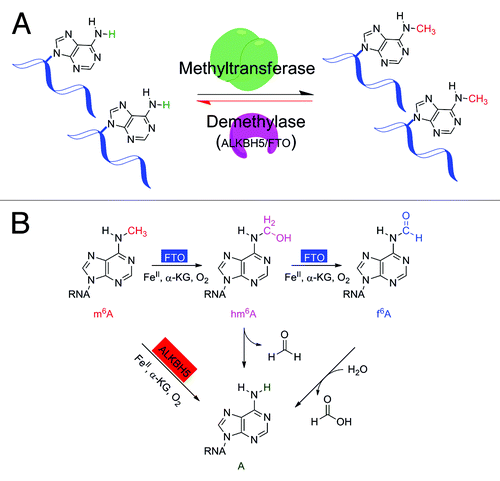
Abstract
More than 100 structurally distinct RNA modifications have been identified in all kingdoms of life.Citation1 These post-transcriptional modifications are widely present in various RNAs, including ribosomal RNA (rRNA), transfer RNA (tRNA), messenger RNA (mRNA), long non-coding RNA (lncRNA), etc. We have shown that the methylation of N6-methyladenine (m6A) can be reversed through the discovery of the first RNA demethylase, the human fat mass and obesity-associated protein, FTO, in 2011.Citation2 Most recently, we have identified a new mammalian RNA demethylase, ALKBH5, which is also able to remove the methyl group of m6A from RNA both in vitro and in vivo (). The ALKBH5 protein colocalizes with nuclear speckles where pre-mRNA processing occurs. This protein is actively involved in mRNA export regulation, in which its demethylation activity seems to play an important role, as well as in RNA synthesis. A knockout of the Alkbh5 gene in mice resulted in impaired male fertility due to compromised spermatogenesis. Importantly, increased m6A levels were observed in mRNA isolated from the Alkbh5-knockout mouse organs compared to those from wild-type littermates. RNA-Seq results indicate aberrant gene expression in spermatogenic cells of the seminoferous tubulus of testes from Alkbh5-deficient mice, thereby showing that the loss of the m6A demethylase influences gene expression, which, in turn, leads to defects in spermatogenesis and increased apoptosis of meiotic cells. Thus, the discovery of FTO and this new RNA demethylase strongly suggests that the methylation of RNA, like DNA and histone modifications, is dynamically regulated and likely to play broad roles in mammalian cells.
AlkB Homologues and ALKBH5
ALKBH5 belongs to the AlkB family of non-heme FeII/ α-ketoglutarate (α-KG)-dependent dioxygenases. Proteins of the AlkB family are widespread from bacteria to humans.Citation3,Citation4 In addition to ALKBH5, eight other mammalian homologues of AlkB have been identified so far (ALKBH1-ALKBH8 and FTO).Citation3,Citation5 Proteins from this family catalyze a wide range of oxidative reactions. AlkB in E. coliCitation6,Citation7 and two human homologues ALKBH2 and ALKBH3Citation8-Citation11 have been shown to act as DNA repair enzymes by demethylating 1-methyladenine (m1A) and 3-methylcytosine (m3C) lesions both in vitro and in vivo. ALKBH1 is a histone dioxygenase that acts specifically on histone H2A, and is likely to be involved in neural differentiation.Citation12 ALKBH8, associated with bladder cancer evasion, catalyzes the hypermodification of a tRNA wobble uridine, which may impact codon selection during translation.Citation13,Citation14 Our laboratory discovered that FTO oxidatively reverses the methylation of m6A, the most prevalent internal modification on mRNA and long non-coding RNA, showing that RNA modification is reversible and may have important regulatory functions.
ALKBH5 has been identified as a nuclear α-KG-dependent oxygenase that is capable of oxidizing α-KG to succinate and CO2 in the presence of co-factors iron(II) and ascorbic acid. Hypoxia-inducible factor 1α (HIF-1α) transcriptionally regulates ALKBH5 in hypoxia conditions.Citation15 Protein arginine methyltransferase 7 (PRMT7) has been shown to negatively regulate the expression of ALKBH5 and other DNA repair proteins of the AlkB family.Citation16 A recent study that profiled the mRNA-bound proteome has revealed ALKBH5 as one of over 800 potential mRNA-binding proteins.Citation17 However, the exact biological function of ALKBH5 has never been revealed until it was identified as a mammalian RNA demethylase involved in mouse spermatogenesis.
The m6A RNA Demethylases
ALKBH5 and FTO, the only two m6A RNA demethylases identified so far, have been shown to possess similar demethylation activity towards m6A in RNA. However, ALKBH5 possesses some unique properties compared to FTO.
While FTO catalyzes the formation of N6-hydroxymethyladenosine (hm6A) and N6-formyladenosine (f6A) during the oxidative demethylation of m6A,Citation18 these intermediates were not detected in the ALKBH5-catalyzed m6A demethylation process. hm6A and f6A have half-lives of ~ 3 h under physiological conditions at 37 °C, and will be ultimately converted to unmodified A as the product of the FTO-mediated oxidative demethylation. Considering the relative stability of hm6A, ALKBH5 is likely to generate unmethylated adenosine in situ by catalyzing the decomposition of hm6A, thus preventing further oxidation of hm6A to f6A and accelerating the demethylation reaction. The different reaction products of ALKBH5 and FTO suggest that their demethylation pathways and functions could be different ().
ALKBH5 may exhibit higher RNA-binding affinity compared to FTO. Indirect immunofluorescence analysis reveals that the expression level of ALKBH5 dramatically decreases after RNase A treatment, indicating a potentially strong protein-RNA interaction.Citation19 Neither traditional RNA pull-down assays using protein immunoprecipitation nor photoactivatable-ribonucleoside-enhanced crosslinking and immunoprecipitation (PAR-CLIP) have succeeded in identifying the bound RNA substrates of FTO from mammalian cells. In contrast, ALKBH5 could efficiently pull down its cellular bound RNA substrates; ALKBH5 has been successfully pulled down with mRNA baits using a photoreactive nucleotide-enhanced UV crosslinking and oligo(dT) purification approach.Citation17 Although over 800 other mRNA-binding proteins were identified in this assay, FTO was not present in this long list. FTO might be involved in a more defined RNA processing complex, whereas ALKBH5 appears to be a more “common” type of RNA processing enzyme.
ALKBH5 and FTO participate in different biological pathways. Both are widely expressed across multiple tissues in the mouse. However, while mouse Alkbh5 is most highly expressed in testis, mouse Fto has the highest expression level in the brain, especially in the hypothalamus where energy homeostasis is balanced. Alkbh5-deficient mice are viable, anatomically normal and reached adulthood. In contrast, Fto-null mice display post-natal growth retardation and high rates of early mortality.Citation20 The loss of Alkbh5 in mouse leads to defects in spermatogenesis without other apparent phenotypes, while the knockout of Fto in mouse results in a significant reduction in adipose tissue and decreased body mass.Citation20 The diverse biological functions regulated by these two demethylases suggest broad roles of m6A in mammals.
While the Alkbh5 gene is only conserved in vertebrates,Citation3 the Fto gene has homologs in vertebrates (from fish to mammals) and marine algae (from unicellular photosynthetic picoplankton to multicellular seaweed), but not in invertebrate animals, fungi, plants, bacteria or archaea.Citation21 Since m6A is a ubiquitous mRNA modification in eukaryotes, including mammals, plants, flies and yeast in the meiotic state, as well as viruses that replicate inside host nuclei,Citation22 m6A demethylases other than FTO and ALKBH5 may exist in other organisms.
Future Directions
m6A was first identified in mammalian mRNA in the 1970sCitation23-Citation25 and it is the most abundant internal mRNA modification in mammals.Citation26 However, the lack of methods to map its location on mRNA poses challenges to the investigation of the function of m6A. m6A has been proposed to exist within a consensus sequence of RRm6ACH,Citation27 where R represents purine and H is a nonguanine base. Because the methylation on the N6 position of adenosine does not alter its Watson-Crick base-pairing property, reverse transcription-based methods are not able to map m6A. In addition, the low abundance of mRNA in contrast to the overwhelming rRNA and tRNA, as well as the non-stoichiometric pattern of m6A on mRNA,Citation28 sets a high standard for detection methods. Until recently, the exact m6A sites have only been determined in two individual mRNAs, Rous sarcoma virus (RSV) and bovine prolactin (bPRL).Citation26
Recently, two groups have independently developed an m6A antibody-based high-throughput sequencing method, which reveals the topology of the human and mouse m6A RNA methylomes for the first time.Citation29,Citation30 Polyadenylated RNAs are first fragmented to ~100 nucleotides (nt). m6A-containing fragments are then immunoprecipitated with an m6A-specific antibody and subjected to sequencing. In this way, over 12,000 m6A sites in the transcripts of more than 7,000 mammalian genes have been identified. m6A sites have been mostly found around the stop codon and 3′UTR of mRNA, and are highly conserved from mouse to human. Some of these sites are dynamically modulated upon stimulus or during development, whose biological significance is yet to be discovered. However, it is difficult to accurately position the methylated adenine on mRNA due to the low resolution and frequent occurrence of consensus sequence. In addition, the modification stoichiometry at each site is not revealed. Therefore, a method for high-throughput sequencing of m6A with single-base resolution, preferentially also with methylation abundance at each modification site, is highly desired. The development of such approaches could potentially define the exact demethylomes of ALKBH5 and FTO.
Although m6A has been detected in all kingdoms of life, only two demethylases have been identified in mammals, leaving open a wide area for exploration into other eukaryotes and prokaryotes. On the other hand, the m6A methyltransferase has yet to be fully characterized. It is proposed to be a multi-component protein complex composed of two large separate components, MT-A (200 kDa) and MT-B (875 kDa), based on biochemical data obtained from HeLa cells.Citation26 Currently, only a 70 kDa SAM-binding subunit of MT-A, MT-A70 (also called METTL3), of this large complex has been identified.Citation31 Homologues of MT-A70 have been reported to play important roles in Saccharomyces cerevisiae,Citation32 ArabidopsisCitation33 and Drosophila.Citation34 Mutation studies of these homologs related the biological function of the putative m6A methyltrasferase to meiosis, gametogenesis, and embryogenesis. However, none of these homologues has been biochemically characterized. Identification of new demethylases would reveal how broad the reversible m6A regulation is, and a full characterization of the methyltransferease complex will help explain the molecular mechanisms underlying the specificity of the methylation.
The discovery of RNA demethylases indicates that, m6A on RNA, similar to 5-methylcytosine on mammalian genomic DNA, is dynamically regulated and encodes important biological information. While the m6A RNA landscape is sculptured by methyltransferases and demethylases, m6A-binding proteins may serve to decipher such information and exhibit downstream functions. Thus, the identification and characterization of m6A-binding proteins would undoubtedly help to unveil the mystery of m6A. The physiological functions of hm6A and f6A, the reaction products of FTO, remain to be explored. These products are likely to have their own specific binding proteins, distinct from m6A-binding proteins, which would contribute further complexity to the regulation network.
Noticeably, both ALKBH5 and FTO are partially colocalized with nuclear speckles. The proper assembly and modification of certain mRNA processing factors in nuclear speckles appear to depend on the catalytic activity of ALKBH5, but not FTO, in HeLa cells. Nuclear speckles are dynamic structures enriched with pre-mRNA splicing factors. Proteins associated with nuclear speckles have been shown to play roles in splicing pre-mRNAs.Citation35 In addition, MT-A70, the only known subunit of the m6A methyltransferase complex, which installs the methyl group onto adenosine of mammalian mRNAs, has been shown to colocalize with nuclear speckles.Citation26 Based on cellular localization, it is tempting to envision that m6A modifications that are dynamically regulated by enzymes with opposing activities might affect pre-mRNAs splicing. The potential effect and the mechanism are questions to be answered. In particular, during spermatogenesis, transcription is stalled while stored mRNAs are used for translation. The major defect of spermatogenesis in Alkbh5-deficient mice strongly indicates that proper methylation on mRNA is critical for correct processing or function of mRNA during spermatogenesis.
In summary, despite decades of efforts, the exact role of m6A in eukaryotic mRNA is largely unknown due to its complex nature and the lack of effective technology. Recent waves of breakthroughs in enzyme identification, functional hypotheses and technology development have shed light on our understanding of its pathways and roles in biological systems. Given the similarity to the well-demonstrated DNA and histone epigenetics, reversible RNA methylation might introduce another piece to the Atlas of epigenetics.
Acknowledgements
This study was supported by NIH GM071440. We thank S.F. Reichard, MA for editing the manuscript.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- Cantara WA, Crain PF, Rozenski J, McCloskey JA, Harris KA, Zhang X, et al. The RNA Modification Database, RNAMDB: 2011 update. Nucleic Acids Res 2011; 39:Database issue D195 - 201; http://dx.doi.org/10.1093/nar/gkq1028; PMID: 21071406
- Jia G, Fu Y, Zhao X, Dai Q, Zheng G, Yang Y, et al. N6-methyladenosine in nuclear RNA is a major substrate of the obesity-associated FTO. Nat Chem Biol 2011; 7:885 - 7; http://dx.doi.org/10.1038/nchembio.687; PMID: 22002720
- Kurowski MA, Bhagwat AS, Papaj G, Bujnicki JM. Phylogenomic identification of five new human homologs of the DNA repair enzyme AlkB. BMC Genomics 2003; 4:48; http://dx.doi.org/10.1186/1471-2164-4-48; PMID: 14667252
- Bratlie MS, Drabløs F. Bioinformatic mapping of AlkB homology domains in viruses. BMC Genomics 2005; 6:1; http://dx.doi.org/10.1186/1471-2164-6-1; PMID: 15627404
- Gerken T, Girard CA, Tung YCL, Webby CJ, Saudek V, Hewitson KS, et al. The obesity-associated FTO gene encodes a 2-oxoglutarate-dependent nucleic acid demethylase. Science 2007; 318:1469 - 72; http://dx.doi.org/10.1126/science.1151710; PMID: 17991826
- Trewick SC, Henshaw TF, Hausinger RP, Lindahl T, Sedgwick B. Oxidative demethylation by Escherichia coli AlkB directly reverts DNA base damage. Nature 2002; 419:174 - 8; http://dx.doi.org/10.1038/nature00908; PMID: 12226667
- Falnes PO, Johansen RF, Seeberg E. AlkB-mediated oxidative demethylation reverses DNA damage in Escherichia coli.. Nature 2002; 419:178 - 82; http://dx.doi.org/10.1038/nature01048; PMID: 12226668
- Duncan T, Trewick SC, Koivisto P, Bates PA, Lindahl T, Sedgwick B. Reversal of DNA alkylation damage by two human dioxygenases. Proc Natl Acad Sci USA 2002; 99:16660 - 5; http://dx.doi.org/10.1073/pnas.262589799; PMID: 12486230
- Aas PA, Otterlei M, Falnes PO, Vågbø CB, Skorpen F, Akbari M, et al. Human and bacterial oxidative demethylases repair alkylation damage in both RNA and DNA. Nature 2003; 421:859 - 63; http://dx.doi.org/10.1038/nature01363; PMID: 12594517
- Falnes PO. Repair of 3-methylthymine and 1-methylguanine lesions by bacterial and human AlkB proteins. Nucleic Acids Res 2004; 32:6260 - 7; http://dx.doi.org/10.1093/nar/gkh964; PMID: 15576352
- Dango S, Mosammaparast N, Sowa ME, Xiong LJ, Wu F, Park K, et al. DNA unwinding by ASCC3 helicase is coupled to ALKBH3-dependent DNA alkylation repair and cancer cell proliferation. Mol Cell 2011; 44:373 - 84; http://dx.doi.org/10.1016/j.molcel.2011.08.039; PMID: 22055184
- Ougland R, Lando D, Jonson I, Dahl JA, Moen MN, Nordstrand LM, et al. ALKBH1 is a histone H2A dioxygenase involved in neural differentiation. Stem Cells 2012; 30:2672 - 82; http://dx.doi.org/10.1002/stem.1228; PMID: 22961808
- Fu Y, Dai Q, Zhang W, Ren J, Pan T, He C. The AlkB domain of mammalian ABH8 catalyzes hydroxylation of 5-methoxycarbonylmethyluridine at the wobble position of tRNA. Angew Chem Int Ed Engl 2010; 49:8885 - 8; http://dx.doi.org/10.1002/anie.201001242; PMID: 20583019
- van den Born E, Vågbø CB, Songe-Møller L, Leihne V, Lien GF, Leszczynska G, et al. ALKBH8-mediated formation of a novel diastereomeric pair of wobble nucleosides in mammalian tRNA. Nat Commun 2011; 2:172; http://dx.doi.org/10.1038/ncomms1173; PMID: 21285950
- Thalhammer A, Bencokova Z, Poole R, Loenarz C, Adam J, O’Flaherty L, et al. Human AlkB homologue 5 is a nuclear 2-oxoglutarate dependent oxygenase and a direct target of hypoxia-inducible factor 1α (HIF-1α). PLoS One 2011; 6:e16210; http://dx.doi.org/10.1371/journal.pone.0016210; PMID: 21264265
- Karkhanis V, Wang L, Tae S, Hu YJ, Imbalzano AN, Sif S. Protein arginine methyltransferase 7 regulates cellular response to DNA damage by methylating promoter histones H2A and H4 of the polymerase δ catalytic subunit gene, POLD1. J Biol Chem 2012; 287:29801 - 14; http://dx.doi.org/10.1074/jbc.M112.378281; PMID: 22761421
- Baltz AG, Munschauer M, Schwanhäusser B, Vasile A, Murakawa Y, Schueler M, et al. The mRNA-bound proteome and its global occupancy profile on protein-coding transcripts. Mol Cell 2012; 46:674 - 90; http://dx.doi.org/10.1016/j.molcel.2012.05.021; PMID: 22681889
- Fu Y, Jia G, Pang X, Wang R, Wang X, Li C, et al. FTO-Mediated Formation of N6-Hydroxymethyladenosine and N6-Formyladenosine in Mammalian RNA. Nature Communication 2013. DOI http://dx.doi.org/10.1038/ncomms2822.
- Ricciardi S, Kilstrup-Nielsen C, Bienvenu T, Jacquette A, Landsberger N, Broccoli V. CDKL5 influences RNA splicing activity by its association to the nuclear speckle molecular machinery. Hum Mol Genet 2009; 18:4590 - 602; http://dx.doi.org/10.1093/hmg/ddp426; PMID: 19740913
- Fischer J, Koch L, Emmerling C, Vierkotten J, Peters T, Brüning JC, et al. Inactivation of the Fto gene protects from obesity. Nature 2009; 458:894 - 8; http://dx.doi.org/10.1038/nature07848; PMID: 19234441
- Robbens S, Rouzé P, Cock JM, Spring J, Worden AZ, Van de Peer Y. The FTO gene, implicated in human obesity, is found only in vertebrates and marine algae. J Mol Evol 2008; 66:80 - 4; http://dx.doi.org/10.1007/s00239-007-9059-z; PMID: 18058156
- Jia G, Fu Y, He C. Reversible RNA adenosine methylation in biological regulation. Trends Genet 2013; 29:108 - 15; http://dx.doi.org/10.1016/j.tig.2012.11.003; PMID: 23218460
- Wei CM, Gershowitz A, Moss B. Methylated nucleotides block 5′ terminus of HeLa cell messenger RNA. Cell 1975; 4:379 - 86; http://dx.doi.org/10.1016/0092-8674(75)90158-0; PMID: 164293
- Perry RP, Kelley DE, Friderici K, Rottman F. The methylated constituents of L cell messenger RNA: evidence for an unusual cluster at the 5′ terminus. Cell 1975; 4:387 - 94; http://dx.doi.org/10.1016/0092-8674(75)90159-2; PMID: 1168101
- Desrosiers RC, Friderici KH, Rottman FM. Characterization of Novikoff hepatoma mRNA methylation and heterogeneity in the methylated 5′ terminus. Biochemistry 1975; 14:4367 - 74; http://dx.doi.org/10.1021/bi00691a004; PMID: 169893
- Bokar, JA. Fine-tuning of RNA functions by modification and editing Berlin; New York: Springer, 2005; 141-77.
- Schibler U, Kelley DE, Perry RP. Comparison of methylated sequences in messenger RNA and heterogeneous nuclear RNA from mouse L cells. J Mol Biol 1977; 115:695 - 714; http://dx.doi.org/10.1016/0022-2836(77)90110-3; PMID: 592376
- Kane SE, Beemon K. Precise localization of m6A in Rous sarcoma virus RNA reveals clustering of methylation sites: implications for RNA processing. Mol Cell Biol 1985; 5:2298 - 306; PMID: 3016525
- Dominissini D, Moshitch-Moshkovitz S, Schwartz S, Salmon-Divon M, Ungar L, Osenberg S, et al. Topology of the human and mouse m6A RNA methylomes revealed by m6A-seq. Nature 2012; 485:201 - 6; http://dx.doi.org/10.1038/nature11112; PMID: 22575960
- Meyer KD, Saletore Y, Zumbo P, Elemento O, Mason CE, Jaffrey SR. Comprehensive analysis of mRNA methylation reveals enrichment in 3′ UTRs and near stop codons. Cell 2012; 149:1635 - 46; http://dx.doi.org/10.1016/j.cell.2012.05.003; PMID: 22608085
- Bokar JA, Shambaugh ME, Polayes D, Matera AG, Rottman FM. Purification and cDNA cloning of the AdoMet-binding subunit of the human mRNA (N6-adenosine)-methyltransferase. RNA 1997; 3:1233 - 47; PMID: 9409616
- Clancy MJ, Shambaugh ME, Timpte CS, Bokar JA. Induction of sporulation in Saccharomyces cerevisiae leads to the formation of N6-methyladenosine in mRNA: a potential mechanism for the activity of the IME4 gene. Nucleic Acids Res 2002; 30:4509 - 18; http://dx.doi.org/10.1093/nar/gkf573; PMID: 12384598
- Zhong S, Li H, Bodi Z, Button J, Vespa L, Herzog M, et al. MTA is an Arabidopsis messenger RNA adenosine methylase and interacts with a homolog of a sex-specific splicing factor. Plant Cell 2008; 20:1278 - 88; http://dx.doi.org/10.1105/tpc.108.058883; PMID: 18505803
- Hongay CF, Orr-Weaver TL. Drosophila Inducer of MEiosis 4 (IME4) is required for Notch signaling during oogenesis. Proc Natl Acad Sci USA 2011; 108:14855 - 60; http://dx.doi.org/10.1073/pnas.1111577108; PMID: 21873203
- Spector DL, Lamond AI. Nuclear speckles. Cold Spring Harb Perspect Biol 2011; 3; http://dx.doi.org/10.1101/cshperspect.a000646; PMID: 20926517