Abstract
hnRNP A2 is a cellular protein that is important for nucleocytoplasmic and cytosolic trafficking of the HIV-1 genomic RNA. Both hnRNP A2’s interaction with HIV-1 RNA and its expression levels influence the activities of Rev in mediating nucleocytoplasmic export of the HIV-1 genomic RNA. While the lack of Rev expression during HIV-1 gene expression results in nuclear retention of HIV-1 genomic RNA, we show here by fluorescence in situ hybridization and fractionation studies that the genomic RNA translocates to the cytoplasm when hnRNP A2/B1 are depleted from cells. Polyribosome analyses revealed that the genomic RNA was shunted into a cytoplasmic, dense polyribosomal fraction. This fraction contained several RNA-binding proteins involved in viral gene expression and RNA trafficking but did not contain the translation initiation factor, eIF4G1. Amino acid incorporation into nascent polypeptides in this fraction was also greatly reduced, demonstrating that this fraction contains mRNAs that are poorly translated. These results demonstrate that hnRNP A2/B1 expression plays roles in the nuclear retention of the HIV-1 genomic RNA in the absence of Rev and in the release of the genomic RNA from translationally inactive, cytoplasmic RNP complexes.
Introduction
The trafficking of HIV-1 RNAs is characterized by the overlapping activities of several host and viral proteins during their transit from the nucleus into the cytoplasm, in the cytoplasm, and eventually, in the case of the 9 kilobasepair (kb) genomic, unspliced genomic RNA (vRNA), from the cytoplasm into progeny virions.Citation1 Previous work has demonstrated a critical role for the HIV-1 protein, Rev in the regulated export of the vRNA from the nucleus. This RNA is unspliced, harboring introns, and might be as a consequence retained in the nucleus or degraded by the cellular RNA surveillance machinery. Nuclear retention may also rely on the presence of cis-acting instability (INS) and nuclear retention sequences in the HIV-1 RNA that are bound by cellular proteins to promote nuclear retention.Citation2-Citation4 However, the vRNA (as well as the singly-spliced HIV-1 RNA species) harbors a Rev-responsive element (RRE) to overcome nuclear retention of the RRE-containing RNAs by its association with Rev.Citation5 This event is critical for efficient expression of HIV-1 structural and auxiliary proteins encoded by these mRNAs.Citation6-Citation8
Several host proteins have been shown to influence Rev’s activity by direct interaction or by influencing the ribonucleoprotein (RNP) context of the RNA.Citation9-Citation12 Rev also has effects on splicing regulationCitation13,Citation14 as well as on downstream gene expression events at the translation level.Citation15-Citation17 Efforts to understand Rev’s functions during viral replication cycle have provided several key leads in the identification of potential therapeutic strategies and targetsCitation18,Citation19 and have provided critical information on the processes governing nuclear RNA export in general.Citation20-Citation22
Proteins of the heterogeneous nuclear ribonucleoprotein (hnRNP) family have been shown to influence Rev activity. hnRNP A1 was shown to act synergistically with Rev to contribute to nucleocytoplasmic transport of RRE-containing HIV-1 RNAs.Citation23 The capacity of hnRNP A1 relied on the presence of an hnRNP A1-binding site within the INS in the N-terminal Matrix domain of HIV-1 Gag.Citation23 Additional roles for hnRNP A1 and other hnRNPs in retroviral splicing regulation rely largely on their ability to bind sequences adjacent to and within splicing enhancer and negative splicing regulatory sequences.Citation18,Citation24,Citation25 These roles in retroviral RNA splicing and nucleocytoplasmic trafficking might also be functionally linkedCitation26 a notion that is supported by newer work that shows that several functionally distinct hnRNPs interact with Rev.Citation27 Although principally nuclear, several hnRNPs indeed have cytoplasmic functions that contribute to the localization, the utilization, and fate of RNA in the cell.Citation28-Citation31 Recent work for example emphasizes the importance for hnRNPs that translocate to the cytoplasm in an HIV-1-dependent manner to contribute to vRNA stability and translation in the cytoplasm.Citation32-Citation34
Transcription of the HNRPA2B1 gene generates a primary transcript that is alternatively spliced to generate two mRNAs. One of these mRNAs encodes the 36 kDa, hnRNP A2. hnRNP A2 expression levels have also been shown to influence HIV-1 Rev activity and function to export vRNA into the cytoplasm. For example, the disruption of the interaction between hnRNP A2 with its cognate binding sequence in the HIV-1 RNA, the hnRNP A2 response element (A2RE) leads to nuclear retention of the vRNA despite the expression of Rev.Citation35 This result suggested that hnRNP A2’s association to the vRNA through the A2RE plays a permissive role in enabling Rev’s overriding function in mediating nuclear RNA export of unspliced or incompletely spliced viral mRNAs. The activity of hnRNP A2/B1 was confirmed by demonstrating that efficient depletion of hnRNP A2 in these conditions (i.e., Rev-) enabled the A2RE proviral mutant vRNA to be released into the cytoplasm.Citation31 These studies revealed two possible roles for hnRNP A2, one in nuclear retention that relies on its association to the vRNA and another in nucleocytoplasmic trafficking that depends on its expression levels in cells. As such, these data underscore a potentially important association between hnRNP A2 expression levels and Rev function during the expression phases of HIV-1.
Our later work revealed that the depletion of hnRNP A2 by small interfering (si)RNA (and the splice variant hnRNP B1 due to the identical targeted sequence in the mRNAs) from cells resulted in a juxtanuclear accumulation of the vRNA at the microtubule organizing center (MTOC) but had no detectable effects on other aspects of HIV-1 gene expression such as splicing or translationCitation31,Citation35 although this was met by a modest increase in vRNA encapsidation and lowered virus production.Citation31,Citation36 Expression levels of the major structural protein, 55 kDa precursor group-specific antigen or pr55Gag that is encoded by the vRNA were not detectably modified when hnRNP A2/B1 were depleted.Citation31
In this work, we characterize additional roles for hnRNP A2/B1 in HIV-1 vRNA biogenesis (hnRNP A2/B1 is used herein since we target both isoforms with the siRNA). Expression of a Rev-negative provirus (Rev-) resulted in predominant nuclear localization of the vRNA. However, when hnRNP A2/B1 were depleted from cells in these conditions, the vRNA translocated to the cytoplasm. The vRNA was found almost exclusively in the cytoplasm, yet pr55Gag expression remained undetectable. Thus, in the absence of Rev, hnRNP A2/B1 expression is necessary to maintain the nuclear localization of vRNA. The cytoplasmic accumulation of the vRNA was reflected by its presence in a dense polyribosome fraction. This fraction contained resident RNA trafficking granule proteins such as Staufen1, PABP1, eEF1α, ribosomal protein L7,Citation33,Citation37-Citation39 was virtually devoid of the translation initiation factor eIF4G1 and was severely compromised in translational activity. Our data support the notion that hnRNP A2/B1 expression has an important accessory role to play with Rev in promoting nuclear RNA retention and nucleocytoplasmic RNA transport. Moreover, we demonstrate that hnRNP A2/B1 expression promotes the recruitment of HIV-1 RNAs from a translationally deficient polyribosomal fraction that could impact on viral assembly and vRNA encapsidation.
Results
hnRNP A2/B1 depletion promotes cytoplasmic localization of vRNA in Rev--expressing cells
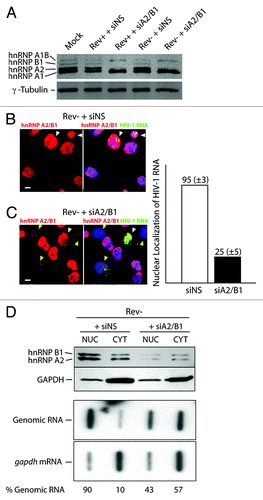
In cases when the A2RE is mutated, vRNA is retained in the nucleus despite the expression of Rev, while in cells in which hnRNP A2/B1 is depleted in cells, the vRNA is localized to the cytosol.Citation31 To explore this interaction further, we examined the localization of the HIV-1 vRNA in cells depleted of hnRNP A2/B1 during the expression a Rev- provirus that results in the retention of vRNA in the nucleus. Cells were mock transfected or transfected with provirus, HxBRU (Rev+ provirus) or a Rev- provirus [pcMRev(-)]. Cells were treated with either a non-silencing control siRNA (siNS) or one that results in the depletion of hnRNP A2 (and hnRNP B1) from cells (siA2/B1). Gels were run long enough to distinctly separate hnRNPs and their isoforms on western blots. In mock- and siNS-treated cells, the expression of hnRNP A1 (34 kDa, and its isoform hnRNP A1B, 39 kDa) and hnRNP A2 (36 kDa, and its isoform hnRNP B1, 38 kDa) is readily detectable by western blotting using a pan-specific antibody that recognizes a common epitope in all of these hnRNPs (). The depletion of hnRNP A2, as well as hnRNP B1 by siA2/B1, was consistently as high as 90–97% as shown in this blot related to the loading control protein, γ-tubulin. When we examined the localization of vRNA by fluorescence in situ hybridization (FISH) as described in Materials and Methods, siNS-treated cells showed a punctate distribution of vRNA and siA2/B1 treatment resulted in a phenotype identical to that observed previously with the vRNA accumulating at a juxtanuclear position, identified as the MTOC in our earlier work with Gag distributed throughout the cell and at the periphery in wild-type, Rev+-expressing cells (Fig. S1A; ref. Citation31). In Rev--expressing cells, Gag expression was undetectable by both immunofluorescence and western blotting analyses but Gag expression was readily rescued by supplying Rev in trans using the expression vector pCMV-Rev (ref. Citation40 [not shown; Fig. S1B]). In the context of pcMRev(-) expression, cells were co-stained for hnRNP A2/B1 using a specific rabbit anti-hnRNP A2/B1 antiserum (Act-2) by immunofluorescence and vRNA by FISH. In Rev--expressing and siNS-treated cells (Rev- + siNS), all cells abundantly expressed hnRNP A2/B1 and the vRNA was predominantly found in the nucleus as expected in HIV-1-expressing cells (white arrowheads in ). In siA2/B1-treated and Rev--expressing cells (Rev- + siA2/B1), hnRNP A2/B1-depleted cells were identified by those in which red fluorescence was faint or undetectable (yellow arrowheads in ). Surprisingly, in hnRNP A2/B1-depleted cells, the localization of vRNA was principally cytoplasmic, whereas cells in which a detectable signal for hnRNP A2/B1 was found exhibited the characteristic nuclear localization of vRNA (white arrowhead in upper portion of panels of ). There was a tight correlation between cells that were depleted of hnRNP A2/B1 and the cytoplasmic localization of vRNA in four independently performed experiments (n = 110 cells, 86 ± 5%, S.D.). A minor proportion of cells exhibited intermediate phenotypes indicated by low but detectable hnRNP A2/B1 expression levels and nuclear and cytoplasmic localization of vRNA. We then performed cell fractionation studies to confirm the changes in the localization of vRNA in siA2/B1 conditions. Cells were transfected with pcMRev(-) in siNS or siA2/B1 followed by nuclear and cytoplasmic fractionation. We used hnRNP A2/B1 and GAPDH as nuclear (principally) and cytoplasmic marker proteins in this analysis. hnRNPs were found predominantly in the nuclear fraction and GAPDH was found in the cytoplasmic fraction demonstrating efficient biochemical separation of these compartments (). In siA2/B1 conditions, hnRNP A2/B1 were noticeably reduced by 80% in both compartments due to siRNA treatment (). When we assessed the distribution of HIV-1 vRNA in these fractions, this revealed that whereas it was predominantly nuclear in Rev- conditions as expected (90%, average from two experiments), there was a 5–6-fold enhancement in vRNA abundance in the cytoplasmic fraction (). This result correlated well with our in situ data shown above () and demonstrates that hnRNP A2/B1 depletion promotes nuclear export of retained vRNA. gapdh mRNA fractionated almost exclusively to the cytoplasmic fraction in both conditions ().
Figure 1. Depletion of hnRNP A2/B1 leads to cytoplasmic localization of HIV-1 vRNA in Rev- conditions. HeLa cells were mock transfected or transfected with nonsilencing siRNAs (siNS) or siRNA to deplete hnRNP A2/B1 (siA2/B1) in cells. HxBru (Rev+) or pcMRev(-) (Rev-) proviral DNAs were then transfected. (A) The expression levels of hnRNP A1, A2, B1, and A1B and γ-tubulin (as loading control) were determined by western blot analysis. The distribution of hnRNP A2 (and hnRNP B1 due to the common epitope recognized in these protein isoforms; red fluorescence) and HIV-1 vRNA (green fluorescence) in Rev--expressing cells (siNS or siA2/B1 conditions) is shown and was determined by immunofluorescence using an hnRNP A2/B1-specific antiserum (Act-2) and FISH using a pol-specific digoxigenin-labeled RNA probe. All siNS-treated cells in (B) express hnRNP A2/B1 abundantly. White arrowheads in (B and C) identify cells in the vRNA localizes in the nucleus due to a lack of Rev expression. (C) shows cells treated with siA2/B1. Yellow arrowheads identify hnRNP A2/B1-depleted cells with noticeable reduction in hnRNP A2/B1 staining (red). White bar = 10 µm, Cells were fractionated into nuclear (NUC) and cytoplasmic (CYT) fractions following transfection with pcMRev(-) and siNS or siA2/B1 (D). Extracts from each of these fractions were analyzed for GAPDH and hnRNP A2/B1 content as well as for gapdh and genomic mRNAs. The average determination for the abundance of vRNA (presented as % RNA in each fraction) in each subcellular fraction is shown from two experiments. This figure is supplemented by Figure S1 in supplemental material.
hnRNP A2/B1 depletion promotes the accumulation of HIV-1 RNA in a dense polyribosome population
In earlier work, Rev was shown to enhance polyribosomal loading of HIV-1 RNAs to enhance Gag expression.Citation17 Consistently, Gag expression was not detectable in either immunofluorescence or western blotting experiments when Rev was not expressed (ref. Citation35 and Fig. S1B). Nevertheless, we were interested in determining the state of translational readiness of the vRNA since it was now found in the cytoplasm in cells depleted of hnRNP A2/B1 in Rev- conditions. Importantly, because Gag was not expressed despite cytoplasmic localization of its cognate mRNA, we suspected that HIV-1 vRNA must also be found as a cytoplasmic RNP complex that is translationally incompetent/silent in Rev- conditions. Polyribosome profile analysis has been used to identify this type of translationally inactive, cytoplasmic RNP in cells that were shown to sediment as dense RNP complexes.Citation41 Therefore, cells were treated with siNS or siA2/B1 and transfected with pcMRev(-) as described in Materials and Methods. At 30–36 h, cells were harvested for polyribosome profile analyses exactly as described.Citation42 Ten fractions from the bottom of each gradient were collected, fraction 1 being the most dense and fraction 10 the least. Fractions 7–8 represent the monosome sedimentation peak as determined by spectrophotometry (M, in and ) or by the sedimentation of ribosomal protein L7 in small to larger polyribosomes (see ). RNA was isolated from each fraction and separated on denaturing agarose gels and transferred to nylon membranes for northern blotting. HIV-1 RNAs were identified using a radiolabelled TAR cDNA probe to identify all three RNA species (2, 4, and 9 kb; ) and gapdh mRNA was identified as described before (; ref. Citation43). In Rev- + siNS cells, the vRNA was predominantly found in the nucleus and little is detectable in the cytoplasm by our in situ methods () and only about 10% is found to be cytoplasm using biochemical fractionation techniques (). Importantly, RRE-containing HIV-1 RNAs have not been shown to be strictly dependent on Rev for nuclear export and are indeed found in the cytoplasm in undefined complexes.Citation16 They are nevertheless dependent on Rev for efficient expression (Fig. S1). This early observation is also corroborated by the weak signal obtained for the 9 kb vRNA that was found in the least dense fraction of the polyribosome gradient (, left panels). This likely represents a soluble, non-polyribosome bound cytoplasmic mRNP fraction consistent with the undetectable Gag expression levels in these cells. In all experiments (n = 6), the RRE-containing 4 kb as well as the fully spliced 2 kb RNAs were predominantly found in fractions 1–6, within polyribosomal populations (). When we depleted hnRNP A2/B1 from these cells (, right panels), the cytoplasmic, resident vRNA shifted from fraction 10 to the most dense fraction of the gradient (fraction 1) as shown in a long exposure of the northern blot, although this did not result in any change in Gag expression levels (Fig. S1B). These results suggest that hnRNP A2/B1 expression levels in cells dictate the compartmentalization of the vRNA between a non-polyribosomal compartment (fraction 10) and a distinctly dense, polyribosomal fraction (fraction 1).
Figure 2. siA2/B1 treatment results in the redistribution of vRNA to a dense polyribosomal fraction. Cells were treated with siNS or siA2/B1 and transfected with pcMRev(-) and harvested for polyribosome analyses as described in Materials and Methods. RNA was purified from each gradient fraction and separated on agarose gels followed by northern blotting. (A) Continuous OD254 polyribosome profiles are shown. The monosome peak is indicated (M). (B) Northern blotting was performed to identify the three HIV-1 RNA size species (9, 4, and 2 kb). (C) The gapdh mRNA polyribosome profile is shown. Fraction 1 represents the most dense fraction (bottom of gradient) and fraction 10, the least dense, likely containing soluble RNA and proteins and material that has not entered sucrose gradients.
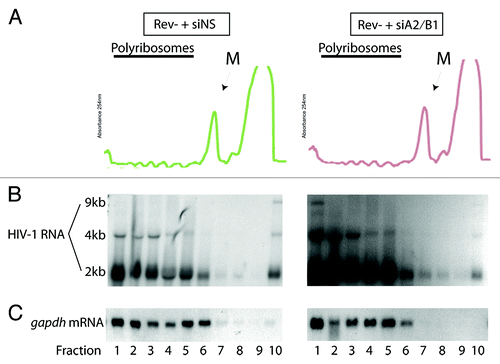
Figure 3. siA2/B1 treatment results in the redistribution of vRNA to a dense polyribosomal fraction in Rev+ conditions. Cells were transfected as described in the legend of except that the proviral DNA, HxBRU (Rev+) replaced pcMRev(-) in transfections. (A) Continuous OD254 polyribosome profiles are shown. The monosome peak is indicated (M). (B) Short exposure of northern blots to identify 9, 4, and 2 kb HIV-1 RNA species. A longer exposure of the autoradiogram (OE) is shown below to visualize more clearly the 9 kb vRNA. (C) gapdh mRNA was identified as a loading control as well as a polyribosomal distribution control in these analyses.
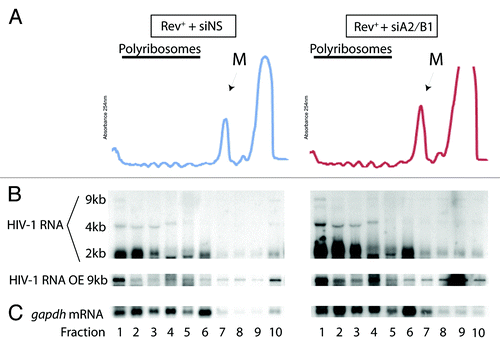
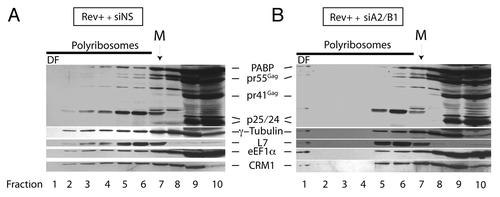
Figure 4. siA2/B1 treatment results in the redistribution of several proteins to a dense polyribosomal fraction (DF). Cells were treated as described in the legend to . Following sucrose polyribosome gradient fractionation, proteins in all fractions were precipitated with TCA and precipitated proteins were resuspended in SDS-containing buffer. Extracts from each fraction were resolved on SDS-PAGE gels and probed for PABP, Gag (pr55Gag, pr41Gag, p25, and p24), γ-tubulin, ribosomal protein L7, eIF1α, and CRM1. The location of the monosome peak (M) is indicated on top.
Our data demonstrate that the vRNA is translated when cells are depleted of hnRNP A2/B1 with no apparent changes in Gag synthesis levels during the expression of a Rev+ proviral DNA. In siA2/B1-knockdown conditions, the vRNA also localizes to the MTOC.Citation31 We therefore performed polyribosome profile analysis in these conditions. The OD254 profiles were identical in Rev+ and Rev--expressing cells (). The distributions of the 4 and 2 kb HIV-1 RNAs were similar between Rev+ and Rev- condition with the bulk of these RNA species sedimenting in fractions 1–6 (). The distribution of the vRNA, while not clearly visible in the exposure shown in the top panel in , was found throughout the gradient in siNS-treated cells (see overexposed [OE] image in bottom inset). gapdh mRNA was found primarily in the polyribosomal fractions 1–6 and did not markedly move with siRNA treatment. A population of vRNA was indeed found in the dense fraction in this gradient, and a smaller proportion was found in the least dense fraction 10. In siA2/B1-treated cells, the distribution of 4 and 2 kb HIV-1 RNAs and gapdh mRNA were nearly identical. However, again there was a striking accumulation of vRNA in the densest polyribosome fraction 1 in hnRNP A2/B1-depleted cells, similar to what we observed in the Rev- conditions (). In this case, there was a 2.8 (n = 4, ± 0.4, S.D.)-fold increase in the proportion of vRNA found in fraction 1 when related to the total RNA signal from all fractions in siA2/B1 conditions (). Thus, a population of vRNA is shunted into this dense polyribosomal population when hnRNP A2/B1 is depleted from cells revealing that at steady-state, hnRNP A2/B1 impacts on the polyribosomal distribution of vRNA. In Rev- conditions, this appears to occur via the recruitment of vRNA from a soluble compartment (noticeable at the top of the sucrose gradients, fraction 10, ). Treatment of cells with puromycin to release ribosomes from polyribosomes or of cell extracts with EDTA prior to loading led to the expected accumulated material in monosomes and RNPs, respectively as determined by spectrophotometry (Fig. S2). However, whereas puromycin led to the sequestration of the HIV-1 vRNA into translationally silent stress granules,Citation44 the vRNA was found to be refractory to EDTA treatment (ref. Citation45; and Valiente-Echeverria et al., submitted). The vRNA found in the DF is therefore polyribosomal and can be dissociated with puromycin but not entirely with EDTA. For experiments performed in the presence of Rev, there was also a loss of vRNA from fraction 10 and a concomitant increase in the relative level of vRNA into fraction 1 (, OE). Because the distribution of RNA is widespread in the gradient, it is not possible to determine from where RNA is being recruited into fraction 1, although there is quantitatively less vRNA in fraction 10 (, OE).
siA2/B1 depletion causes a redistribution of RNA-binding proteins
In our attempts to define the dense fraction 1 in which vRNA accumulates under siA2/B1 conditions, we performed polyribosome profile analysis again but examined protein content in each fraction. Following sedimentation in sucrose gradients, each polyribosome fraction was precipitated using TCA as described in Materials and Methods. Following resuspension, proteins were loaded on SDS-PAGE gels for western blotting. Gag proteins, except p25 or p24, were found in the top 5–6 fractions whereas p24 was found primarily in the soluble fractions 9 and 10. We posited that the dense fraction 1 was a translationally incompetent/silent complex whose composition would be reflected by the presence of multiple RNA-binding proteins contained in this type of complex.Citation1,Citation37,Citation46 We therefore probed for PABP, Tubulin, L7, and eEF1α, most of which are components of typical RNA trafficking granules that are considered to be translationally silent until RNA is trafficked to the right destination in cells.Citation47 When we assessed their sedimentation pattern in polyribosome gradients (), we noted that most of these proteins had wide sedimentation patterns, indicating that they are either ribosome/polyribosome-bound proteins, sediment in complexes of similar density, and/or are involved in various aspects of ribosome function and translation. Ribosomal protein L7 and this protein was found within monosomes and adjacent to the monosome peak likely in small polyribosomes of the gradient (peak at fraction 6), as shown earlier.Citation48 However, while many of these proteins sedimented throughout the gradients, hnRNP A2/B1 depletion also led to the appearance of several of these proteins in the dense fraction 1 (). In fact, pr55Gag (but not any of the smaller processed or mature forms of Gag), PABP, Tubulin, L7, eIF1α, as well as CRM1 accumulated in dense fraction 1. This recruitment corresponded to the marked shift of vRNA to this fraction in Rev- and Rev+ conditions. Thus, hnRNP A2/B1 depletion favored the accumulation of several proteins in the dense fraction 1. CRM1 was probed because it has been found associated with Rev-mediated nucleocytoplasmic transport as well, it has been localized to the MTOC region,Citation49 a region at which RNAs accumulate in siA2/B1 conditions, with and without Rev ( and ref. Citation31). PABP has also been found in translationally silent RNA trafficking granules and intracellular stress granules.Citation38,Citation44,Citation46
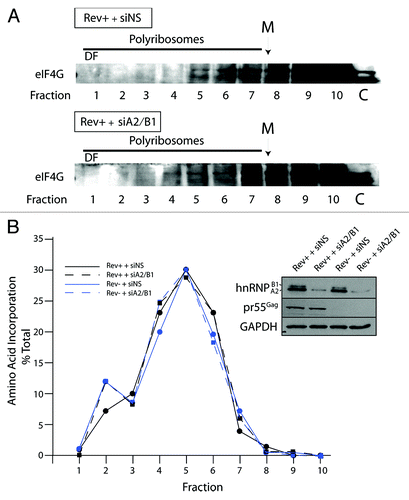
The dense polyribosomal fraction 1 contains poorly translated mRNAs
In order to investigate if the fraction 1 represented a translationally silent, dense polyribosomal fraction/RNP complex, we blotted for eIF4G1, a critical translation initiation factor that bridges 5′ and 3′ regions of the RNA to favor translational initiation.Citation50 This protein was found in the polyribosome gradients in all conditions tested in this manuscript and was distributed between fraction 4–10 (). We never observed eIF4G1 in the dense fraction 1 in any condition tested indicating that translation initiation is limited or absent on RNAs found in this fraction. We confirmed this result by performing acute metabolic labeling of cells immediately prior to harvesting cells for polyribosome analysis. Cells were pulsed with radiolabeled amino acids for 7 min, harvested, and polyribosome profile analysis was performed. The distribution of TCA-precipitable radiolabeled polypeptides were counted in an aliquot from each fraction of the gradient. A peak of amino acid incorporation was found in fractions 4–6 in each gradient and there was little difference found in Rev-/Rev+, siNS, or siA2/B1 conditions (). Most importantly is the low levels of TCA-precipitable counts in the dense polyribosome fractions 1 and 2, representing 7–12% TCA precipitable counts, demonstrating that these dense fractions are translationally deficient, even if they contain PABP, translation factors, ribosomal proteins, and mRNAs (). The lack of eIF4G1 and amino acid incorporation in this dense polyribosomal fraction 1 both demonstrate that HIV-1 vRNA and other RNAs found in this dense fraction are not actively translated, consistent with earlier observations.Citation41
Figure 5. The dense fraction 1 (DF) contains little if any eIF4G and contains translationally silent RNAs. Cells were transfected with siNS or siA2/B1 and HxBRU (Rev+) and polyribosome analyses were performed. Proteins were precipitated from gradient fractions as described above and blotted for eIF4G. The dense fraction 10 is indicated by DF; the monosome peak is identified with M on top. Lane C represents 5–10% input control before sucrose gradient centrifugation. (B) Seven minutes before cell harvesting, cells were pulsed with radiolabelled amino acids. Polyribosome profile analysis was performed as described above. An aliquot from each gradient fraction was TCA precipitated and radiolabelled polypeptides were collected on glass fiber filters and counted. Radioactivity was plotted as the percentage of radioactivity in each fraction related to the total. The background was corrected as described in Materials and Methods. The inset on right shows expression levels for hnRNP A2/B1 (identified using a mouse monoclonal anti-hnRNP A2/B1), pr55Gag and GAPDH (as loading control) in siNS- and siA2/B1-treated cells.
Staufen1—but not hnRNP—is found in dense polyribosome gradient fraction
The polyribosome fraction to which vRNA is recruited when hnRNP A2/B1 is depleted represents not only a dense RNP as determined by sucrose gradient analyses, but is also deficient in translational activity. Because these are both characteristics of RNA trafficking granules, we attempted to identify if any of the classical RNA trafficking granule proteins, including the hnRNPs and Staufen1, were present. Thus, cells were transfected with siNS or siA2/B1 in Rev+ or Rev- conditions and cells were harvested for polyribosome profile analyses. Protein extracts were prepared from each gradient fraction as described earlier and loaded onto SDS-PAGE gels for Western analysis. We blotted for hnRNPs and Staufen1 using antisera that recognize several isoforms of each (A1, A2, B1, A1B, Staufen155 kDa, and Staufen163 kDa). In Rev+ and Rev- conditions (), hnRNPs A1, A2, B1, and AIB were found to be distributed mostly in fractions 5–10, hnRNP A1 and hnRNP A2 were the most readily detectable proteins using our pan-specific antibody. In siA2/B1 conditions in both Rev- and Rev+ conditions, a noticeable gap between hnRNP A1 and A1B protein signals was observed (compare with ), again demonstrating effective depletions of hnRNP A2 and hnRNP B1 proteins in both conditions. siA2/B1 treatment did not appear to significantly modify the sedimentation of the remaining hnRNP proteins in these gradients. However, we did detect the appearance of hnRNP A1 in more dense fractions of the gradient, likely due to the reciprocal increase in the abundance of hnRNP A1 in these siA2/B1 knockdown conditions as noted in earlier work.Citation31,Citation51
Figure 6. The distribution of hnRNPs and Staufen1 in polyribosome gradients. Cells were transfected with siNS or siA2/B1 and HxBRU (REV+) or pcMRev(-) (REV-) and polyribosome profile analyses were performed. Proteins were precipitated from gradient fractions as described above and hnRNPs (A2, B1, A1, and AIB) and Staufen1 (55 kDa and 63 kDa isoforms) were identified in western blots using a rabbit pan-specific and mono-specific antisera, respectively (identified on right of each autoradiogram). The dense fraction 1 is indicated by DF; the location of the monosome peak is identified with M on top. Lane C represents 5–10% input control before sucrose gradient centrifugation.
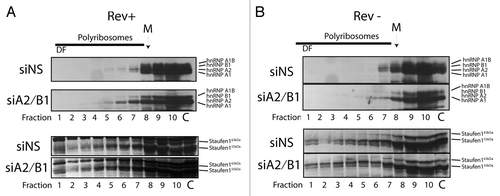
We then assessed the abundance of Staufen1 in polyribosomal gradient fractions because Staufen was found to be a major component of translationally silent complexes in neuronal cells.Citation41 The smaller, more abundant isoform of Staufen1 (Staufen155 kDa) was found in virtually all gradient fractions, including dense fraction 1, peaking at fractions 8/9, coincident with ribosomes, consistent with earlier reportsCitation52,Citation53 (). The larger, less abundant Staufen1 isoform, Staufen163 kDa, appeared only in the fractions 4/5–10, and tapered off toward the denser gradient fractions, in all conditions. The distribution of Staufen1 was unaffected by siA2/B1 treatment, however. While we now understand that there are differential roles for Staufen1 isoforms during HIV-1 replication,Citation44,Citation54 as well as for other Staufen proteins,Citation55-Citation57 only the Staufen155 kDa isoform sedimented to the dense fraction 1 in the polyribosome gradients. The presence of Staufen155 kDa in the dense fractions, in contrast to what we observed for the Staufen163 kDa isoform, implicates the lower molecular isoform in HIV-1 RNP biogenesis. Indeed, this would be consistent with the roles for this isoform in HIV-1 assembly as well as in vRNA packaging that we have defined earlier.Citation33,Citation44 It is possible that Staufen155 kDa governs these processes in the context of a Staufen155 kDa containing and dense RNP that only functions during limited translational activity, as proposed in earlier work.Citation41,Citation44,Citation64
Discussion
The results presented in this manuscript contribute to a more complete understanding on how HIV-1 uses hnRNP A2/B1 during viral replication. Even though vRNA retention is believed to depend on threshold levels of Rev at sites of proviral DNA transcription during the late expression phase of HIV-1,Citation14 hnRNP A2/B1 appears to play a role in this event in two experimental situations. In the first, if the association between hnRNP A2/B1 is blocked by mutating a cognate hnRNP A2 (/B1)-response element (A2RE) found at the N terminus of the vpr coding region, vRNA is partially retained in the nucleus, similar to a Rev- phenotypeCitation35 suggesting a functional importance for the hnRNP A2/B1-vRNA association. The second scenario is presented in this manuscript in which we show that when hnRNP A2/B1 are depleted, nuclear retained vRNA in Rev- conditions is released to the cytoplasm (). This could be achieved by a loss of a direct vRNA interaction and/or by the deficiency of a Rev-interacting partner.Citation27 While we have proposed that the hnRNP A2/B1-A2RE association is important for both nuclear and cytoplasmic vRNA trafficking,Citation35 hnRNP A2/B1 depletion could also indirectly affect the expression or localization of critical nuclear vRNA retention factors, a scenario that will deserve further study (Zolotukhin et al. 2003). In light of the well-characterized roles in post-transcriptional regulation, these results promote the notion that hnRNP A2/B1’s several functions in the control of vRNA fate are coupled.Citation1,Citation58 Furthermore, this work demonstrates that hnRNP A2/B1’s functional role extends into the cytoplasm in which hnRNP A2/B1 are important for the recruitment of the HIV-1 vRNA from cytoplasmic RNPs such that when hnRNP A2/B1 are depleted from cells, there is an accumulation of vRNA in dense, cytoplasmic polyribosomal fractions in both Rev- and Rev+ conditions that correspond to translation-deficient RNP complexes or RNA trafficking granules.Citation37,Citation41,Citation59 These complexes bear many of the hallmarks of RNA trafficking granules because they sediment to dense fractions in sucrose gradients,Citation41 they are translationally silent and they contain classical RNA trafficking granule components.Citation37,Citation41,Citation59,Citation60 It is noteworthy that many of the components of these granules have functions during HIV-1 gene expression stages or are found associated in purified virus particles,Citation1,Citation33 suggesting that these types of granules contribute to viral assembly.
The recruitment of the vRNA from translationally deficient RNPs might imply that hnRNP A2/B1 have effects on gag mRNA (vRNA) translation and Gag synthesis consistent with the reported activities of hnRNP A2 on translation enhancement.Citation61,Citation62 However, hnRNP A2/B1 depletion does not lead to detectable changes to steady-state Gag levels as shown earlierCitation31,Citation35 and in this manuscript (see inset in ; Fig. S1). The shift of a proportion of vRNA in polyribosomes may have a more subtle function than we appreciated in our earlier work, however. Because only a fraction of the total cellular pool of vRNA has been shown to be encapsidated into progeny virionsCitation63 and translational silencing of the vRNA was suggested to be a signal immediately prior to the encapsidation of vRNA into new virus particles,Citation64 a polyribosomal shift of vRNA to a translationally silent pool may be critical in viral assembly in the selection of vRNA for encapsidation. Consistently, depleting hnRNP A2/B1 not only leads to MTOC localization of the vRNA but could explain why a modest increase in vRNA encapsidation is also observed.Citation31 Our results support such a model in the control of vRNA encapsidation that involves subtle changes to the composition and trafficking of the HIV-1 RNP. hnRNP A2/B1 is a common component of several relevant HIV-1 complexes that have been recently characterized at the proteomics levelCitation9,Citation33,Citation65 and these hnRNPs likely exert functions during the transit of HIV-1 complexes from the nucleus to and within the cytoplasm, perhaps acting in concert with Rev at multiple levels.
Polyribosomal dynamics were explored for HIV-1 RNAs in earlier work in which Rev was shown to be a principal player in promoting the loading of most, but not all HIV-1 transcripts on polyribosomes.Citation16 The results shown here highlight the fact that the cytoplasmic location of HIV-1 RNAs (at least that of gag mRNA) is insufficient to promote translation and expression of Gag and support important earlier findings that Rev expression is essential for viral structural protein synthesis in the cytoplasm. The nature of the requirement for Rev expression likely stems in part from a highly structured 5′UTR, which is a strong impediment to translation initiation factors and ribosomes.Citation66 Rev could interact with the RNA packaging signalCitation67 to modify the conformation of this region and the 5′-end of HIV-1 RNA so that it becomes more readily amenable to the cellular translation machinery leading to ribosome loading on HIV-1 RNAs.Citation15,Citation68,Citation69 These types of conformational switches are important for HIV-1 and MLV, for example, in the encapsidation process.Citation70-Citation72 Furthermore, one major finding suggested that a fraction of the gag mRNA (vRNA) is retained in distinct cytoplasmic compartments in both the absence and presence of Rev.Citation17 Our results confirm this finding and also reveal how cellular proteins, like hnRNP A2/B1, influence the dynamic formation of these HIV-1 RNA pools by influencing tightly regulated nuclear and cytoplasmic trafficking events. hnRNP A2/B1’s influence on the polyribosomal distribution of HIV-1 RNA also denotes an important cytoplasmic role for hnRNP A2/B1 in RNA trafficking, viral gene expression, assembly, and encapsidation.
Materials and Methods
Cell culture
HeLa cells were cultured at 37 °C in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum (FBS) and containing 1% penicillin/streptomycin. Sixteen hours prior to transfection, cells were trypsinized, counted, and replated. For sucrose gradient experiments, cells were seeded in 150 cm2 flasks or 6-well plates at a density of 3.5 × 104 cells per cm2. The quantity of cells was doubled for some experiments in order to enhance the detection of endogenous proteins in gradient fractions.
For FISH/IF co-analyses experiments, cells were seeded in 6-well plates (NUNC) containing autoclaved glass coverslips at a density of 1.5 × 104 cells per cm2. Cells collected for western blotting in FISH/IF experiments were plated at a density of 3.5 × 104 cell per cm2. These cells were collected for western blotting in order to determine the efficiency of siRNA-mediated gene silencing.
Transfections and siRNAs
Transfections were performed using Lipofectamine 2000 (Invitrogen) in OptiMEM serum-reduced medium (Invitrogen), according to the manufacturer’s protocol. Knockdown of hnRNP A2/B1 was achieved using a double transfection method. On day 1, cells were transfected with either non-silencing (siNS) 5′ AATTCTCCGA ACGTGTCACG A or hnRNP A2/B1-specific (siA2/B1) 5′ AAGCTTTGAA ACACAGAAGA siRNA duplexes at 25 nM (Qiagen-Xeragon). Twenty-four hours later, cells were transfected a second time with both siRNA and proviral DNA. The proviral DNA construct used in these experiments was HxBru.Citation31,Citation35 The Rev-minus proviral construct, pcMRev(-) was used as described previously (kindly provided via the AIDS Research and Reference Reagent Program, Division of AIDS, NIAID, from Dr Maria SadaieCitation35,Citation73,Citation74). In Rev rescue experiments, pCMV-Rev was used at 0.5 µg per transfectionCitation40 (kindly provided via the AIDS Research and Reference Reagent Program, Division of AIDS, NIAID, [NIH] from Dr Marie-Louise Hammarskjöld and Dr David RekoshCitation40). Mock-transfected cells were transfected with the empty vector pcDNA3 and siNS (25 nM). Four hours after each transfection, the culture medium, containing Lipofectamine and OptiMEM, was removed and replaced with fresh DMEM/FBS without antibiotics. hnRNP A2/B1 knockdowns were effective as of 8 h post-second transfection as described,Citation31 and cells were harvested or fixed at time points as indicated in the text. Knockdown efficiencies were calculated by quantitation of the autoradiographic signals obtained in western blotting with the Molecular Analyst software (Bio-Rad) or using ImageJ software from the National Institutes of Health as described.Citation35
Fluorescence in situ hybridization/immunofluorescence (FISH/IF) co-analyses
Procedural details on FISH/IF co-analyses are completely described elsewhere.Citation75 Briefly, at 30 h post-transfection, the medium was carefully removed and wells containing coverslips were washed twice with PBS. They were then incubated in 4% paraformaldehyde for 20 min at room temperature. Fixed cells were then incubated for 10 min at room temperature with gentle agitation in a solution of 0.1 M glycine in PBS. This step ensures that any remaining aldehyde groups are quenched. Lastly, cells were permeabilized in a 0.2% solution of Triton-X-100 in PBS for 10 min at room temperature, also with gentle agitation. Between fixation, quenching, and permeabilization steps, coverslips were gently washed with PBS. Coverslips were stored in 70% ethanol in sealed plates at 4 °C until examined. FISH/IF and microscopy were performed exactly as described using an Olympus BX-51 fluorescence microscope equipped with an UPlanFI 100X oil objective. Images were captured in black and white with the Spot camera (Diagnostics Instruments) using Spot Advanced Software and Image-Pro-Plus version 4.0.1. Images were pseudo-colored using Adobe Photoshop CS5 (Adobe Systems) in RGB mode and then merged as described.Citation31,Citation35
Polyribosome profile analyses
Polyribosome profile analysis experiments were performed with HeLa cells and were performed exactly as described.Citation42 Thirty to 36 h post-transfection, cells were washed twice and collected in PBS, then spun for 10 min at 1 000 × g at 4 °C to pellet the cells. Supernatant was removed and the cells were resuspended in Polysome Buffer (250 mM KCl, 10 mM MgCl2, 20 mM HEPES [pH 7.5], 0.25 M sucrose, 0.1 mM DTT, 150 μg/mL cycloheximide, 1μL/mL RNase Out (Invitrogen), and 0.5% NP-40). Cells were homogenized mechanically using a sterile Eppendorf pestle and spun at 500 × g for 5 min at 4 °C, in order to pellet the nuclei. The supernatant was then removed and added to a 20% solution of sodium deoxycholic acid in order to liberate membrane-associated proteins in the cell homogenate. Following this, post-mitochondrial supernatants were prepared by centrifugation for 25 min at 16 000 × g (4 °C). Post-mitochondrial supernatants were then transferred to clean Eppendorf tubes, snap frozen in liquid nitrogen and stored at -80 °C. Prior to freezing, an aliquot was removed from each sample for quantitation by spectrophotometry and for western blot analysis to ascertain the efficiency of the knockdown. Continuous sucrose density gradients (10% to 50% wt/vol) were prepared in buffer containing 500 mM KCl, 10 mM MgCl2, 20 mM HEPES (pH 7.5), 2 mM dithiothreitol, 150 μg/mL cycloheximide, and 10 U/mL sodium heparin. Gradients were prepared in 5 mL polyallomer tubes by gently layering 2.2 mL of 10% sucrose in buffer over 2.2 mL of 50% sucrose in buffer. Tubes were then sealed, turned on their sides and left to equilibrate overnight at 4 °C. The next day, 600 μL of sample lysate (containing equal quantities of material as normalized by spectrophotometry, λ = 260 nm) was layered gently on to the gradients and ultracentrifuged in a Beckman Ti55 swing rotor at 83 000 × g for 6 h (4 °C). Continuous OD254 readings for gradients were read from the bottom by piercing and fractions were collected using an ISCO fractionator (Teledyne, ISCO). Ten fractions of 0.5 mL were collected from each gradient. In some experiments, a 250 μL aliquot was taken for RNA analysis while the remaining 250 μL was used for protein analysis. The ribosomal protein L7 was used in western blotting analyses in some experiments and this protein sediments in monosomes and in small polyribosomes, as shown.Citation48
RNA precipitation and northern blotting
RNA was purified from sucrose density gradient fractions exactly as described before using proteinase K digestion, phenol-chloroform extraction and ethanol precipitation.Citation42 Alternatively, TRIzol® LS (Invitrogen) was employed to isolate RNA from the collected fractions according to the manufacturer’s protocol. In both procedures, 5 μg of glycogen (Roche Diagnostics, ON) was used as a carrier in each sample during ethanol precipitation to enhance the yield of RNA. RNA pellets were resuspended in 10 μL of RNase-free water and denatured for 30 min at 65 °C in a solution of 15% sample, 50% formamide, 7.4% formaldehyde, and 5% ethidium bromide in MOPS buffer (20 mM 3-[N-morpholino] propanesulfonic acid, 2 mM sodium acetate, 2 mM EDTA). Samples were then resolved by electrophoresis in a denaturing 0.8% agarose gel containing 1.85% formaldehyde in 1X MOPS buffer. RNA was transferred overnight by capillary action to a Biodyne® B membrane (Pall Corporation) in 20X SSC (3 M NaCl, 300 mM sodium citrate, pH 7.0), then covalently linked using UV radiation. Membranes were hybridized overnight at 65 °C in Church’s Buffer (250 mM sodium phosphate [pH 7.0], 7% SDS, 1 mM EDTA [pH 8.0], 1% bovine serum albumin), and developed by autoradiography. A 32P-labeled cDNA probe specific to the TAR region (this probe recognizes all HIV-1 transcripts) and gapdh mRNA were used as described previously.Citation43,Citation76
For protein content analysis in each fraction, following sedimentation in sucrose gradients, each fraction was mixed with 0.1 volumes of 70% trichloroacetic acid (TCA), and stored on ice for 2 h in order to precipitate the protein components from the sucrose solution in each fraction. After this, samples were spun at 16 000 × g for 15 min (4 °C) and protein pellets were washed twice in ice cold acetone, then dried at room temperature. Dried pellets were resuspended in a solution of 5% SDS in PBS 1X, and stored at -20 °C. Resolution of the protein components in each fraction was accomplished using SDS PAGE (SDS-PAGE). Stacking gels were 4% polyacrylamide while the resolving gel was 12% acrylamide. Proteins were transferred to a nitrocellulose membrane (Pall Corporation) and blocked with 10% fat-free milk for 2 h.
Immunoblotting was performed with the following antibodies: rabbit anti-p24 (1:5000, Trinity Biotech); pan-specific rabbit polyclonal anti-hnRNP, recognizing hnRNP A1, A1b, A2, and B2 (1:5000) generously provided by Benoit Chabôt (Université de Sherbrooke); rabbit anti-eIF4G1 (1:1000), and rabbit anti-PABP1 (1:1000), both generously provided by Dr Nahum Sonenberg (McGill University); rabbit anti-γ-tubulin (1:5000, Sigma-Aldrich), mouse anti-L7 (1:5000, Novus Biologicals), mouse anti-eEF1α (1:1000), rabbit anti-CRM1 (1:5000, Santa Cruz Biotechnologies), and anti-GAPDH (1:5000, Sigma-Aldrich). Horseradish peroxidase-conjugated goat anti-rabbit (1:5000) and sheep anti-mouse (1:5000) were used as secondary antibodies. Finally, signals were exposed by autoradiography following a 1 min incubation with Western Lightning® Chemiluminescence Reagents, as described by the manufacturer (Perkin-Elmer). Antisera to hnRNP A2 (rabbit anti-hnRNP A2 [Act-2, used for immunofluorescence]), EF67 (mouse anti-hnRNP A2; see below) recognize both hnRNP A2 and hnRNP B1 due to common C-terminal epitopes in these isoforms.Citation35
Cell fractionation analyses
HeLa cells transfected with pcMRev(-) and siNS or siA2/B1 and cells were harvested at 30–36 h post-transfection. Cells were fractionated into nuclear and cytoplasmic fractions. Cells were allowed to swell for 5 min in 400 µL hypotonic buffer (20 mM Tris, 10 mM KCl, 1 mM MgCl2, 1 mM dithiothreitol, 0.5 mM PMSF, 0.2% NP-40) and were then dounce homogenized using an Eppendorf pestle. Nuclei were collected at 500 × g for 5 min at 4 °C and the supernatant was recentrifuged and put on ice (cytoplasmic extract). Nuclei were washed once with NP-40-containing buffer (+5 mM EDTA) and once without NP-40 (+5 mM EDTA) and the wash supernatants were discarded. Nuclei were then lysed in 10 mM Tris-100 mM NaCl-1mM EDTA containing 0.5% NP-40, centrifuged at 14 000 × g to remove chromosomal DNA. Aliquots from nuclear and cytoplasmic extracts were taken for western blot analysis. For RNA extraction, extracts were immediately transferred to a tube containing 400 µL phenol-chloroform-isoamyl alcohol (50:50:1) and 200 µL urea-SDS extraction buffer as described previously.Citation43,Citation77 RNA was collected by ethanol precipitation using glycogen as a carrier. RNA was slot-blotted onto nylon membranes and then probed using a HIV-1 vRNA-specific probe as describedCitation31 or one that recognizes gapdh mRNA.Citation43 RNA gel blotting was performed as described above.Citation43
Amino acid incorporation into de novo synthesized polypeptides
Cells were transfected with HxBRU or pcMRev(-) proviral DNA, and siNS or siA2/B1. Cells were washed with pre-warmed PBS and before harvesting cells for polyribosome analyses, cells were pulsed with 1150 µCi Express (SCitation35) Protein labeling mix (Perkin Elmer; 8 mCi/mL) for 7 min in methionine- and cysteine-free DMEM, washed three times with ice-cold PBS, and processed for polyribosome analyses as described above. Following collection of gradient fractions, a 200 µL aliquot was precipitated with 10% TCA, filtered through GF/C Whatman glass fiber filters. Filters were washed extensively with 5% TCA, dried, and radioactivity was counted using a liquid scintillation counter. Background counts in each fraction were derived from mock transfected cells incubated without radiolabelled amino acids. For this set of experiments, a mouse anti-hnRNP A2/B1 monoclonal antibody (EF67) was used to detect hnRNP A2/B1 proteins by western blotting analysis.
| Abbreviations: | ||
| HIV-1 | = | human immunodeficiency virus type 1 |
| hnRNP | = | heterogeneous ribonucleoprotein |
| RNP | = | ribonucleoprotein |
| kb | = | kilobasepair |
| vRNA | = | HIV-1 genomic RNA |
| RRE | = | Rev-responsive element |
| eIF | = | eukaryotic initiation factor |
| siNS | = | non-silencing siRNA |
| Gag | = | group specific antigen |
| MTOC | = | microtubule organising center |
| S.D. | = | standard deviation |
| PABP | = | polyA-binding protein |
| CRM1 | = | chromosome region maintenance 1 |
Additional material
Download Zip (1.9 MB)Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
We especially thank Dr Anne Monette, Melanie Halvorsen, and Martin Lehmann for contributions to experiments reported in this manuscript, Drs Benoit Chabôt, Nahum Sonenberg, Graciella Boccaccio for antibodies, Drs Sadaie, Hammarskjöld, Rekosh, and the AIDS Research and Reference Reagent Program, Division of AIDS, NIAID, NIH for reagents and Alan Cochrane for advice on the fractionation assay. AJM was supported by a Canadian Institutes of Health Research (CIHR) New Investigator Award and this work is supported by grants from the Canadian Foundation for AIDS Research and the CIHR (MOP-56974 & MOP-38111).
Supplemental Materials
Supplemental materials may be found here: www.landesbioscience.com/journals/rnabiology/article/26542
References
- Cochrane AW, McNally MT, Mouland AJ. The retrovirus RNA trafficking granule: from birth to maturity. Retrovirology 2006; 3:18; http://dx.doi.org/10.1186/1742-4690-3-18; PMID: 16545126
- Schneider R, Campbell M, Nasioulas G, Felber BK, Pavlakis GN. Inactivation of the human immunodeficiency virus type 1 inhibitory elements allows Rev-independent expression of Gag and Gag/protease and particle formation. J Virol 1997; 71:4892 - 903; PMID: 9188551
- Schwartz S, Felber BK, Pavlakis GN. Distinct RNA sequences in the gag region of human immunodeficiency virus type 1 decrease RNA stability and inhibit expression in the absence of Rev protein. J Virol 1992; 66:150 - 9; PMID: 1727477
- Suh D, Seguin B, Atkinson S, Ozdamar B, Staffa A, Emili A, Mouland A, Cochrane A. Mapping of determinants required for the function of the HIV-1 env nuclear retention sequence. Virology 2003; 310:85 - 99; http://dx.doi.org/10.1016/S0042-6822(03)00073-4; PMID: 12788633
- Malim MH, Hauber J, Le SY, Maizel JV, Cullen BR. The HIV-1 rev trans-activator acts through a structured target sequence to activate nuclear export of unspliced viral mRNA. Nature 1989; 338:254 - 7; http://dx.doi.org/10.1038/338254a0; PMID: 2784194
- Sodroski J, Goh WC, Rosen C, Dayton A, Terwilliger E, Haseltine W. A second post-transcriptional trans-activator gene required for HTLV-III replication. Nature 1986; 321:412 - 7; http://dx.doi.org/10.1038/321412a0; PMID: 3012355
- Emerman M, Vazeux R, Peden K. The rev gene product of the human immunodeficiency virus affects envelope-specific RNA localization. Cell 1989; 57:1155 - 65; http://dx.doi.org/10.1016/0092-8674(89)90053-6; PMID: 2736624
- Felber BK, Hadzopoulou-Cladaras M, Cladaras C, Copeland T, Pavlakis GN. rev protein of human immunodeficiency virus type 1 affects the stability and transport of the viral mRNA. Proc Natl Acad Sci U S A 1989; 86:1495 - 9; http://dx.doi.org/10.1073/pnas.86.5.1495; PMID: 2784208
- Naji S, Ambrus G, Cimermančič P, Reyes JR, Johnson JR, Filbrandt R, Huber MD, Vesely P, Krogan NJ, Yates JR 3rd, et al. Host cell interactome of HIV-1 Rev includes RNA helicases involved in multiple facets of virus production. Mol Cell Proteomics 2012; 11:M111.015313; http://dx.doi.org/10.1074/mcp.M111.015313; PMID: 22174317
- Kula A, Guerra J, Knezevich A, Kleva D, Myers MP, Marcello A. Characterization of the HIV-1 RNA associated proteome identifies Matrin 3 as a nuclear cofactor of Rev function. Retrovirology 2011; 8:60; http://dx.doi.org/10.1186/1742-4690-8-60; PMID: 21771346
- Kjems J, Askjaer P. Rev protein and its cellular partners. Adv Pharmacol 2000; 48:251 - 98; http://dx.doi.org/10.1016/S1054-3589(00)48009-9; PMID: 10987094
- Zolotukhin AS, Michalowski D, Bear J, Smulevitch SV, Traish AM, Peng R, Patton J, Shatsky IN, Felber BK. PSF acts through the human immunodeficiency virus type 1 mRNA instability elements to regulate virus expression. Mol Cell Biol 2003; 23:6618 - 30; http://dx.doi.org/10.1128/MCB.23.18.6618-6630.2003; PMID: 12944487
- Stoltzfus CM, Madsen JM. Role of viral splicing elements and cellular RNA binding proteins in regulation of HIV-1 alternative RNA splicing. Curr HIV Res 2006; 4:43 - 55; http://dx.doi.org/10.2174/157016206775197655; PMID: 16454710
- Pomerantz RJ, Seshamma T, Trono D. Efficient replication of human immunodeficiency virus type 1 requires a threshold level of Rev: potential implications for latency. J Virol 1992; 66:1809 - 13; PMID: 1738210
- Anderson EC, Lever AM. Human immunodeficiency virus type 1 Gag polyprotein modulates its own translation. J Virol 2006; 80:10478 - 86; http://dx.doi.org/10.1128/JVI.02596-05; PMID: 17041220
- Arrigo SJ, Chen IS. Rev is necessary for translation but not cytoplasmic accumulation of HIV-1 vif, vpr, and env/vpu 2 RNAs. Genes Dev 1991; 5:808 - 19; http://dx.doi.org/10.1101/gad.5.5.808; PMID: 1827422
- D’Agostino DM, Felber BK, Harrison JE, Pavlakis GN. The Rev protein of human immunodeficiency virus type 1 promotes polysomal association and translation of gag/pol and vpu/env mRNAs. Mol Cell Biol 1992; 12:1375 - 86; PMID: 1545819
- Cochrane A. Controlling HIV-1 Rev function. Curr Drug Targets Immune Endocr Metabol Disord 2004; 4:287 - 95; http://dx.doi.org/10.2174/1568008043339677; PMID: 15578979
- Stauber RH, Afonina E, Gulnik S, Erickson J, Pavlakis GN. Analysis of intracellular trafficking and interactions of cytoplasmic HIV-1 Rev mutants in living cells. Virology 1998; 251:38 - 48; http://dx.doi.org/10.1006/viro.1998.9295; PMID: 9813201
- Cullen BR. Nuclear mRNA export: insights from virology. Trends Biochem Sci 2003; 28:419 - 24; http://dx.doi.org/10.1016/S0968-0004(03)00142-7; PMID: 12932730
- Lawrence JB, Cochrane AW, Johnson CV, Perkins A, Rosen CA. The HIV-1 Rev protein: a model system for coupled RNA transport and translation. New Biol 1991; 3:1220 - 32; PMID: 1725960
- Malim MH, Cullen BR. Rev and the fate of pre-mRNA in the nucleus: implications for the regulation of RNA processing in eukaryotes. Mol Cell Biol 1993; 13:6180 - 9; PMID: 8105371
- Najera I, Krieg M, Karn J. Synergistic stimulation of HIV-1 rev-dependent export of unspliced mRNA to the cytoplasm by hnRNP A1. J Mol Biol 1999; 285:1951 - 64; http://dx.doi.org/10.1006/jmbi.1998.2473; PMID: 9925777
- Jablonski JA, Caputi M. Role of cellular RNA processing factors in human immunodeficiency virus type 1 mRNA metabolism, replication, and infectivity. J Virol 2009; 83:981 - 92; http://dx.doi.org/10.1128/JVI.01801-08; PMID: 19004959
- Kress E, Baydoun HH, Bex F, Gazzolo L, Duc Dodon M. Critical role of hnRNP A1 in HTLV-1 replication in human transformed T lymphocytes. Retrovirology 2005; 2:8; http://dx.doi.org/10.1186/1742-4690-2-8; PMID: 15703079
- Huang Y, Wimler KM, Carmichael GG. Intronless mRNA transport elements may affect multiple steps of pre-mRNA processing. EMBO J 1999; 18:1642 - 52; http://dx.doi.org/10.1093/emboj/18.6.1642; PMID: 10075934
- Hadian K, Vincendeau M, Mäusbacher N, Nagel D, Hauck SM, Ueffing M, Loyter A, Werner T, Wolff H, Brack-Werner R. Identification of a heterogeneous nuclear ribonucleoprotein-recognition region in the HIV Rev protein. J Biol Chem 2009; 284:33384 - 91; http://dx.doi.org/10.1074/jbc.M109.021659; PMID: 19808671
- Brumwell C, Antolik C, Carson JH, Barbarese E. Intracellular trafficking of hnRNP A2 in oligodendrocytes. Exp Cell Res 2002; 279:310 - 20; http://dx.doi.org/10.1006/excr.2002.5604; PMID: 12243756
- Huang YS, Carson JH, Barbarese E, Richter JD. Facilitation of dendritic mRNA transport by CPEB. Genes Dev 2003; 17:638 - 53; http://dx.doi.org/10.1101/gad.1053003; PMID: 12629046
- Gu W, Pan F, Zhang H, Bassell GJ, Singer RH. A predominantly nuclear protein affecting cytoplasmic localization of beta-actin mRNA in fibroblasts and neurons. J Cell Biol 2002; 156:41 - 51; http://dx.doi.org/10.1083/jcb.200105133; PMID: 11781334
- Lévesque K, Halvorsen M, Abrahamyan L, Chatel-Chaix L, Poupon V, Gordon H, DesGroseillers L, Gatignol A, Mouland AJ. Trafficking of HIV-1 RNA is mediated by heterogeneous nuclear ribonucleoprotein A2 expression and impacts on viral assembly. Traffic 2006; 7:1177 - 93; http://dx.doi.org/10.1111/j.1600-0854.2006.00461.x; PMID: 17004321
- Lund N, Milev MP, Wong R, Sanmuganantham T, Woolaway K, Chabot B, Abou Elela S, Mouland AJ, Cochrane A. Differential effects of hnRNP D/AUF1 isoforms on HIV-1 gene expression. Nucleic Acids Res 2012; 40:3663 - 75; http://dx.doi.org/10.1093/nar/gkr1238; PMID: 22187150
- Milev MP, Ravichandran M, Khan MF, Schriemer DC, Mouland AJ. Characterization of staufen1 ribonucleoproteins by mass spectrometry and biochemical analyses reveal the presence of diverse host proteins associated with human immunodeficiency virus type 1. Front Microbiol 2012; 3:367; http://dx.doi.org/10.3389/fmicb.2012.00367; PMID: 23125841
- Monette A, Ajamian L, López-Lastra M, Mouland AJ. Human immunodeficiency virus type 1 (HIV-1) induces the cytoplasmic retention of heterogeneous nuclear ribonucleoprotein A1 by disrupting nuclear import: implications for HIV-1 gene expression. J Biol Chem 2009; 284:31350 - 62; http://dx.doi.org/10.1074/jbc.M109.048736; PMID: 19737937
- Bériault V, Clément JF, Lévesque K, Lebel C, Yong X, Chabot B, Cohen EA, Cochrane AW, Rigby WF, Mouland AJ. A late role for the association of hnRNP A2 with the HIV-1 hnRNP A2 response elements in genomic RNA, Gag, and Vpr localization. J Biol Chem 2004; 279:44141 - 53; http://dx.doi.org/10.1074/jbc.M404691200; PMID: 15294897
- Lehmann M, Milev MP, Abrahamyan L, Yao XJ, Pante N, Mouland AJ. Intracellular transport of human immunodeficiency virus type 1 genomic RNA and viral production are dependent on dynein motor function and late endosome positioning. J Biol Chem 2009; 284:14572 - 85; http://dx.doi.org/10.1074/jbc.M808531200; PMID: 19286658
- Kanai Y, Dohmae N, Hirokawa N. Kinesin transports RNA: isolation and characterization of an RNA-transporting granule. Neuron 2004; 43:513 - 25; http://dx.doi.org/10.1016/j.neuron.2004.07.022; PMID: 15312650
- Villacé P, Marión RM, Ortín J. The composition of Staufen-containing RNA granules from human cells indicates their role in the regulated transport and translation of messenger RNAs. Nucleic Acids Res 2004; 32:2411 - 20; http://dx.doi.org/10.1093/nar/gkh552; PMID: 15121898
- Mallardo M, Deitinghoff A, Müller J, Goetze B, Macchi P, Peters C, Kiebler MA. Isolation and characterization of Staufen-containing ribonucleoprotein particles from rat brain. Proc Natl Acad Sci U S A 2003; 100:2100 - 5; http://dx.doi.org/10.1073/pnas.0334355100; PMID: 12592035
- Lewis N, Williams J, Rekosh D, Hammarskjöld ML. Identification of a cis-acting element in human immunodeficiency virus type 2 (HIV-2) that is responsive to the HIV-1 rev and human T-cell leukemia virus types I and II rex proteins. J Virol 1990; 64:1690 - 7; PMID: 2157051
- Krichevsky AM, Kosik KS. Neuronal RNA granules: a link between RNA localization and stimulation-dependent translation. Neuron 2001; 32:683 - 96; http://dx.doi.org/10.1016/S0896-6273(01)00508-6; PMID: 11719208
- Mouland AJ, Hendy GN. 1,25-Dihydroxycholecalciferol regulates chromogranin-A translatability in bovine parathyroid cells. Mol Endocrinol 1992; 6:1781 - 8; http://dx.doi.org/10.1210/me.6.11.1781; PMID: 1480170
- Mouland AJ, Coady M, Yao XJ, Cohen ÉA. Hypophosphorylation of poly(A) polymerase and increased polyadenylation activity are associated with human immunodeficiency virus type 1 Vpr expression. Virology 2002; 292:321 - 30; http://dx.doi.org/10.1006/viro.2001.1261; PMID: 11878934
- Abrahamyan LG, Chatel-Chaix L, Ajamian L, Milev MP, Monette A, Clément JF, Song R, Lehmann M, DesGroseillers L, Laughrea M, et al. Novel Staufen1 ribonucleoproteins prevent formation of stress granules but favour encapsidation of HIV-1 genomic RNA. J Cell Sci 2010; 123:369 - 83; http://dx.doi.org/10.1242/jcs.055897; PMID: 20053637
- Sharma A, Yilmaz A, Marsh K, Cochrane A, Boris-Lawrie K. Thriving under stress: selective translation of HIV-1 structural protein mRNA during Vpr-mediated impairment of eIF4E translation activity. PLoS Pathog 2012; 8:e1002612; http://dx.doi.org/10.1371/journal.ppat.1002612; PMID: 22457629
- Anderson P, Kedersha N. RNA granules. J Cell Biol 2006; 172:803 - 8; http://dx.doi.org/10.1083/jcb.200512082; PMID: 16520386
- Rodriguez AJ, Shenoy SM, Singer RH, Condeelis J. Visualization of mRNA translation in living cells. J Cell Biol 2006; 175:67 - 76; http://dx.doi.org/10.1083/jcb.200512137; PMID: 17030983
- Ko JR, Wu JY, Kirby R, Li IF, Lin A. Mapping the essential structures of human ribosomal protein L7 for nuclear entry, ribosome assembly and function. FEBS Lett 2006; 580:3804 - 10; http://dx.doi.org/10.1016/j.febslet.2006.05.073; PMID: 16797011
- Forgues M, Difilippantonio MJ, Linke SP, Ried T, Nagashima K, Feden J, Valerie K, Fukasawa K, Wang XW. Involvement of Crm1 in hepatitis B virus X protein-induced aberrant centriole replication and abnormal mitotic spindles. Mol Cell Biol 2003; 23:5282 - 92; http://dx.doi.org/10.1128/MCB.23.15.5282-5292.2003; PMID: 12861014
- Gross JD, Moerke NJ, von der Haar T, Lugovskoy AA, Sachs AB, McCarthy JE, Wagner G. Ribosome loading onto the mRNA cap is driven by conformational coupling between eIF4G and eIF4E. Cell 2003; 115:739 - 50; http://dx.doi.org/10.1016/S0092-8674(03)00975-9; PMID: 14675538
- Patry C, Bouchard L, Labrecque P, Gendron D, Lemieux B, Toutant J, Lapointe E, Wellinger R, Chabot B. Small interfering RNA-mediated reduction in heterogeneous nuclear ribonucleoparticule A1/A2 proteins induces apoptosis in human cancer cells but not in normal mortal cell lines. Cancer Res 2003; 63:7679 - 88; PMID: 14633690
- Marión RM, Fortes P, Beloso A, Dotti C, Ortín J. A human sequence homologue of Staufen is an RNA-binding protein that is associated with polysomes and localizes to the rough endoplasmic reticulum. Mol Cell Biol 1999; 19:2212 - 9; PMID: 10022908
- Wickham L, Duchaîne T, Luo M, Nabi IR, DesGroseillers L. Mammalian staufen is a double-stranded-RNA- and tubulin-binding protein which localizes to the rough endoplasmic reticulum. Mol Cell Biol 1999; 19:2220 - 30; PMID: 10022909
- Duchaîne T, Wang HJ, Luo M, Steinberg SV, Nabi IR, DesGroseillers L. A novel murine Staufen isoform modulates the RNA content of Staufen complexes. Mol Cell Biol 2000; 20:5592 - 601; http://dx.doi.org/10.1128/MCB.20.15.5592-5601.2000; PMID: 10891497
- Furic L, Maher-Laporte M, DesGroseillers L. A genome-wide approach identifies distinct but overlapping subsets of cellular mRNAs associated with Staufen1- and Staufen2-containing ribonucleoprotein complexes. RNA 2008; 14:324 - 35; http://dx.doi.org/10.1261/rna.720308; PMID: 18094122
- Duchaîne TF, Hemraj I, Furic L, Deitinghoff A, Kiebler MA, DesGroseillers L. Staufen2 isoforms localize to the somatodendritic domain of neurons and interact with different organelles. J Cell Sci 2002; 115:3285 - 95; PMID: 12140260
- Macchi P, Brownawell AM, Grunewald B, DesGroseillers L, Macara IG, Kiebler MA. The brain-specific double-stranded RNA-binding protein Staufen2: nucleolar accumulation and isoform-specific exportin-5-dependent export. J Biol Chem 2004; 279:31440 - 4; http://dx.doi.org/10.1074/jbc.C400226200; PMID: 15166236
- Shyu AB, Wilkinson MF. The double lives of shuttling mRNA binding proteins. Cell 2000; 102:135 - 8; http://dx.doi.org/10.1016/S0092-8674(00)00018-0; PMID: 10943833
- Kosik KS, Krichevsky AM. The message and the messenger: delivering RNA in neurons. Sci STKE 2002; 2002:pe16; http://dx.doi.org/10.1126/stke.2002.126.pe16; PMID: 11930084
- Barbarese E, Koppel DE, Deutscher MP, Smith CL, Ainger K, Morgan F, Carson JH. Protein translation components are colocalized in granules in oligodendrocytes. J Cell Sci 1995; 108:2781 - 90; PMID: 7593319
- Kosturko LD, Maggipinto MJ, Korza G, Lee JW, Carson JH, Barbarese E. Heterogeneous nuclear ribonucleoprotein (hnRNP) E1 binds to hnRNP A2 and inhibits translation of A2 response element mRNAs. Mol Biol Cell 2006; 17:3521 - 33; http://dx.doi.org/10.1091/mbc.E05-10-0946; PMID: 16775011
- Kwon S, Barbarese E, Carson JH. The cis-acting RNA trafficking signal from myelin basic protein mRNA and its cognate trans-acting ligand hnRNP A2 enhance cap-dependent translation. J Cell Biol 1999; 147:247 - 56; http://dx.doi.org/10.1083/jcb.147.2.247; PMID: 10525532
- Poole E, Strappe P, Mok HP, Hicks R, Lever AM. HIV-1 Gag-RNA interaction occurs at a perinuclear/centrosomal site; analysis by confocal microscopy and FRET. Traffic 2005; 6:741 - 55; http://dx.doi.org/10.1111/j.1600-0854.2005.00312.x; PMID: 16101678
- Cimarelli A, Luban J. Translation elongation factor 1-alpha interacts specifically with the human immunodeficiency virus type 1 Gag polyprotein. J Virol 1999; 73:5388 - 401; PMID: 10364286
- Jäger S, Cimermancic P, Gulbahce N, Johnson JR, McGovern KE, Clarke SC, Shales M, Mercenne G, Pache L, Li K, et al. Global landscape of HIV-human protein complexes. Nature 2012; 481:365 - 70; PMID: 22190034
- Parkin NT, Cohen EA, Darveau A, Rosen C, Haseltine W, Sonenberg N. Mutational analysis of the 5′ non-coding region of human immunodeficiency virus type 1: effects of secondary structure on translation. EMBO J 1988; 7:2831 - 7; PMID: 3181141
- Gallego J, Greatorex J, Zhang H, Yang B, Arunachalam S, Fang J, Seamons J, Lea S, Pomerantz RJ, Lever AM. Rev binds specifically to a purine loop in the SL1 region of the HIV-1 leader RNA. J Biol Chem 2003; 278:40385 - 91; http://dx.doi.org/10.1074/jbc.M301041200; PMID: 12851400
- Groom HC, Anderson EC, Dangerfield JA, Lever AM. Rev regulates translation of human immunodeficiency virus type 1 RNAs. J Gen Virol 2009; 90:1141 - 7; http://dx.doi.org/10.1099/vir.0.007963-0; PMID: 19264599
- Groom HC, Anderson EC, Lever AM. Rev: beyond nuclear export. J Gen Virol 2009; 90:1303 - 18; http://dx.doi.org/10.1099/vir.0.011460-0; PMID: 19321757
- Paillart JC, Dettenhofer M, Yu XF, Ehresmann C, Ehresmann B, Marquet R. First snapshots of the HIV-1 RNA structure in infected cells and in virions. J Biol Chem 2004; 279:48397 - 403; http://dx.doi.org/10.1074/jbc.M408294200; PMID: 15355993
- Abbink TE, Berkhout B. A novel long distance base-pairing interaction in human immunodeficiency virus type 1 RNA occludes the Gag start codon. J Biol Chem 2003; 278:11601 - 11; http://dx.doi.org/10.1074/jbc.M210291200; PMID: 12458192
- Ooms M, Huthoff H, Russell R, Liang C, Berkhout B. A riboswitch regulates RNA dimerization and packaging in human immunodeficiency virus type 1 virions. J Virol 2004; 78:10814 - 9; http://dx.doi.org/10.1128/JVI.78.19.10814-10819.2004; PMID: 15367648
- Sadaie MR, Benter T, Wong-Staal F. Site-directed mutagenesis of two trans-regulatory genes (tat-III,trs) of HIV-1. Science 1988; 239:910 - 3; http://dx.doi.org/10.1126/science.3277284; PMID: 3277284
- Sadaie MR, Rappaport J, Benter T, Josephs SF, Willis R, Wong-Staal F. Missense mutations in an infectious human immunodeficiency viral genome: functional mapping of tat and identification of the rev splice acceptor. Proc Natl Acad Sci U S A 1988; 85:9224 - 8; http://dx.doi.org/10.1073/pnas.85.23.9224; PMID: 3194421
- Vyboh K, Ajamian L, Mouland AJ. Detection of viral RNA by Fluorescence in situ Hybridization (FISH). Journal of Visualized Experiments 2012; http://www.jove.com/video/4002/.
- Yao XJ, Mouland AJ, Subbramanian RA, Forget J, Rougeau N, Bergeron D, Cohen EA. Vpr stimulates viral expression and induces cell killing in human immunodeficiency virus type 1-infected dividing Jurkat T cells. J Virol 1998; 72:4686 - 93; PMID: 9573232
- Mouland AJ, Hendy GN. Regulation of synthesis and secretion of chromogranin-A by calcium and 1,25-dihydroxycholecalciferol in cultured bovine parathyroid cells. Endocrinology 1991; 128:441 - 9; http://dx.doi.org/10.1210/endo-128-1-441; PMID: 1986936