig.2005.10.003; PMID: 16290135
- Wang J, Zhang J, Zheng H, Li J, Liu D, Li H, et al. Mouse transcriptome: neutral evolution of 'non-coding' complementary DNAs. Nature 2004; 431:757; http://dx.doi.org/10.1038/nature03016; PMID: 15483594
- van Bakel H, Nislow C, Blencowe BJ, Hughes TR. Most “dark matter” transcripts are associated with known genes. PLoS Biol 2010; 8:e1000371; http://dx.doi.org/10.1371/journal.pbio.1000371; PMID: 20502517
- Cabili MN, Trapnell C, Goff L, Koziol M, Tazon-Vega B, Regev A, et al. Integrative annotation of human large intergenic noncoding RNAs reveals global properties and specific subclasses. Genes Dev 2011; 25:1915 - 27; http://dx.doi.org/10.1101/gad.17446611; PMID: 21890647
- Clark MB, Mattick JS. Long noncoding RNAs in cell biology. Semin Cell Dev Biol 2011; 22:366 - 76; http://dx.doi.org/10.1016/j.semcdb.2011.01.001; PMID: 21256239
- Ponting CP, Oliver PL, Reik W. Evolution and functions of long noncoding RNAs. Cell 2009; 136:629 - 41; http://dx.doi.org/10.1016/j.cell.2009.02.006; PMID: 19239885
- Martianov I, Ramadass A, Serra Barros A, Chow N, Akoulitchev A. Repression of the human dihydrofolate reductase gene by a non-coding interfering transcript. Nature 2007; 445:666 - 70; http://dx.doi.org/10.1038/nature05519; PMID: 17237763
- Rinn JL, Kertesz M, Wang JK, Squazzo SL, Xu X, Brugmann SA, et al. Functional demarcation of active and silent chromatin domains in human HOX loci by noncoding RNAs. Cell 2007; 129:1311 - 23; http://dx.doi.org/10.1016/j.cell.2007.05.022; PMID: 17604720
- Rintala-Maki ND, Sutherland LC. Identification and characterisation of a novel antisense non-coding RNA from the RBM5 gene locus. Gene 2009; 445:7 - 16; http://dx.doi.org/10.1016/j.gene.2009.06.009; PMID: 19559772
- Tripathi V, Ellis JD, Shen Z, Song DY, Pan Q, Watt AT, et al. The nuclear-retained noncoding RNA MALAT1 regulates alternative splicing by modulating SR splicing factor phosphorylation. Mol Cell 2010; 39:925 - 38; http://dx.doi.org/10.1016/j.molcel.2010.08.011; PMID: 20797886
- Muddashetty R, Khanam T, Kondrashov A, Bundman M, Iacoangeli A, Kremerskothen J, et al. Poly(A)-binding protein is associated with neuronal BC1 and BC200 ribonucleoprotein particles. J Mol Biol 2002; 321:433 - 45; http://dx.doi.org/10.1016/S0022-2836(02)00655-1; PMID: 12162957
- Beltran M, Puig I, Peña C, García JM, Alvarez AB, Peña R, et al. A natural antisense transcript regulates Zeb2/Sip1 gene expression during Snail1-induced epithelial-mesenchymal transition. Genes Dev 2008; 22:756 - 69; http://dx.doi.org/10.1101/gad.455708; PMID: 18347095
- Watanabe Y, Yamamoto MS. S. pombe mei2+ encodes an RNA-binding protein essential for premeiotic DNA synthesis and meiosis I, which cooperates with a novel RNA species meiRNA. Cell 1994; 78:487 - 98; http://dx.doi.org/10.1016/0092-8674(94)90426-X; PMID: 7520368
- Campalans A, Kondorosi A, Crespi M. Enod40, a short open reading frame-containing mRNA, induces cytoplasmic localization of a nuclear RNA binding protein in Medicago truncatula.. Plant Cell 2004; 16:1047 - 59; http://dx.doi.org/10.1105/tpc.019406; PMID: 15037734
- Kloc M, Wilk K, Vargas D, Shirato Y, Bilinski S, Etkin LD. Potential structural role of non-coding and coding RNAs in the organization of the cytoskeleton at the vegetal cortex of Xenopus oocytes. Development 2005; 132:3445 - 57; http://dx.doi.org/10.1242/dev.01919; PMID: 16000384
- Sunwoo H, Dinger ME, Wilusz JE, Amaral PP, Mattick JS, Spector DL. MEN epsilon/beta nuclear-retained non-coding RNAs are up-regulated upon muscle differentiation and are essential components of paraspeckles. Genome Res 2009; 19:347 - 59; http://dx.doi.org/10.1101/gr.087775.108; PMID: 19106332
- Brockdorff N, Ashworth A, Kay GF, Cooper P, Smith S, McCabe VM, et al. Conservation of position and exclusive expression of mouse Xist from the inactive X chromosome. Nature 1991; 351:329 - 31; http://dx.doi.org/10.1038/351329a0; PMID: 2034279
- Brown CJ, Ballabio A, Rupert JL, Lafreniere RG, Grompe M, Tonlorenzi R, et al. A gene from the region of the human X inactivation centre is expressed exclusively from the inactive X chromosome. Nature 1991; 349:38 - 44; http://dx.doi.org/10.1038/349038a0; PMID: 1985261
- Bartolomei MS, Zemel S, Tilghman SM. Parental imprinting of the mouse H19 gene. Nature 1991; 351:153 - 5; http://dx.doi.org/10.1038/351153a0; PMID: 1709450
- Ginger MR, Shore AN, Contreras A, Rijnkels M, Miller J, Gonzalez-Rimbau MF, et al. A noncoding RNA is a potential marker of cell fate during mammary gland development. Proc Natl Acad Sci U S A 2006; 103:5781 - 6; http://dx.doi.org/10.1073/pnas.0600745103; PMID: 16574773
- Mourtada-Maarabouni M, Hedge VL, Kirkham L, Farzaneh F, Williams GT. Growth arrest in human T-cells is controlled by the non-coding RNA growth-arrest-specific transcript 5 (GAS5). J Cell Sci 2008; 121:939 - 46; http://dx.doi.org/10.1242/jcs.024646; PMID: 18354083
- Huarte M, Guttman M, Feldser D, Garber M, Koziol MJ, Kenzelmann-Broz D, et al. A large intergenic noncoding RNA induced by p53 mediates global gene repression in the p53 response. Cell 2010; 142:409 - 19; http://dx.doi.org/10.1016/j.cell.2010.06.040; PMID: 20673990
- Sheik Mohamed J, Gaughwin PM, Lim B, Robson P, Lipovich L. Conserved long noncoding RNAs transcriptionally regulated by Oct4 and Nanog modulate pluripotency in mouse embryonic stem cells. RNA 2010; 16:324 - 37; http://dx.doi.org/10.1261/rna.1441510; PMID: 20026622
- Loewer S, Cabili MN, Guttman M, Loh YH, Thomas K, Park IH, et al. Large intergenic non-coding RNA-RoR modulates reprogramming of human induced pluripotent stem cells. Nat Genet 2010; 42:1113 - 7; http://dx.doi.org/10.1038/ng.710; PMID: 21057500
- Espinoza CA, Allen TA, Hieb AR, Kugel JF, Goodrich JA. B2 RNA binds directly to RNA polymerase II to repress transcript synthesis. Nat Struct Mol Biol 2004; 11:822 - 9; http://dx.doi.org/10.1038/nsmb812; PMID: 15300239
- Shamovsky I, Ivannikov M, Kandel ES, Gershon D, Nudler E. RNA-mediated response to heat shock in mammalian cells. Nature 2006; 440:556 - 60; http://dx.doi.org/10.1038/nature04518; PMID: 16554823
- Tano K, Akimitsu N. Long non-coding RNAs in cancer progression. Front Genet 2012; 3:219; http://dx.doi.org/10.3389/fgene.2012.00219; PMID: 23109937
- Wapinski O, Chang HY. Long noncoding RNAs and human disease. Trends Cell Biol 2011; 21:354 - 61; http://dx.doi.org/10.1016/j.tcb.2011.04.001; PMID: 21550244
- Guttman M, Garber M, Levin JZ, Donaghey J, Robinson J, Adiconis X, et al. Ab initio reconstruction of cell type-specific transcriptomes in mouse reveals the conserved multi-exonic structure of lincRNAs. Nat Biotechnol 2010; 28:503 - 10; http://dx.doi.org/10.1038/nbt.1633; PMID: 20436462
- Guttman M, Amit I, Garber M, French C, Lin MF, Feldser D, et al. Chromatin signature reveals over a thousand highly conserved large non-coding RNAs in mammals. Nature 2009; 458:223 - 7; http://dx.doi.org/10.1038/nature07672; PMID: 19182780
- Kampa D, Cheng J, Kapranov P, Yamanaka M, Brubaker S, Cawley S, et al. Novel RNAs identified from an in-depth analysis of the transcriptome of human chromosomes 21 and 22. Genome Res 2004; 14:331 - 42; http://dx.doi.org/10.1101/gr.2094104; PMID: 14993201
- Cheng J, Kapranov P, Drenkow J, Dike S, Brubaker S, Patel S, et al. Transcriptional maps of 10 human chromosomes at 5-nucleotide resolution. Science 2005; 308:1149 - 54; http://dx.doi.org/10.1126/science.1108625; PMID: 15790807
- Katayama S, Tomaru Y, Kasukawa T, Waki K, Nakanishi M, Nakamura M, et al, RIKEN Genome Exploration Research Group, Genome Science Group (Genome Network Project Core Group), FANTOM Consortium. Antisense transcription in the mammalian transcriptome. Science 2005; 309:1564 - 6; http://dx.doi.org/10.1126/science.1112009; PMID: 16141073
- Bu D, Yu K, Sun S, Xie C, Skogerbø G, Miao R, et al. NONCODE v3.0: integrative annotation of long noncoding RNAs. Nucleic Acids Res 2012; 40:Database issue D210 - 5; http://dx.doi.org/10.1093/nar/gkr1175; PMID: 22135294
- Lander ES, Linton LM, Birren B, Nusbaum C, Zody MC, Baldwin J, et al, International Human Genome Sequencing Consortium. Initial sequencing and analysis of the human genome. Nature 2001; 409:860 - 921; http://dx.doi.org/10.1038/35057062; PMID: 11237011
- Venter JC, Adams MD, Myers EW, Li PW, Mural RJ, Sutton GG, et al. The sequence of the human genome. Science 2001; 291:1304 - 51; http://dx.doi.org/10.1126/science.1058040; PMID: 11181995
- Prensner JR, Iyer MK, Balbin OA, Dhanasekaran SM, Cao Q, Brenner JC, et al. Transcriptome sequencing across a prostate cancer cohort identifies PCAT-1, an unannotated lincRNA implicated in disease progression. Nat Biotechnol 2011; 29:742 - 9; http://dx.doi.org/10.1038/nbt.1914; PMID: 21804560
- Ørom UA, Derrien T, Beringer M, Gumireddy K, Gardini A, Bussotti G, et al. Long noncoding RNAs with enhancer-like function in human cells. Cell 2010; 143:46 - 58; http://dx.doi.org/10.1016/j.cell.2010.09.001; PMID: 20887892
- Khalil AM, Guttman M, Huarte M, Garber M, Raj A, Rivea Morales D, et al. Many human large intergenic noncoding RNAs associate with chromatin-modifying complexes and affect gene expression. Proc Natl Acad Sci U S A 2009; 106:11667 - 72; http://dx.doi.org/10.1073/pnas.0904715106; PMID: 19571010
- Clark MB, Johnston RL, Inostroza-Ponta M, Fox AH, Fortini E, Moscato P, et al. Genome-wide analysis of long noncoding RNA stability. Genome Res 2012; 22:885 - 98; http://dx.doi.org/10.1101/gr.131037.111; PMID: 22406755
- Zhang X, Rice K, Wang Y, Chen W, Zhong Y, Nakayama Y, et al. Maternally expressed gene 3 (MEG3) noncoding ribonucleic acid: isoform structure, expression, and functions. Endocrinology 2010; 151:939 - 47; http://dx.doi.org/10.1210/en.2009-0657; PMID: 20032057
- Leygue E. Steroid receptor RNA activator (SRA1): unusual bifaceted gene products with suspected relevance to breast cancer. Nucl Recept Signal 2007; 5:e006; PMID: 17710122
- Girard G, Roussis A, Gultyaev AP, Pleij CW, Spaink HP. Structural motifs in the RNA encoded by the early nodulation gene enod40 of soybean. Nucleic Acids Res 2003; 31:5003 - 15; http://dx.doi.org/10.1093/nar/gkg721; PMID: 12930950
- Martens JA, Laprade L, Winston F. Intergenic transcription is required to repress the Saccharomyces cerevisiae SER3 gene. Nature 2004; 429:571 - 4; http://dx.doi.org/10.1038/nature02538; PMID: 15175754
- Thompson DM, Parker R. Cytoplasmic decay of intergenic transcripts in Saccharomyces cerevisiae.. Mol Cell Biol 2007; 27:92 - 101; http://dx.doi.org/10.1128/MCB.01023-06; PMID: 17074811
- Heo JB, Sung S. Vernalization-mediated epigenetic silencing by a long intronic noncoding RNA. Science 2011; 331:76 - 9; http://dx.doi.org/10.1126/science.1197349; PMID: 21127216
- Blume SW, Meng Z, Shrestha K, Snyder RC, Emanuel PD. The 5′-untranslated RNA of the human dhfr minor transcript alters transcription pre-initiation complex assembly at the major (core) promoter. J Cell Biochem 2003; 88:165 - 80; http://dx.doi.org/10.1002/jcb.10326; PMID: 12461786
- Maenner S, Blaud M, Fouillen L, Savoye A, Marchand V, Dubois A, et al. 2-D structure of the A region of Xist RNA and its implication for PRC2 association. PLoS Biol 2010; 8:e1000276; http://dx.doi.org/10.1371/journal.pbio.1000276; PMID: 20052282
- Hirota K, Miyoshi T, Kugou K, Hoffman CS, Shibata T, Ohta K. Stepwise chromatin remodelling by a cascade of transcription initiation of non-coding RNAs. Nature 2008; 456:130 - 4; http://dx.doi.org/10.1038/nature07348; PMID: 18820678
- Zhao J, Ohsumi TK, Kung JT, Ogawa Y, Grau DJ, Sarma K, et al. Genome-wide identification of polycomb-associated RNAs by RIP-seq. Mol Cell 2010; 40:939 - 53; http://dx.doi.org/10.1016/j.molcel.2010.12.011; PMID: 21172659
- Wang KC, Yang YW, Liu B, Sanyal A, Corces-Zimmerman R, Chen Y, et al. A long noncoding RNA maintains active chromatin to coordinate homeotic gene expression. Nature 2011; 472:120 - 4; http://dx.doi.org/10.1038/nature09819; PMID: 21423168
- Tsai MC, Manor O, Wan Y, Mosammaparast N, Wang JK, Lan F, et al. Long noncoding RNA as modular scaffold of histone modification complexes. Science 2010; 329:689 - 93; http://dx.doi.org/10.1126/science.1192002; PMID: 20616235
- Chu C, Qu K, Zhong FL, Artandi SE, Chang HY. Genomic maps of long noncoding RNA occupancy reveal principles of RNA-chromatin interactions. Mol Cell 2011; 44:667 - 78; http://dx.doi.org/10.1016/j.molcel.2011.08.027; PMID: 21963238
- Nguyen VT, Kiss T, Michels AA, Bensaude O. 7SK small nuclear RNA binds to and inhibits the activity of CDK9/cyclin T complexes. Nature 2001; 414:322 - 5; http://dx.doi.org/10.1038/35104581; PMID: 11713533
- Yang Z, Zhu Q, Luo K, Zhou Q. The 7SK small nuclear RNA inhibits the CDK9/cyclin T1 kinase to control transcription. Nature 2001; 414:317 - 22; http://dx.doi.org/10.1038/35104575; PMID: 11713532
- Feng J, Bi C, Clark BS, Mady R, Shah P, Kohtz JD. The Evf-2 noncoding RNA is transcribed from the Dlx-5/6 ultraconserved region and functions as a Dlx-2 transcriptional coactivator. Genes Dev 2006; 20:1470 - 84; http://dx.doi.org/10.1101/gad.1416106; PMID: 16705037
- Tasheva ES, Roufa DJ. Regulation of human RPS14 transcription by intronic antisense RNAs and ribosomal protein S14. Genes Dev 1995; 9:304 - 16; http://dx.doi.org/10.1101/gad.9.3.304; PMID: 7867928
- Tsuiji H, Yoshimoto R, Hasegawa Y, Furuno M, Yoshida M, Nakagawa S. Competition between a noncoding exon and introns: Gomafu contains tandem UACUAAC repeats and associates with splicing factor-1. Genes Cells 2011; 16:479 - 90; http://dx.doi.org/10.1111/j.1365-2443.2011.01502.x; PMID: 21463453
- Lin D, Pestova TV, Hellen CU, Tiedge H. Translational control by a small RNA: dendritic BC1 RNA targets the eukaryotic initiation factor 4A helicase mechanism. Mol Cell Biol 2008; 28:3008 - 19; http://dx.doi.org/10.1128/MCB.01800-07; PMID: 18316401
- Parrott AM, Tsai M, Batchu P, Ryan K, Ozer HL, Tian B, et al. The evolution and expression of the snaR family of small non-coding RNAs. Nucleic Acids Res 2011; 39:1485 - 500; http://dx.doi.org/10.1093/nar/gkq856; PMID: 20935053
- Crawford DR, Schools GP, Salmon SL, Davies KJ. Hydrogen peroxide induces the expression of adapt15, a novel RNA associated with polysomes in hamster HA-1 cells. Arch Biochem Biophys 1996; 325:256 - 64; http://dx.doi.org/10.1006/abbi.1996.0032; PMID: 8561505
- Pagano A, Castelnuovo M, Tortelli F, Ferrari R, Dieci G, Cancedda R. New small nuclear RNA gene-like transcriptional units as sources of regulatory transcripts. PLoS Genet 2007; 3:e1; http://dx.doi.org/10.1371/journal.pgen.0030001; PMID: 17274687
- Gong C, Maquat LE. lncRNAs transactivate STAU1-mediated mRNA decay by duplexing with 3′ UTRs via Alu elements. Nature 2011; 470:284 - 8; http://dx.doi.org/10.1038/nature09701; PMID: 21307942
- Cesana M, Cacchiarelli D, Legnini I, Santini T, Sthandier O, Chinappi M, et al. A long noncoding RNA controls muscle differentiation by functioning as a competing endogenous RNA. Cell 2011; 147:358 - 69; http://dx.doi.org/10.1016/j.cell.2011.09.028; PMID: 22000014
- Sumazin P, Yang X, Chiu HS, Chung WJ, Iyer A, Llobet-Navas D, et al. An extensive microRNA-mediated network of RNA-RNA interactions regulates established oncogenic pathways in glioblastoma. Cell 2011; 147:370 - 81; http://dx.doi.org/10.1016/j.cell.2011.09.041; PMID: 22000015
- Salmena L, Poliseno L, Tay Y, Kats L, Pandolfi PP. A ceRNA hypothesis: the Rosetta Stone of a hidden RNA language?. Cell 2011; 146:353 - 8; http://dx.doi.org/10.1016/j.cell.2011.07.014; PMID: 21802130
- Tay Y, Kats L, Salmena L, Weiss D, Tan SM, Ala U, et al. Coding-independent regulation of the tumor suppressor PTEN by competing endogenous mRNAs. Cell 2011; 147:344 - 57; http://dx.doi.org/10.1016/j.cell.2011.09.029; PMID: 22000013
- Franco-Zorrilla JM, Valli A, Todesco M, Mateos I, Puga MI, Rubio-Somoza I, et al. Target mimicry provides a new mechanism for regulation of microRNA activity. Nat Genet 2007; 39:1033 - 7; http://dx.doi.org/10.1038/ng2079; PMID: 17643101
- Wang J, Liu X, Wu H, Ni P, Gu Z, Qiao Y, et al. CREB up-regulates long non-coding RNA, HULC expression through interaction with microRNA-372 in liver cancer. Nucleic Acids Res 2010; 38:5366 - 83; http://dx.doi.org/10.1093/nar/gkq285; PMID: 20423907
- Poliseno L, Salmena L, Zhang J, Carver B, Haveman WJ, Pandolfi PP. A coding-independent function of gene and pseudogene mRNAs regulates tumour biology. Nature 2010; 465:1033 - 8; http://dx.doi.org/10.1038/nature09144; PMID: 20577206
- Faghihi MA, Modarresi F, Khalil AM, Wood DE, Sahagan BG, Morgan TE, et al. Expression of a noncoding RNA is elevated in Alzheimer’s disease and drives rapid feed-forward regulation of beta-secretase. Nat Med 2008; 14:723 - 30; http://dx.doi.org/10.1038/nm1784; PMID: 18587408
- Faghihi MA, Zhang M, Huang J, Modarresi F, Van der Brug MP, Nalls MA, et al. Evidence for natural antisense transcript-mediated inhibition of microRNA function. Genome Biol 2010; 11:R56; http://dx.doi.org/10.1186/gb-2010-11-5-r56; PMID: 20507594
- Feng J, Funk WD, Wang SS, Weinrich SL, Avilion AA, Chiu CP, et al. The RNA component of human telomerase. Science 1995; 269:1236 - 41; http://dx.doi.org/10.1126/science.7544491; PMID: 7544491
- Hellwig S, Bass BL. A starvation-induced noncoding RNA modulates expression of Dicer-regulated genes. Proc Natl Acad Sci U S A 2008; 105:12897 - 902; http://dx.doi.org/10.1073/pnas.0805118105; PMID: 18723671
- Nowacki M, Landweber LF. Epigenetic inheritance in ciliates. Curr Opin Microbiol 2009; 12:638 - 43; http://dx.doi.org/10.1016/j.mib.2009.09.012; PMID: 19879799
- Wang KC, Chang HY. Molecular mechanisms of long noncoding RNAs. Mol Cell 2011; 43:904 - 14; http://dx.doi.org/10.1016/j.molcel.2011.08.018; PMID: 21925379
- Hung T, Wang Y, Lin MF, Koegel AK, Kotake Y, Grant GD, et al. Extensive and coordinated transcription of noncoding RNAs within cell-cycle promoters. Nat Genet 2011; 43:621 - 9; http://dx.doi.org/10.1038/ng.848; PMID: 21642992
- Zwieb C, van Nues RW, Rosenblad MA, Brown JD, Samuelsson T. A nomenclature for all signal recognition particle RNAs. RNA 2005; 11:7 - 13; http://dx.doi.org/10.1261/rna.7203605; PMID: 15611297
- Walter P, Blobel G. Signal recognition particle contains a 7S RNA essential for protein translocation across the endoplasmic reticulum. Nature 1982; 299:691 - 8; http://dx.doi.org/10.1038/299691a0; PMID: 6181418
- Hung T, Chang HY. Long noncoding RNA in genome regulation: prospects and mechanisms. RNA Biol 2010; 7:582 - 5; http://dx.doi.org/10.4161/rna.7.5.13216; PMID: 20930520
- Nagano T, Mitchell JA, Sanz LA, Pauler FM, Ferguson-Smith AC, Feil R, et al. The Air noncoding RNA epigenetically silences transcription by targeting G9a to chromatin. Science 2008; 322:1717 - 20; http://dx.doi.org/10.1126/science.1163802; PMID: 18988810
- Ma L, Huang Y, Zhu W, Zhou S, Zhou J, Zeng F, et al. An integrated analysis of miRNA and mRNA expressions in non-small cell lung cancers. PLoS One 2011; 6:e26502; http://dx.doi.org/10.1371/journal.pone.0026502; PMID: 22046296
- Cai X, Cullen BR. The imprinted H19 noncoding RNA is a primary microRNA precursor. RNA 2007; 13:313 - 6; http://dx.doi.org/10.1261/rna.351707; PMID: 17237358
- Augoff K, McCue B, Plow EF, Sossey-Alaoui K. miR-31 and its host gene lncRNA LOC554202 are regulated by promoter hypermethylation in triple-negative breast cancer. Mol Cancer 2012; 11:5; http://dx.doi.org/10.1186/1476-4598-11-5; PMID: 22289355
- Hagan JP, O’Neill BL, Stewart CL, Kozlov SV, Croce CM. At least ten genes define the imprinted Dlk1-Dio3 cluster on mouse chromosome 12qF1. PLoS One 2009; 4:e4352; http://dx.doi.org/10.1371/journal.pone.0004352; PMID: 19194500
- Kino T, Hurt DE, Ichijo T, Nader N, Chrousos GP. Noncoding RNA gas5 is a growth arrest- and starvation-associated repressor of the glucocorticoid receptor. Sci Signal 2010; 3:ra8; http://dx.doi.org/10.1126/scisignal.2000568; PMID: 20124551