Abstract
RNA editing by ADARs can change the coding potential of protein-coding mRNAs. So far, this type of RNA editing has mainly been shown to affect RNAs expressed in the nervous system with much lower editing levels being observed in other tissues.
The actin crosslinking proteins filamin α and filamin β are widely expressed in most tissues. The mRNAs encoding either protein are edited at the same position leading to a conserved Q to R exchange in both proteins. Using bar-coded next generation sequencing, we show that editing of filamin α is most abundant in the gastrointestinal tract and only to a lesser extent in the nervous system. Using knockout mice, we show that ADARB1 (ADAR2) is responsible for the majority of FLNA editing, while ADAR1 can edit filamin α mRNA in some tissues quite efficiently. Interestingly, editing levels of filamin α and β do not follow the same trend across tissues, suggesting a substrate-specific regulation of editing.
Introduction
Adenosine to inosine deamination of RNAs is a conserved RNA-editing mechanism that is widespread in metazoa. The enzymatic reaction is performed by adenosine deaminases that act on RNA (ADARs) that recognize structured and double-stranded (ds) RNA.Citation1 Inosines are recognized as guanosines by many cellular processes and, therefore, editing can alter the amino acid sequence of encoded proteins, change splice patterns, alter RNA-folding, or modify binding sites for associated proteins.Citation2 In all metazoa tested so far, ADARs have been shown to be essential for development and normal life.Citation2,Citation3
Mice have two active ADAR enzymes, ADAR1 and ADARB1 (generally referred to as ADAR2), which exhibit overlapping, yet distinct substrate specificity.Citation4-Citation6 So far, editing events that lead to an amino acid exchange in the encoded protein were primarily observed in RNAs transcribed in neuronal tissues that encode neuronal receptors and ion channels.Citation7 In most cases, these editing events have significant impact on protein structure and function.Citation8-Citation13 The majority of editing events observed in neuronally expressed protein coding regions are mediated by ADAR2. Editing events in protein coding regions are less abundant outside the nervous system and may also be attributed to ADAR1 activity.Citation14,Citation15 Moreover, abundant editing is found in non-coding regions of mRNAs such as introns and UTRs where repetitive elements lead to the formation of extensive double-stranded regions.Citation16-Citation18 Editing in UTRs can be performed by both ADAR1 and ADAR2.
Consistently, lack of ADAR1 or ADAR2 leads to different phenotypes. ADAR1−/− mice die at embryonic stage (E12.5) due to liver disintegration and defects in hematopoiesis, whereas ADAR2−/− mice are prone to seizures and die shortly after birth.Citation12,Citation19-Citation21 Altered editing patterns are also found in patients suffering from psychiatric disorders, neurodegenerative diseases, or malignant gliomas.Citation22-Citation24 Following editing patterns throughout development, it was also shown that editing frequencies in the nervous system increase, despite a constant expression of ADARs.Citation25
Comprehensive computational and sequence analysis has revealed novel editing events in the coding region of mRNAs outside neuronal tissues.Citation26,Citation27 One editing event that is found highly conserved among vertebrates affects the widely expressed cytoskeletal protein Filamin A (FLNA).Citation26,Citation28
Filamin A is a dimeric protein that binds and crosslinks F-actin.Citation29 Besides its function as an actin crosslinker, novel roles for Filamin A have been described that can be attributed to many interactions at its C-terminal part. More than 90 binding partners including channels, receptors, intracellular signaling molecules, and even transcription factors have been reported to interact with Filamin A.Citation30 Thus, Filamin A may anchor membrane receptors to the actin cytoskeleton and regulate their precise location and transport.Citation31-Citation33 Moreover, Filamin A also acts as a co-localization factor for signaling pathways and a mechanical element for caveolae and membrane ruffle formation.Citation34-Citation39
The Filamin A transcript is edited at a single position in exon 42, which leads to a Q/R substitution in Ig-repeat 22 of the encoded protein.Citation26 This site is located in the very interactive rod 2 domain, implying that it could affect the FLNA interactome. The editing site and sequence context have been found conserved from birds to mammals suggesting that FLNA editing must have functional consequences, which are currently not known.Citation26 Moreover, the very same position is also edited in the highly conserved filamin B pre-mRNA, also giving rise to a Q/R substitution in repeat 22 of the encoded protein.Citation27 To get some clues on the functional relevance of FLNA editing, to determine the extent of editing in different tissues and developmental stages, and to determine the enzyme responsible for editing we applied Illumina deep sequencing to FLNA amplicons of spliced mRNAs isolated from different tissues throughout development. Based on these data we generated the spatio-temporal profile of Filamin A editing in mature mRNAs.
Results and Discussion
Previous studies have shown that A to I editing levels for protein-coding targets expressed in the mammalian brain increase gradually during development, from embryo to adulthood.Citation25,Citation40 To test whether this trend would also apply to Filamin A and whether editing can also be found in other organs of non-neuronal origin we determined FLNA editing levels using Illumina deep sequencing. Using bar-coded amplicons of FLNA cDNA we compared editing levels in various tissues from developmental stages E15, P0, P20, and adult (> 4 mo) mice. Depending on the sample, read depths varied from a minimum of 450 reads to 961000 reads maximum with an average of 161500 reads per sample. As the overall mistakes in base calling were around 0.3%, A to G transitions that were lower than 0.5% were not considered for further analyses.
Due to the small size of E15 embryos we separated head, brain, whole thorax and abdomen, and abdomen only in these early stages. In contrast to the majority of mammalian protein coding targets of A to I editing, filamin A mRNA showed highest editing in the abdomen, reaching up to 12%, while editing levels in the brain were around 2%, similar to the editing levels previously observed for the R/G site in Gria2Citation25 (; ).
Figure 1. Editing levels in filamin α in wild-type (A) and ADAR2-knockout (B) mice at E15.5. (A) In the embryo, editing levels are highest in the abdomen and thorax, reaching up to 12% while editing in the brain only reaches 2%. (B) In ADAR2−/− animals, editing levels are dramatically reduced, only reaching 1.3% in thorax and abdomen while editing in all other parts is around the base calling error of 0.3%.
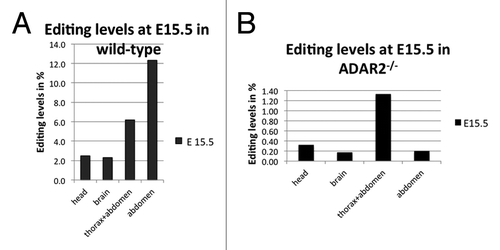
Table 1. Editing levels at E 15.5 in wild-type mice
Filamin A editing levels increased with age and, hence, higher editing levels were observed in young and adult mice. Most interestingly, the overall frequency distribution of editing events varied dramatically from previous studies on other substrates.Citation41 Our analysis revealed that editing levels of Filamin A mRNA are highest in 4-mo-old mice with stomach showing 95% A–G transitions, large intestine (86% A–G transitions), and lung (68% A–G transitions). Nevertheless, editing levels were also high in different parts of the brain (30–40% of A–G transitions), as well as in heart (56% A–G transitions), and bladder (55% A-G transitions) (; ). To verify the high editing levels observed in non-neuronal tissues and to compensate for any inter-individual differences, we have tested editing levels in the gastro-intestinal tract by Sanger sequencing on three additional mice. These data independently confirmed the very high editing levels in stomach (~85–90%), large intestine (~80%), and heart (~60%) (data not shown). Thus, while direct Sanger sequencing generally detected slightly less editing events the general observation of very high editing levels in the gastrointestinal tract could be verified. This also demonstrates that inter-individual differences are low, as already discussed elsewhere.Citation42
Figure 2. Post-natal editing levels in filamin in wild-type (A) and ADAR2-knockout animals (B). (A) In the wild-type, post-natal editing levels in filamin A increase from P0 to P120. Editing levels are highest in the gastrointestinal tract, reaching more than 90% in the stomach. Editing in neuronal tissue is comparatively modest, reaching almost 40% in the cerebellum. (B) In ADAR2−/− animals, editing rates are strongly reduced and decrease from P0 to P120 in spleen pancreas and liver while editing increases in the nervous system. Highest editing levels are reached in spleen, pancreas, liver, and bone, reaching at least 25% in the spleen.
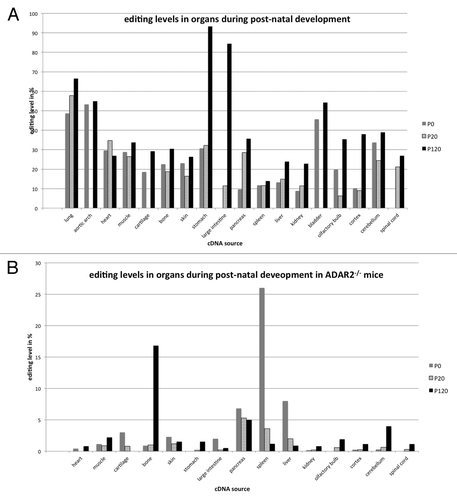
Table 2. Editing levels in filamin A pre-mRNA during post-natal development
FLNA editing levels had also been determined for a few human embryonic and adult tissues.Citation43 The limited number of human tissues investigated makes a direct comparison of human and mouse editing levels difficult. However, in humans—like in the mouse—embryonic editing levels were very low. In adult human brain, liver, kidney, and spleen editing reached about 25%. In the mouse, in contrast, editing levels of up to 40% were observed in the brain, while editing levels in kidney, liver and spleen were in a similar range between 15–25%. Whether humans also show high editing levels in the gastrointestinal tract remains to be determined.
ADAR1 and ADAR2 have different, yet overlapping target specificities.Citation6,Citation44 Moreover, ADAR1 and ADAR2 show different but partially overlapping expression patterns.Citation45-Citation47 We therefore wanted to determine which enzyme would be largely responsible for the observed editing events. In a previous study, we had already shown that editing of FLNA in post-natal brain can be achieved by both ADAR1 and ADAR2.Citation4
We therefore performed deep sequencing of organ and development-specific cDNA samples derived from ADAR2−/− mice and embryos. In the absence of ADAR2, editing levels were strongly reduced, indicating that ADAR2 is the main enzyme responsible for editing of FLNA RNA. In embryos lacking ADAR2, the rate of A to G transitions dropped below the background level in head and brain (; ). The only significant editing levels of 1.2% were detectable in samples derived from thorax and abdomen. In post-natal tissues only few organs showed editing levels well above 1% (; ). The highest editing levels were detected in bone, pancreas, spleen, and liver. In the spleen of P0 animals editing reached 26%, followed by 17% editing detected in adult bone. Editing in liver varied from 8% to 1% while editing in pancreas varied from 7 to 5% between P0 and P120, respectively (; ). Again, editing in neuronal tissues was lower than in other organs but reached at least 4% in adult cerebellum.
Table 3. Editing levels at E 15.5 in ADAR2−/− mice
Table 4. Editing levels in filamin A pre-mRNA during post-natal development in ADAR2−/− mice
Taken together, our data indicates that ADAR2 is the primary enzyme responsible for editing of FLNA mRNA. Nonetheless, in some tissues and some developmental stages editing by ADAR1 still reaches significant levels, even in the absence of ADAR2. In spleen, for instance, editing levels peak at 14% at p120 in the wild type, but can increase to 26% at P0 in ADAR2−/− knockout animals. Also, in bone, editing levels reach 30% in the wild type vs. 16% in the ADAR2−/− knockout.
We have obviously considered the possibility of barcoding or basecalling problems. We have therefore confirmed the most important samples derived from ADAR2−/− samples by conventional Sanger sequencing (data not shown). Increased editing in the absence of one ADAR enzyme suggests competitive binding of different ADARs to one and the same site. Lack of one ADAR would then facilitate access to an editing site for another ADAR enzyme that might act more efficiently at this site. Similar phenomena had been described before.Citation4
Both, in the presence and absence of ADAR2, editing levels in most tissues increase with age. However, in some non-neuronal tissues like large intestine, cartilage, skin, liver, or spleen, editing levels decreased from P0 to P120; sometimes even below the levels of basecalling errors, which we defined around 0.3% (compare ).
Recently, it was noted that the mRNA encoding the paralogous protein filamin B is edited at exactly the same position as filamin A, also leading to a Q to R exchange in Ig repeat 22.Citation27 We therefore wanted to test whether editing levels in FLNB mRNA would follow the same trend as editing levels in FLNA. We therefore amplified and sequenced the FLNB mRNA from the same cDNAs used for the analysis of FLNA editing. cDNAs from stomach, large intestine, heart, bladder, and lung were analyzed using Sanger sequencing. Interestingly, while FLNA editing levels were highest in stomach, followed by large intestine, lung, bladder, and heart, the situation was different in filamin B. FLNB editing was highest in heart, followed by stomach, lung, large intestine, and bladder (; ). As the very same cDNA samples to determine editing in filamin A and filamin B mRNAs were used, the observed differences in editing levels cannot reflect differences in expression of ADARs in these samples. We therefore compared the ratios of FLNA and ADAR2 expression in a few isolated tissues (). However, no clear trend between editing levels and FLNA/ADAR2 ratios could be observed. Stomach, showing very high editing levels had a very high FLNA to ADAR2 ratio while cerebellum with also high editing levels had a low FLNA to ADAR2 ratio. It therefore appears more likely that other factors that can enhance ore repress editing in a tissue- and substrate-specific manner will show different expression levels in those tissues that have a different trend for FLNA and FLNB editing. In fact, we have recently shown that some RNA-binding proteins can selectively modulate editing of some substrates, most likely by competitive binding to editing sites.Citation48,Citation49 Therefore, if some RNA-binding proteins can specifically interact with the editing sites in either FLNA or FLNB pre-mRNAs, editing in either one of these substrates might be specifically repressed.
Figure 3. Different levels of filamin A and filamin B editing. (A) Filamin B cDNAs from selected tissues used in the analysis shown in were amplified, subjected to Sanger sequencing, and editing levels were determined by calculating A to G peak ratios. Filamin B editing was highest in heart, followed (in decreasing order) by stomach, lung, large intestine, and bladder. Therefore, the extent of filamin A and filamin B editing does not correlate in these tissues. (B) Expression ratios of filamin A and ADAR2 do not correlate with editing levels. To determine whether the ratios of substrate to enzyme might govern editing levels, expression levels of filamin A and ADAR2 were determined relative to actin mRNA using qPCR. Highest substrate (FLNA) to ADAR2 ratio was observed in spleen of P20 animals and in stomach of P120 animals. These two tissues show 10 and 90% editing levels, respectively. Adult neuronal tissues, in contrast, showed a rather low substrate to enzyme ratio but only exhibited moderate editing levels of 30–40%. Hence, differences in editing levels cannot be explained by different ratios of substrate RNA to editing enzyme.
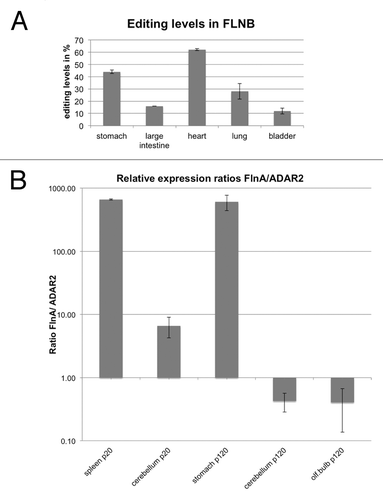
Table 5. Editing levels in FLNB at P120 in wild-type mice
In summary, our comprehensive analysis of editing in the filamin A encoding mRNA shows very high editing levels outside the nervous system. Our study shows further that FLNA editing is mainly achieved by ADAR2 but that in some cases ADAR1 can efficiently compensate for ADAR2. High editing levels in some tissues in the absence of ADAR2 might possibly result from facilitating access of ADAR1.Citation4 However, clarification of this point will require further experimentation. Most interestingly, our finding that editing levels in FLNA are highest outside neuronal tissues suggests further novel and unexplored roles for RNA editing. Given the many functions of filamin A, ranging from actin crosslinking to receptor interaction it may be too premature to speculate about the function of FLNA editing. However, editing may well alter the interaction repertoire of FLNA. With editing levels as high as 90% in the gastrointestinal tract of adult mice dropping to a mere 4–5% of editing in ADAR2−/− mice and editing levels of more than 12% in the abdomen of wild-type embryos it is unlikely that the high editing levels in the gastrointestinal tract represent an ADAR1-mediated anti-inflammatory response. Instead, a constitutive function for the edited version of FLNA seems most likely.
Materials and Methods
Tissue isolation
Adarb1−/− (ADAR2)-knockout mice were a kind gift of Peter Seeburg (MPI Heidelberg).Citation12 Tissues expressing both active versions of ADAR were obtained from c57bl/6 wt mouse. Mice were sacrificed and organs were dissected and kept in liquid nitrogen. The tissues were macerated using 10% SDS and 0.5M EDTA before RNA isolation. For deep sequencing, tissues from single mice were analyzed per time point. For some tissues (stomach, intestine, heart), sequences from three additional mice were determined by Sanger sequencing.
RNA isolation, reverse transcription (RT), and Filamin amplification
Total RNA was isolated from homogenized organs by using PeqGOLD TRIzol reagent (PEQLAB Biotechnologie GmbH) using manufacturers protocols. Aliquots of isolated RNAs were treated with DNase I (Fermentas 1 U/μL, 10U) at 37 °C for 1 h and kept in 96% ethanol prior to reverse transcription. cDNAs were synthesized using M-MLV Reverse Transcriptase kit (Invitrogen) and random hexamer primers in a total volume of 20 μl. Filamin A fragments were amplified in a 15 cycle PCR reactions using Phusion High Fidelity Polymerase (Finnzymes). Forward primers were designed to bind Filamin A exon 42 and contained the sequencing primer sequence and a 4-nucleotide barcode. The forward primer sequence was ACACGACGCT CTTCCGATCT NNNNCCGCCTT ACTGTTTCTA GTCT. The sequencing primer part is in italics while NNNN is the position of the barcode. The FLNA hybridizing part is underlined. The reverse primer was designed to bind Filamin A exon 43 and had the sequence GCTGGTTGAC CTTTAACCCT G. PCR products (50 nt long, spanning spliced exons 42–43) were purified from polyacrylamide gels by elution with 2.5M NH4OAc o/n at +4 °C, followed by ethanol precipitation. The eluted samples were used for second round of PCR with Phusion High Fidelity Polymerase (Finnzymes) and Illumina sequencing adaptor primers (15 cycles). Forward primer sequence AATGATACGG CGACCACCGA GATCTACACT CTTTCCCTAC ACGACGCTCT TCCGATCT; reverse primer sequence: CAAGCAGAAG ACGGCATACG AGCTCTTCCG ATCTGCTGGT TGACCTTTAA CCCTG. PCR fragments were again purified by PAGE and resuspended in water.
The same cDNAs were used to amplify filamin B with the forward primer in exon 41: CAGCCCTTAC CTGGTGCCCG T and the reverse primer in exon 42: CTTTGCATCA ATCTTCCCTT TCG
Deep sequencing
Cluster generation and DNA sequencing was performed using the Illumina Genome analyzer (GA) IIx system. After sequencing, the adaptor and barcode sequences were removed using Cutadapt.Citation50 Sequences were aligned to the predicted spliced product. Sequences not fitting the predicted spliced product except for the edited nucleotide were discarded.
Basecalling tables were used to define the level of noise at 0.3% for all possible transitions over the entire sequence length. A to G transitions above 0.5% at position 3 of the actual sequence, representing the editing site, were considered as true editing events.
Real-time PCR
To quantify the expression of filamin A, ADAR2, and actin in selected tissues, RNAs prepared for the deep sequencing was DNase I digested (Fermentas 1 U/μl, 10 U) and reverse transcribed using M-MLV Reverse Transcriptase kit (Invitrogen) and random hexamer primers in a total volume of 20 μl. Real-time PCR was performed on an Eppendorf realplex 2 mastercycler, whereas using a GoTaq qPCR master mix (Promega) and following primers and reaction conditions:
Filamin A sense 5′-GGTGACGCCC GCCGCCTTAC- 3′,
anti- sense 5′-AAGATGCTGG CTGGTTGACC-3′,
ADARb1 sense 5′-TCCTGCAGTG ACAAGATAGC A-3′,
anti-sense 5′- GGTTCCACGA AAATGCTGAG 3′
Actin β sense 5′-CTTTGCAGCT CCTTCGTTGC-3′,
antisense 5′ ACGATGGAGG GGAATACAGC 3′
40 cycles of 95 °C/15 s, 57.5 °C/30 s, 72 °C/1 min, preceded by denaturation at 95 °C/3 min.
Relative difference in expression of FilaminA and ADARb1 was calculated by the ΔCt method using actin β as a reference gene.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
The authors would like to thank the Vienna Campus Support Facility (CSF) for performing the Illumina deep sequencing, de-barcoding, and aligning of sequences. The authors would also like to thank Prof Peter Seeburg, MPI Heidelberg for the kind gift of ADAR2-knockout mice. This work was supported by the Austrian Science Foundation grant Nr. SFB 4313 to MFJ. MS was supported by the Austrian Science Foundation Doctoral Program W1207.
References
- Bass BL. RNA editing and hypermutation by adenosine deamination. [published erratum appears in Trends Biochem Sci 1997 Jul;22(7):278] Trends Biochem Sci 1997; 22:157 - 62; http://dx.doi.org/10.1016/S0968-0004(97)01035-9; PMID: 9175473
- Nishikura K. Functions and regulation of RNA editing by ADAR deaminases. Annu Rev Biochem 2010; 79:321 - 49; http://dx.doi.org/10.1146/annurev-biochem-060208-105251; PMID: 20192758
- Bass BL. RNA editing by adenosine deaminases that act on RNA. Annu Rev Biochem 2002; 71:817 - 46; http://dx.doi.org/10.1146/annurev.biochem.71.110601.135501; PMID: 12045112
- Riedmann EM, Schopoff S, Hartner JC, Jantsch MF. Specificity of ADAR-mediated RNA editing in newly identified targets. RNA 2008; 14:1110 - 8; http://dx.doi.org/10.1261/rna.923308; PMID: 18430892
- Wong SK, Sato S, Lazinski DW. Substrate recognition by ADAR1 and ADAR2. RNA 2001; 7:846 - 58; http://dx.doi.org/10.1017/S135583820101007X; PMID: 11421361
- Eggington JM, Greene T, Bass BL. Predicting sites of ADAR editing in double-stranded RNA. Nat Commun 2011; 2:319; http://dx.doi.org/10.1038/ncomms1324; PMID: 21587236
- Tariq A, Jantsch MF. Transcript diversification in the nervous system: a to I RNA editing in CNS function and disease development. Front Neurosci 2012; 6:99; http://dx.doi.org/10.3389/fnins.2012.00099; PMID: 22787438
- Morabito MV, Abbas AI, Hood JL, Kesterson RA, Jacobs MM, Kump DS, Hachey DL, Roth BL, Emeson RB. Mice with altered serotonin 2C receptor RNA editing display characteristics of Prader-Willi syndrome. Neurobiol Dis 2010; 39:169 - 80; http://dx.doi.org/10.1016/j.nbd.2010.04.004; PMID: 20394819
- Burns CM, Chu H, Rueter SM, Hutchinson LK, Canton H, Sanders-Bush E, Emeson RB. Regulation of serotonin-2C receptor G-protein coupling by RNA editing. Nature 1997; 387:303 - 8; http://dx.doi.org/10.1038/387303a0; PMID: 9153397
- Kawahara Y, Grimberg A, Teegarden S, Mombereau C, Liu S, Bale TL, Blendy JA, Nishikura K. Dysregulated editing of serotonin 2C receptor mRNAs results in energy dissipation and loss of fat mass. J Neurosci 2008; 28:12834 - 44; http://dx.doi.org/10.1523/JNEUROSCI.3896-08.2008; PMID: 19036977
- Olaghere da Silva UB, Morabito MV, Canal CE, Airey DC, Emeson RB, Sanders-Bush E. Impact of RNA editing on functions of the serotonin 2C receptor in vivo. Front Neurosci 2010; 4:26; PMID: 20582266
- Brusa R, Zimmermann F, Koh DS, Feldmeyer D, Gass P, Seeburg PH, Sprengel R. Early-onset epilepsy and postnatal lethality associated with an editing-deficient GluR-B allele in mice. Science 1995; 270:1677 - 80; http://dx.doi.org/10.1126/science.270.5242.1677; PMID: 7502080
- Maas S, Rich A, Nishikura K. A-to-I RNA editing: recent news and residual mysteries. J Biol Chem 2003; 278:1391 - 4; http://dx.doi.org/10.1074/jbc.R200025200; PMID: 12446659
- Yeo J, Goodman RA, Schirle NT, David SS, Beal PA. RNA editing changes the lesion specificity for the DNA repair enzyme NEIL1. Proc Natl Acad Sci U S A 2010; 107:20715 - 9; http://dx.doi.org/10.1073/pnas.1009231107; PMID: 21068368
- Jayan GC, Casey JL. Inhibition of hepatitis delta virus RNA editing by short inhibitory RNA-mediated knockdown of ADAR1 but not ADAR2 expression. J Virol 2002; 76:12399 - 404; http://dx.doi.org/10.1128/JVI.76.23.12399-12404.2002; PMID: 12414985
- Levanon EY, Eisenberg E, Yelin R, Nemzer S, Hallegger M, Shemesh R, Fligelman ZY, Shoshan A, Pollock SR, Sztybel D, et al. Systematic identification of abundant A-to-I editing sites in the human transcriptome. Nat Biotechnol 2004; 22:1001 - 5; http://dx.doi.org/10.1038/nbt996; PMID: 15258596
- Athanasiadis A, Rich A, Maas S. Widespread A-to-I RNA editing of Alu-containing mRNAs in the human transcriptome. PLoS Biol 2004; 2:e391; http://dx.doi.org/10.1371/journal.pbio.0020391; PMID: 15534692
- Nishikura K. Editing the message from A to I. Nat Biotechnol 2004; 22:962 - 3; http://dx.doi.org/10.1038/nbt0804-962; PMID: 15286646
- Horsch M, Seeburg PH, Adler T, Aguilar-Pimentel JA, Becker L, Calzada-Wack J, Garrett L, Götz A, Hans W, Higuchi M, et al. Requirement of the RNA-editing enzyme ADAR2 for normal physiology in mice. J Biol Chem 2011; 286:18614 - 22; http://dx.doi.org/10.1074/jbc.M110.200881; PMID: 21467037
- Wang Q, Miyakoda M, Yang W, Khillan J, Stachura DL, Weiss MJ, Nishikura K. Stress-induced apoptosis associated with null mutation of ADAR1 RNA editing deaminase gene. J Biol Chem 2004; 279:4952 - 61; http://dx.doi.org/10.1074/jbc.M310162200; PMID: 14613934
- Higuchi M, Maas S, Single FN, Hartner J, Rozov A, Burnashev N, Feldmeyer D, Sprengel R, Seeburg PH. Point mutation in an AMPA receptor gene rescues lethality in mice deficient in the RNA-editing enzyme ADAR2. Nature 2000; 406:78 - 81; http://dx.doi.org/10.1038/35017558; PMID: 10894545
- Hideyama T, Yamashita T, Suzuki T, Tsuji S, Higuchi M, Seeburg PH, Takahashi R, Misawa H, Kwak S. Induced loss of ADAR2 engenders slow death of motor neurons from Q/R site-unedited GluR2. J Neurosci 2010; 30:11917 - 25; http://dx.doi.org/10.1523/JNEUROSCI.2021-10.2010; PMID: 20826656
- Silberberg G, Lundin D, Navon R, Öhman M. Deregulation of the A-to-I RNA editing mechanism in psychiatric disorders. Hum Mol Genet 2012; 21:311 - 21; http://dx.doi.org/10.1093/hmg/ddr461; PMID: 21984433
- Maas S, Patt S, Schrey M, Rich A. Underediting of glutamate receptor GluR-B mRNA in malignant gliomas. Proc Natl Acad Sci U S A 2001; 98:14687 - 92; http://dx.doi.org/10.1073/pnas.251531398; PMID: 11717408
- Wahlstedt H, Daniel C, Ensterö M, Ohman M. Large-scale mRNA sequencing determines global regulation of RNA editing during brain development. Genome Res 2009; 19:978 - 86; http://dx.doi.org/10.1101/gr.089409.108; PMID: 19420382
- Levanon EY, Hallegger M, Kinar Y, Shemesh R, Djinovic-Carugo K, Rechavi G, Jantsch MF, Eisenberg E. Evolutionarily conserved human targets of adenosine to inosine RNA editing. Nucleic Acids Res 2005; 33:1162 - 8; http://dx.doi.org/10.1093/nar/gki239; PMID: 15731336
- Li JB, Levanon EY, Yoon JK, Aach J, Xie B, Leproust E, Zhang K, Gao Y, Church GM. Genome-wide identification of human RNA editing sites by parallel DNA capturing and sequencing. Science 2009; 324:1210 - 3; http://dx.doi.org/10.1126/science.1170995; PMID: 19478186
- Feng Y, Walsh CA. The many faces of filamin: a versatile molecular scaffold for cell motility and signalling. Nat Cell Biol 2004; 6:1034 - 8; http://dx.doi.org/10.1038/ncb1104-1034; PMID: 15516996
- Hartwig JH, Stossel TP. Isolation and properties of actin, myosin, and a new actinbinding protein in rabbit alveolar macrophages. J Biol Chem 1975; 250:5696 - 705; PMID: 124734
- Nakamura F, Stossel TP, Hartwig JH. The filamins: organizers of cell structure and function. Cell Adh Migr 2011; 5:160 - 9; http://dx.doi.org/10.4161/cam.5.2.14401; PMID: 21169733
- Smith L, Page RC, Xu Z, Kohli E, Litman P, Nix JC, Ithychanda SS, Liu J, Qin J, Misra S, et al. Biochemical basis of the interaction between cystic fibrosis transmembrane conductance regulator and immunoglobulin-like repeats of filamin. J Biol Chem 2010; 285:17166 - 76; http://dx.doi.org/10.1074/jbc.M109.080911; PMID: 20351101
- Wang HY, Burns LH. Naloxone’s pentapeptide binding site on filamin A blocks Mu opioid receptor-Gs coupling and CREB activation of acute morphine. PLoS One 2009; 4:e4282; http://dx.doi.org/10.1371/journal.pone.0004282; PMID: 19172190
- Jiménez-Baranda S, Gómez-Moutón C, Rojas A, Martínez-Prats L, Mira E, Ana Lacalle R, Valencia A, Dimitrov DS, Viola A, Delgado R, et al. Filamin-A regulates actin-dependent clustering of HIV receptors. Nat Cell Biol 2007; 9:838 - 46; http://dx.doi.org/10.1038/ncb1610; PMID: 17572668
- Muriel O, Echarri A, Hellriegel C, Pavón DM, Beccari L, Del Pozo MA. Phosphorylated filamin A regulates actin-linked caveolae dynamics. J Cell Sci 2011; 124:2763 - 76; http://dx.doi.org/10.1242/jcs.080804; PMID: 21807941
- Sverdlov M, Shinin V, Place AT, Castellon M, Minshall RD. Filamin A regulates caveolae internalization and trafficking in endothelial cells. Mol Biol Cell 2009; 20:4531 - 40; http://dx.doi.org/10.1091/mbc.E08-10-0997; PMID: 19759182
- Ravid D, Chuderland D, Landsman L, Lavie Y, Reich R, Liscovitch M. Filamin A is a novel caveolin-1-dependent target in IGF-I-stimulated cancer cell migration. Exp Cell Res 2008; 314:2762 - 73; http://dx.doi.org/10.1016/j.yexcr.2008.06.004; PMID: 18598695
- Head BP, Patel HH, Roth DM, Murray F, Swaney JS, Niesman IR, Farquhar MG, Insel PA. Microtubules and actin microfilaments regulate lipid raft/caveolae localization of adenylyl cyclase signaling components. J Biol Chem 2006; 281:26391 - 9; http://dx.doi.org/10.1074/jbc.M602577200; PMID: 16818493
- Hjälm G, MacLeod RJ, Kifor O, Chattopadhyay N, Brown EM. Filamin-A binds to the carboxyl-terminal tail of the calcium-sensing receptor, an interaction that participates in CaR-mediated activation of mitogen-activated protein kinase. J Biol Chem 2001; 276:34880 - 7; http://dx.doi.org/10.1074/jbc.M100784200; PMID: 11390380
- Fiori JL, Zhu TN, O’Connell MP, Hoek KS, Indig FE, Frank BP, Morris C, Kole S, Hasskamp J, Elias G, et al. Filamin A modulates kinase activation and intracellular trafficking of epidermal growth factor receptors in human melanoma cells. Endocrinology 2009; 150:2551 - 60; http://dx.doi.org/10.1210/en.2008-1344; PMID: 19213840
- Venø MT, Bramsen JB, Bendixen C, Panitz F, Holm IE, Öhman M, Kjems J. Spatio-temporal regulation of ADAR editing during development in porcine neural tissues. RNA Biol 2012; 9:1054 - 65; http://dx.doi.org/10.4161/rna.21082; PMID: 22858680
- Danecek P, Nellåker C, McIntyre RE, Buendia-Buendia JE, Bumpstead S, Ponting CP, Flint J, Durbin R, Keane TM, Adams DJ. High levels of RNA-editing site conservation amongst 15 laboratory mouse strains. Genome Biol 2012; 13:26; http://dx.doi.org/10.1186/gb-2012-13-4-r26; PMID: 22524474
- Greenberger S, Levanon EY, Paz-Yaacov N, Barzilai A, Safran M, Osenberg S, Amariglio N, Rechavi G, Eisenberg E. Consistent levels of A-to-I RNA editing across individuals in coding sequences and non-conserved Alu repeats. BMC Genomics 2010; 11:608; http://dx.doi.org/10.1186/1471-2164-11-608; PMID: 21029430
- Shtrichman R, Germanguz I, Mandel R, Ziskind A, Nahor I, Safran M, Osenberg S, Sherf O, Rechavi G, Itskovitz-Eldor J. Altered A-to-I RNA editing in human embryogenesis. PLoS One 2012; 7:e41576; http://dx.doi.org/10.1371/journal.pone.0041576; PMID: 22859999
- Lehmann KA, Bass BL. Double-stranded RNA adenosine deaminases ADAR1 and ADAR2 have overlapping specificities. Biochemistry 2000; 39:12875 - 84; http://dx.doi.org/10.1021/bi001383g; PMID: 11041852
- Melcher T, Maas S, Herb A, Sprengel R, Seeburg PH, Higuchi M. A mammalian RNA editing enzyme. Nature 1996; 379:460 - 4; http://dx.doi.org/10.1038/379460a0; PMID: 8559253
- Jacobs MM, Fogg RL, Emeson RB, Stanwood GD. ADAR1 and ADAR2 expression and editing activity during forebrain development. Dev Neurosci 2009; 31:223 - 37; http://dx.doi.org/10.1159/000210185; PMID: 19325227
- Hartner JC, Schmittwolf C, Kispert A, Müller AM, Higuchi M, Seeburg PH. Liver disintegration in the mouse embryo caused by deficiency in the RNA-editing enzyme ADAR1. J Biol Chem 2004; 279:4894 - 902; http://dx.doi.org/10.1074/jbc.M311347200; PMID: 14615479
- Garncarz W, Tariq A, Handl C, Pusch O, Jantsch MF. A high-throughput screen to identify enhancers of ADAR-mediated RNA-editing. RNA Biol 2013; 10:192 - 204; http://dx.doi.org/10.4161/rna.23208; PMID: 23353575
- Tariq A, Garncarz W, Handl C, Balik A, Pusch O, Jantsch MF. RNA-interacting proteins act as site-specific repressors of ADAR2-mediated RNA editing and fluctuate upon neuronal stimulation. Nucleic Acids Res 2013; 41:2581 - 93; http://dx.doi.org/10.1093/nar/gks1353; PMID: 23275536
- Martin M. Cutadapt removes adapter sequences from high-throughput sequencing. EMBnetjournal 2011; 17:10 - 2