 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
The analysis of binding interactions between small molecules and biopolymers is important for understanding biological processes. While fluorescence correlation spectroscopy (FCS) requires fluorescence labeling on the small molecule, which often interferes with binding, in microscale thermophoresis (MST) the label can be placed on the biopolymer. Ribozymes have not been analyzed by MST so far. The Diels-Alderase ribozyme (DAse) is a true catalyst, facilitating the Diels-Alder reaction between two free small substrates, anthracene dienes, and maleimide dienophiles. Despite high efforts, the determination of the dissociation constant (KD) of maleimide dienophiles to the DAse by FCS has been unsuccessful. Here, we determined the binding interactions of the DAse to its substrates and the Diels-Alder product using MST. The results supported a positive cooperativity for substrate binding to the DAse. By varying the temperature, we furthermore studied the thermodynamics of dienophile dissociation. The entropic contribution was found to be the energetic driving force for the binding of the dienophile to the DAse.
Introduction
The characterization of RNA-small molecule interactions is of utmost interest for understanding the catalytic mechanisms of ribozymes. Among a large number of natural and in vitro-evolved catalytic RNAs, the Diels-Alderase (DAse) is one of the most thoroughly characterized ribozymes. In contrast to most ribozymes, the DAse undergoes multiple catalytic cycles. It can utilize two freely diffusing substrates, an anthracene and a maleimide derivative, in trans with multiple turnovers.Citation1,Citation2 While exhaustive data on the structureCitation3,Citation4 (crystal structure, ) and folding dynamics of the DAseCitation5 exist, insights into its catalytic mechanism are scarce, and the limited solubility of the substrates so far prevented determination of the binding constants and order. Yet, a comprehensive investigation of the enzymatic mechanism of the DAse would be facilitated considerably by a direct and independent determination of KD values of all chemical species involved in the reaction, namely the anthracene and the maleimide, as well as the corresponding Diels-Alder product. Fluorescence-based approaches, such as anisotropy measurements,Citation6 quenching assays,Citation7,Citation8 and FCS,Citation9-Citation11 have been frequently applied to determine dissociation constants of RNA-aptamer/ligand complexes. Previously, binding of anthracene-fluorophore conjugates was analyzed by FCS,Citation12 but no direct binding data on the maleimide moiety could be obtained. When we initiated the current study, we undertook another attempt to measure the binding of dye-labeled maleimides to the DAse (Fig. S1), just to learn that the chemical nature of the fluorophore, rather than the substrate moiety, governs the binding properties in a specific manner (Fig. S2). This is a general disadvantage of FCS-based approaches as they depend on the fluorescent labeling of the smaller interacting partner. We reasoned that this shortcoming could only be overcome by an experimental setup that would be compatible with fluorescent labeling of the larger interacting partner, i.e., the ribozyme, leaving the substrate unaltered. MST is a rather new method for biomolecular interaction analysis in which the binding constants are determined based on the differential directed movement of biomolecules in a microscale temperature gradient.Citation13-Citation16 Typically, the biomolecule is fluorescently labeled. A binding event changes the hydration shell of the biomolecules, and thereby modulates the movement of the biomolecule along the temperature gradient. Since the method is based on fluorescence, it is highly sensitive and permits measurements using small amounts of the labeled biomolecule. MST has been shown to be an effective method for the determination of binding constants for protein/protein,Citation17 small molecule/protein,Citation18 small molecule/DNA aptamer,Citation19 protein/DNA,Citation19-Citation22 and protein/RNA complexes,Citation23,Citation24 but has never been used on ribozymes. Ribozymes, and in particular those evolved by in vitro selection, typically have higher KM-values for their cognate substrates than typical protein enzymes. Previous kinetic studies by our research group revealed KM-values for the dienophile substrates in the low millimolar range.Citation2 In classical fluorescence-based approaches, very high concentrations of the ribozyme would have to be provided to saturate the fluorescent substrate. Therefore, in addition to the misleading structural effects of the dye-label, enormous concentrations of the ribozyme would have to be supplied, which is incompatible with spectrometric and viscous homogeneity of the sample. Considering these challenging boundary conditions, we expected MST to be a suitable tool to analyze binding events in this class of biomolecules.
Figure 1. (A) 3D structure of the DAse ribozyme bound to a Diels-Alder product (adapted from PDB: 1YKV). The 3′ end of the DAse was labeled with Cy5. (B) Labeling on the DAse does not hamper its catalytic activity where DAse-3′-Cy5 (red line) had similar activity compared to unlabeled DAse (blue line). The activity was measured by a fluorescence assay using 1-AB (5 µM) and NPM (500 µM) as substrates (DAse: 0.67 µM). The increase in fluorescence (Ex: 460 nm Em: 510 nm) is due to disruption of the anthracene π-system, which quenched BODIPY fluorescence due to photoinduced electron transfer (PeT). Uncatalyzed background reaction (kapp,background = [0.036 ± 0.008] 10−5 s−1 - black line) and photoreaction (kapp,photo = [0.03 ± 0.01] 10−5 s−1 - green line) were significantly slow compared to the catalyzed reactions.
![Figure 1. (A) 3D structure of the DAse ribozyme bound to a Diels-Alder product (adapted from PDB: 1YKV). The 3′ end of the DAse was labeled with Cy5. (B) Labeling on the DAse does not hamper its catalytic activity where DAse-3′-Cy5 (red line) had similar activity compared to unlabeled DAse (blue line). The activity was measured by a fluorescence assay using 1-AB (5 µM) and NPM (500 µM) as substrates (DAse: 0.67 µM). The increase in fluorescence (Ex: 460 nm Em: 510 nm) is due to disruption of the anthracene π-system, which quenched BODIPY fluorescence due to photoinduced electron transfer (PeT). Uncatalyzed background reaction (kapp,background = [0.036 ± 0.008] 10−5 s−1 - black line) and photoreaction (kapp,photo = [0.03 ± 0.01] 10−5 s−1 - green line) were significantly slow compared to the catalyzed reactions.](/cms/asset/ba897881-c849-41a6-85fe-310a8dd32c89/krnb_a_10927101_f0001.gif)
Here, we studied substrate binding to the DAse ribozyme to broaden the scope of MST. First, we showed that MST could be used reliably for the DAse, compared with previously published FCS binding data.Citation12 Then, we analyzed the binding of dienes and dienophiles to the DAse, and the thermodynamics of dienophile binding. Hydrophobic interactions and entropy played an important role in dienophile binding. Finally, a positive cooperativity was observed between the substrates of the DAse.
Results and Discussion
Labeling on the DAse does not change its catalytic activity
Fluorescent labeling on the larger bio-molecule is the main advantage of MST. For MST measurements, the 3′ terminus of the DAse was labeled with Cy5. As shown in , this position is far away from the active site and therefore unlikely to interfere with catalysis, which could be confirmed by activity determinations using the fluorescent anthracene-BODIPY turn-on probe 1-AB in combination with N-pentylmaleimide (NPM).Citation12 The apparent pseudo-first order rate constants for the labeled DAse-3′-Cy5 (kapp = 0.015 ± 0.002 s−1) and the unlabeled DAse (kapp = 0.014 ± 0.002 s−1) were essentially identical, indicating that DAse-3′-Cy5 showed “wild-type”-like behavior ().
MST is reliable and comparable to FCS
Previously, the binding interactions between the DAse and diene substrates or Diels-Alder product were analyzed by FCS.Citation12 To assess the suitability of MST for determining ribozyme binding constants, we first analyzed non-fluorescent anthracene (A) and Diels-Alder product (DAP) derivatives containing hexaethylene glycol (HEG) or hexaethylene glycol phosphate (HEG-P) chains (1-AHEG-P, 9-AHEG-P and 9-DAP-HEG, respectively, ). The KD values of non-fluorescent compounds determined by MST () were in good agreement with the FCS-derived data measured with fluorescent BODIPY derivatives ().Citation12 Overall, the results showed that MST is a reliable and comparable method to FCS.
Figure 2. Comparison of substrates and product of the DAse used in FCS and MST measurements. (A) Fluorescent BODIPY molecules used for FCS measurements,Citation12 first row, and non-fluorescent analogs for MST measurements, second row (B) MST analysis of DAse-3′-Cy5 (50 nM) binding to 9-DAP-HEG (black circles), 1-AHEG-P (dark gray triangles), and 9-AHEG-P (light gray squares). The solid curves are the fit of the data points to the Hill equation (n = 1). The error bars represent the standard error in triplicate measurements.
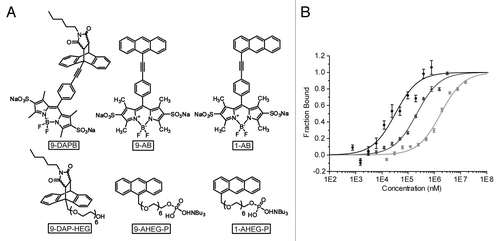
Table 1. Comparison of dissociation constants determined by FCS and MST techniques
MST enables measurement of KD-values of dienophiles
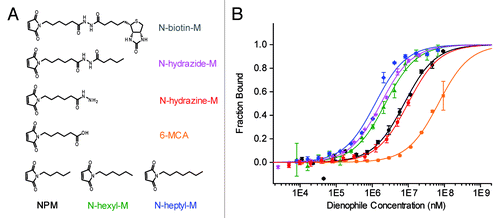
The dissociation constants of the maleimide substrates in DAse buffer (300 mM NaCl, 30 mM TRIS-HCl, 80 mM MgCl2, 2% DMSO, and 8% EtOH, pH 7.4) were determined that had been inaccessible so far (). Maleimide dienophiles that were known to be accepted as substrates were chosen based on their polarities.Citation2,Citation25 The measurements yielded regular binding isotherms with KD values (at 22 °C) ranging from 1.2 ± 0.1 mM to more than 15 mM (; Table S1). The catalytic pocket of the DAse is highly hydrophobic.Citation3 Notably, we found that increasingly long, non-polar substitution on the maleimide nitrogen facilitated the maleimide binding considerably. Introduction of a negative charge into the aliphatic side-chain of NPM (6-MCA) essentially suppressed the binding, which could be restored by functionalization to the corresponding hydrazine (N-hydrazine-M). In agreement with our previous observation, aliphatic N′-substitution on the hydrazine (N-hydrazide-M) improved binding. Unfortunately, N-biotin-M had drastically lower solubility compared with other maleimides, hence no binding data could be obtained. These findings revealed a clear preference of the DAse for hydrophobic substitution on the maleimide nitrogen. Although N-biotin-M was used in the in vitro evolution and selection of DAse, NPM (KD,NPM = 7.7 ± 0.9 mM) is known to be the best dienophile for catalysis,Citation2 therefore we used this substrate for further thermodynamic analysis.
Figure 3. (A) Reactive maleimide dienophile substrates. (B) Binding of maleimide dienophiles to 50 nM DAse-3′-Cy5 (N-heptyl-M, blue diamonds; N-hydrazide-M, magenta stars; N-hexyl-M, green triangles; NPM, black circles; N-hydrazine-M, red pentagons; 6-MCA, orange squares) The solid curves are the fits of the data points to the Hill equation (n = 1). The orange and red solid curves are only to guide the eye since a plateau could not be reached due to solubility limit of 6-MCA and N-hydrazine-M. Error bars represent the standard error in triplicate measurements.
Thermodynamic analysis of NPM dissociation by MST revealed the importance of bound water
Thermodynamic information on binding can be determined, using small amounts of sample (in comparison to isothermal titration calorimetry), by MST under temperature control. According to van’t Hoff’s equation,
eq 1
enthalpy and entropy can be obtained by determining KD at different temperatures. We measured the KD,NPM values at various temperatures between 293 K and 305 K (Fig. S3) and plotted them using van’t Hoff’s equation (). The linear fit to eq 1 was then used to calculate enthalpy (-8.159 kcal/mol), entropy (-37.3 cal/K∙mol), and Gibbs free energy at 300 K (3.031 kcal/mol) for the dissociation of NPM from the DAse. Opposing enthalpic and entropic contributions were observed for the dissociation of NPM from the catalytic pocket. A negative enthalpic value favored the dissociation. At ambient temperatures, however, the -T∆S value was always larger than the enthalpic term, making the dissociation overall unfavorable (a positive ∆G value of 3.031 kcal/mol at 300 K). As a result, the entropic contribution was the energetic driving force for the binding of NPM to the DAse.
Figure 4. (A) van’t Hoff Plot of NPM dissociation from DAse-3′-Cy5. The dependence of the dissociation constant of NPM to DAse-3′-Cy5 was plotted at different temperatures according to eq 1. The linear fit of the eq 1 (black solid line) yielded a slope of -∆H/R and an intercept at ∆S. The error bars represent the standard deviation in triplicate measurements. (B) Proposed mechanism for NPM binding supported by thermodynamic measurements. The dissociation of water from the catalytic site is the energetic driving force for the dienophile binding.
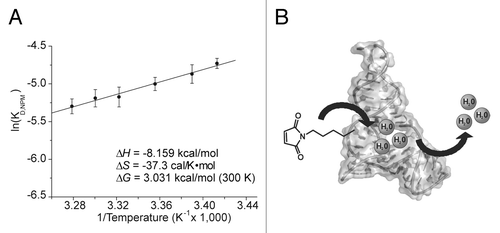
∆S for the dissociation of NPM from the DAse was found to be -37.3 cal/K∙mol (a decrease in disorder). When a small molecule dissociates from the binding site, a positive ∆S (increase in disorder) is usually expected due to the gain of translational and rotational freedom. However, this expectation might not be realistic since in biological systems the molecules are surrounded and solvated by water. The importance of bound water in the catalytic site of enzymes and ribozymes has emerged as a current research theme. The entropy of the system can, thus, decrease as a result of a dissociation event due to ordered water.Citation26-Citation28 Single-molecule FRET measurements and MD simulations on the apo-DAse revealed a dynamic ribozyme structure where the distribution of states was dependent on the Mg2+ concentration.Citation5,Citation29 In those MD simulations, the catalytic pocket was found to have two possible conformations, an open and a closed state. In the open state, up to 23 water molecules were observed inside the catalytic pocket, while in the closed state the catalytic pocket had collapsed and no water molecules could be accommodated.Citation29 Therefore, binding of NPM to the open state DAse would result a priori in a loss of bound water from the catalytical pocket (). This can explain the unexpected negative entropy values for the dissociation of the NPM from the catalytic site, as well as the preference for non-polar maleimide substrates.
MST measurements with an inhibitor shed light on the cooperativity of substrate binding to the DAse
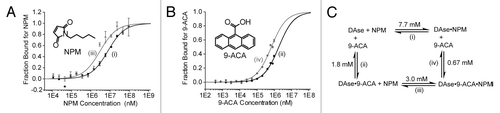
Previously, an ordered substrate binding mechanism (dienophile binding first) had been proposed based on MD simulationsCitation30 but not experimentally tested. A full kinetic analysis is needed to unravel the exact kinetic scheme and the catalytic mechanism of the DAse. Unfortunately, this has been highly challenging due to the poor solubility of the substrates and the limited detection options available for kinetic measurements.Citation12 Although the order of binding for catalysis cannot be obtained from the binding measurements in thermodynamic equilibrium, like in MST, cooperativity of substrate binding analysis is accessible (eq S4). For this purpose, we used anthracene-9-carboxylic acid (9-ACA), which was previously shown to be inactive as a substrate but a potent inhibitor of the DAse-catalyzed reaction.Citation25 This suggests that 9-ACA can still bind into the catalytic pocket, but the electron-withdrawing effect of the carboxylate group reduces electron density and thereby reactivity. As a result, 9-ACA binds to the DAse along with NPM but the resultant complex is practically inactive, even at saturating concentrations of NPM and 9-ACA (Fig. S4). We measured NPM binding to the DAse in the presence and in the absence of saturating concentrations of 9-ACA (1.1 mM) (). The binding of NPM was favored 2.6-fold when 9-ACA was present. Similarly, the binding of 9-ACA to DAse was determined in the absence and in the presence of saturating concentrations of NPM (92 mM) (). A similar positive cooperativity (synergy) of 2.7-fold was observed. Previously, the potential of mean force (PMF) (or the free energy change)Citation31 for releasing the NPM in the presence of 9-(hydroxymethyl)anthracene as the diene was calculated as 6.5 kcal/mol at 300 K.Citation30 In comparison, we found a ∆G value of 3.031 kcal/mol at 300 K for NPM dissociation in the absence of a diene. This lower value indicated that the presence of a bound diene inside the catalytic pocket made NPM dissociation less favorable. This result is in agreement with the decrease in dissociation constant of NPM observed at saturating concentrations of 9-ACA ().
Figure 5. (A) Binding of NPM (black circles), and NPM in the presence of saturating concentrations of 9-ACA (1.1 mM, gray triangles) to 50 nM DAse-3′-Cy5. (B) The binding of 9-ACA (black pentagons), and 9-ACA in the presence of saturating concentrations of NPM (92 mM, gray squares) to 50 nM DAse-3′-Cy5. For both (A and B), gray and black solid curves are the fits of the data points to the Hill equation (n = 1). The error bars represent the standard error in triplicate measurements. (C) The binding scheme for NPM to the DAse (1), 9-ACA to the DAse (2), NPM to DAse-9-ACA complex (DAse•9-ACA) (3), and 9-ACA to DAse-NPM complex (DAse•NPM) (4). 1–4 are the dissociation constants.
Conclusion
In this article, we demonstrated that MST could be used to reliably measure the KD of small molecules to DAse ribozymes where FCS failed due to unspecific binding of the fluorophore. Binding of the maleimide substrates to the DAse revealed the importance of hydrophobic interactions. Thermodynamic analysis by MST showed that NPM binding to the DAse was entropically favored. This is most likely a result of ordered water molecules released from the catalytic site. Moreover, a positive cooperativity of 2.6-fold was observed between the diene and dienophile binding to the DAse (). To our knowledge, this is the first study where the KD, cooperativity, and thermodynamic information about a ribozyme are obtained by MST. All KD values for the substrates and product of the DAse are summarized in Table S1. Given the fact that MST requires small volumes and low concentrations of the biomolecule, it has, in our opinion, the potential to become a valuable tool for analyzing ribozymes and for screening of in vitro evolved ribozymes.
Materials and Methods
Comparison of the kinetic behavior of the Cy5-labeled DAse and DAse by fluorescence kinetic measurement
The experiment was performed similar to previously published procedure with minor modifications.Citation12,Citation32 10 µM stocks of RNA (DAse: 5′-GGAGCUCGCU UCGGCGAGGC CGUGCCAGCU CUUCGGAGCA AUACUCGGC-3′, (CCS Chemical Synthesis Services) or DAse-3′-Cy5 with Cy5 connected by C6-NH2-spacer (IBA) were prepared in water and refolded by heating for 2 min at 75 °C and controlled cooling in a thermoshaker (Eppendorf) within 20 min to RT. RNAs were kept on ice until the measurement. Stock solutions of 5 × standard DAse buffer, Trolox (30 mM in ethanol), NPM (5 mM in ethanol, prepared from 1 M NPM in DMSO), and 1-AB (25 µM in water) were prepared. Fluorescence measurements were conducted with a Jasco FP-6500 fluorophotometer (Jasco Inc.) with a built-in thermoelectric controller (ETC-273T) and an external Julabo F25 thermostat both set to 25 °C. The fluorophotometer settings were: excitation (460 nm), excitation bandwidth (3 nm), emission (510 nm), emission bandwidth (5 nm), response time (2.0 s), data pitch (1.0 s), and sensitivity (high). For the catalyzed reactions, water, 5 × standard DAse buffer, and RNA were mixed and equilibrated at room temperature for 3 min. Then, Trolox and 1-AB were added and equilibrated at room temperature for 2 min. The reaction was started with the addition of NPM. The final concentrations were DAse (0.67 µM), 1-AB (5 µM), NPM (500 µM), Trolox (2 mM) in 1 × standard DAse buffer (300 mM NaCl, 30 mM TRIS-HCl, 80 mM MgCl2) in 15 µl. For the background reaction, the DAse was omitted. For the photoreaction, both NPM and DAse were omitted. All measurements were done in triplicates. Fluorescence curves were recorded and three consecutive measurements were accumulated using the fluorophotometer software. Mathematical fitting of the kinetic curves to monoexponentials was performed and plotted using Origin Pro v8.0 (OriginLab). The apparent catalytic activity of the DAse was determined by calculating the initial slopes (at the reaction time = 0 s). Linear regression was used for the background reactions to calculate apparent pseudo first order rate constants.
MST measurements
The MST measurements were performed using a Monolith NT.115 Green Red MST instrument (Nanotemper technologies) with MST grade standard treated NT.115 capillaries. In this instrument, an infrared laser beam and light (fluorescence excitation and emission) was coupled with a dichroic mirror and focused on the sample. When the IR-laser was on, it heated a small area of the sample and created a temperature gradient. The total fluorescence of the focused area was measured when the IR laser was off (Fcold) and on (Fhot). The capillaries were filled with the sample (less than 5 µl was required for each capillary). The MST settings were: LED power (20%, Red – Ex: 625 nm, Em: 680 nm), MST laser power (40%), fluorescence before (5 s), MST on (30 s), fluorescence after (3 s), and run number (3). The temperature of the instrument was set to 22 °C for all measurements except for the thermodynamic measurements (the temperatures used were presented in the van’t Hoff plot, ). After the capillary scan, thermophoresis of the DAse-3′-Cy5 (50 nM) was measured for 30 s in the presence of the varied concentrations of binding partner (e.g., substrates or product). Fnorm = Fhot/Fcold was analyzed and plotted by NT Analysis software (Nanotemper technologies). The theory of thermophoresis was described in the literature.Citation13,Citation14,Citation19 Fraction bound = (Fnorm – Fnorm(unbound))/(Fnorm(bound) – Fnorm(unbound)) was calculated and the binding data were fitted to a Hill function (n = 1) using Origin Pro. All measurements were conducted as triplicates and the errors were presented as the standard error of the triplicates. The sample preparations for each MST measurements and the binding cooperativity analysis are presented in the supporting information.
| Abbreviations: | ||
| 1-AB | = | anthracen-1-yl-BODIPY |
| 1-AHEG-P | = | tetrabutylammonium 1-(anthracen-1-yl)-2,5,8,11,14,17-hexaoxanonadecan-19-yl hydrogen phosphate |
| 9-AB | = | anthracen-9-yl-BODIPY |
| 9-ACA | = | anthracene-9-carboxylic acid |
| 9-AHEG-P | = | tetrabutylammonium 1-(anthracen-9-yl)-2,5,8,11,14,17-hexaoxanonadecan-19-yl hydrogen phosphate |
| BODIPY | = | 4,4-difluoro-4-bora-3a,4a-diaza-s-indacene |
| 9-DAP-HEG | = | Diels-Alder product of 1-(anthracen-9-yl)-2,5,8,11,14,17-hexaoxanonadecan-19-ol |
| 9-DAPB | = | Diels-Alder product of anthracen-9-yl-BODIPY |
| DAse | = | Diels-Alderase |
| DAse-3′-Cy5 | = | Diels-Alderase labeled with Cy5 fluorescent dye at its 3′-position |
| DMSO | = | dimethyl sulfoxide |
| FCS | = | fluorescence correlation spectroscopy |
| FRET | = | fluorescence resonance energy transfer |
| 6-MCA | = | 6-maleimidocaproic acid |
| MD | = | molecular dynamics |
| MST | = | microscale thermophoresis |
| N-biotin-M | = | N-biotinoyl-N′-(6-maleimidohexanoyl)hydrazide |
| N-heptyl-M | = | N-heptylmaleimide |
| N-hexyl-M | = | N-hexylmaleimide |
| N | = | N-hydrazide-M, N-pentanoyl-N′-(6-maleimidohexanoyl)hydrazide |
| N-hydrazine-M | = | 6-maleimidocaproic acid hydrazide |
| NPM | = | N-pentylmaleimide |
| PeT | = | photoinduced electron transfer |
| PMF | = | potential mean force |
| RT | = | room temperature |
| Trolox | = | 6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid |
Additional material
Download Zip (1.1 MB)Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank Dr Felix Wieland (Heidelberg University) for access to MST equipment, and Dr Jochen Reinstein (Max Planck Institute for Medical Research) for helpful scientific discussions. We are grateful to Sandra Suhm for technical support. Jäschke A is supported by the Deutsche Forschungsgemeinschaft, SFB 623, BMBF, and the Helmholtz Initiative on Synthetic Biology. Gaffarogullari EC is supported by a Landesgraduiertenförderung (LGFG) fellowship and the Hartmut Hoffmann-Berling international graduate school of molecular and cellular biology (HBIGS).
Supplemental Materials
Supplemental materials may be found here: www.landesbioscience.com/journals/rnabiology/article/27101/
References
- Seelig B, Jäschke A. A small catalytic RNA motif with Diels-Alderase activity. Chem Biol 1999; 6:167 - 76; http://dx.doi.org/10.1016/S1074-5521(99)89008-5; PMID: 10074465
- Seelig B, Keiper S, Stuhlmann F, Jäschke A. Enantioselective Ribozyme Catalysis of a Bimolecular Cycloaddition Reaction . Angew Chem Int Ed Engl 2000; 39:4576 - 9; http://dx.doi.org/10.1002/1521-3773(20001215)39:24<4576::AID-ANIE4576>3.0.CO;2-J; PMID: 11169675
- Serganov A, Keiper S, Malinina L, Tereshko V, Skripkin E, Höbartner C, Polonskaia A, Phan AT, Wombacher R, Micura R, et al. Structural basis for Diels-Alder ribozyme-catalyzed carbon-carbon bond formation. Nat Struct Mol Biol 2005; 12:218 - 24; http://dx.doi.org/10.1038/nsmb906; PMID: 15723077
- Kraut S, Bebenroth D, Nierth A, Kobitski AY, Nienhaus GU, Jäschke A. Three critical hydrogen bonds determine the catalytic activity of the Diels-Alderase ribozyme. Nucleic Acids Res 2012; 40:1318 - 30; http://dx.doi.org/10.1093/nar/gkr812; PMID: 21976731
- Kobitski AY, Nierth A, Helm M, Jäschke A, Nienhaus GU. Mg2+-dependent folding of a Diels-Alderase ribozyme probed by single-molecule FRET analysis. Nucleic Acids Res 2007; 35:2047 - 59; http://dx.doi.org/10.1093/nar/gkm072; PMID: 17344321
- Wang Y, Killian J, Hamasaki K, Rando RR. RNA molecules that specifically and stoichiometrically bind aminoglycoside antibiotics with high affinities. Biochemistry 1996; 35:12338 - 46; http://dx.doi.org/10.1021/bi960878w; PMID: 8823168
- Wang Y, Rando RR. Specific binding of aminoglycoside antibiotics to RNA. Chem Biol 1995; 2:281 - 90; http://dx.doi.org/10.1016/1074-5521(95)90047-0; PMID: 9383430
- Gilbert BA, Sha M, Wathen ST, Rando RR. RNA aptamers that specifically bind to a K Ras-derived farnesylated peptide. Bioorg Med Chem 1997; 5:1115 - 22; http://dx.doi.org/10.1016/S0968-0896(97)00047-3; PMID: 9222505
- Schürer H, Buchynskyy A, Korn K, Famulok M, Welzei P, Hahn U. Fluorescence correlation spectroscopy as a new method for the investigation of aptamer/target interactions. Biol Chem 2001; 382:479 - 81; http://dx.doi.org/10.1515/BC.2001.058; PMID: 11347896
- Eydeler K, Magbanua E, Werner A, Ziegelmüller P, Hahn U. Fluorophore binding aptamers as a tool for RNA visualization. Biophys J 2009; 96:3703 - 7; http://dx.doi.org/10.1016/j.bpj.2009.01.041; PMID: 19413975
- Werner A, Konarev PV, Svergun DI, Hahn U. Characterization of a fluorophore binding RNA aptamer by fluorescence correlation spectroscopy and small angle X-ray scattering. Anal Biochem 2009; 389:52 - 62; http://dx.doi.org/10.1016/j.ab.2009.03.018; PMID: 19303859
- Nierth A, Kobitski AY, Nienhaus GU, Jäschke A. Anthracene-BODIPY dyads as fluorescent sensors for biocatalytic Diels-Alder reactions. J Am Chem Soc 2010; 132:2646 - 54; http://dx.doi.org/10.1021/ja9084397; PMID: 20131767
- Duhr S, Braun D. Why molecules move along a temperature gradient. Proc Natl Acad Sci U S A 2006; 103:19678 - 82; http://dx.doi.org/10.1073/pnas.0603873103; PMID: 17164337
- Duhr S, Braun D. Thermophoretic depletion follows Boltzmann distribution. Phys Rev Lett 2006; 96:168301; http://dx.doi.org/10.1103/PhysRevLett.96.168301; PMID: 16712279
- Iacopini S, Piazza R. Thermophoresis in protein solutions. Europhys Lett 2003; 63:247 - 53; http://dx.doi.org/10.1209/epl/i2003-00520-y
- Reineck P, Wienken CJ, Braun D. Thermophoresis of single stranded DNA. Electrophoresis 2010; 31:279 - 86; http://dx.doi.org/10.1002/elps.200900505; PMID: 20084627
- Lippok S, Seidel SAI, Duhr S, Uhland K, Holthoff HP, Jenne D, Braun D. Direct detection of antibody concentration and affinity in human serum using microscale thermophoresis. Anal Chem 2012; 84:3523 - 30; http://dx.doi.org/10.1021/ac202923j; PMID: 22397688
- Seidel SAI, Wienken CJ, Geissler S, Jerabek-Willemsen M, Duhr S, Reiter A, Trauner D, Braun D, Baaske P. Label-free microscale thermophoresis discriminates sites and affinity of protein-ligand binding. Angew Chem Int Ed Engl 2012; 51:10656 - 9; http://dx.doi.org/10.1002/anie.201204268; PMID: 23001866
- Baaske P, Wienken CJ, Reineck P, Duhr S, Braun D. Optical thermophoresis for quantifying the buffer dependence of aptamer binding. Angew Chem Int Ed Engl 2010; 49:2238 - 41; http://dx.doi.org/10.1002/anie.200903998; PMID: 20186894
- Silvers R, Saxena K, Kudlinzki D, Schwalbe H. Recombinant expression and purification of human TATA binding protein using a chimeric fusion. Protein Expr Purif 2012; 85:142 - 7; http://dx.doi.org/10.1016/j.pep.2012.07.006; PMID: 22841618
- Timofeeva OA, Chasovskikh S, Lonskaya I, Tarasova NI, Khavrutskii L, Tarasov SG, Zhang X, Korostyshevskiy VR, Cheema A, Zhang L, et al. Mechanisms of unphosphorylated STAT3 transcription factor binding to DNA. J Biol Chem 2012; 287:14192 - 200; http://dx.doi.org/10.1074/jbc.M111.323899; PMID: 22378781
- Jerabek-Willemsen M, Wienken CJ, Braun D, Baaske P, Duhr S. Molecular interaction studies using microscale thermophoresis. Assay Drug Dev Technol 2011; 9:342 - 53; http://dx.doi.org/10.1089/adt.2011.0380; PMID: 21812660
- Aumiller V, Graebsch A, Kremmer E, Niessing D, Förstemann K. Drosophila Pur-α binds to trinucleotide-repeat containing cellular RNAs and translocates to the early oocyte. RNA Biol 2012; 9:633 - 43; http://dx.doi.org/10.4161/rna.19760; PMID: 22614836
- Feddersen A, Dedic E, Poulsen EG, Schmid M, Van LB, Jensen TH, Brodersen DE. Saccharomyces cerevisiae Ngl3p is an active 3′-5′ exonuclease with a specificity towards poly-A RNA reminiscent of cellular deadenylases. Nucleic Acids Res 2012; 40:837 - 46; http://dx.doi.org/10.1093/nar/gkr782; PMID: 21965533
- Stuhlmann F, Jäschke A. Characterization of an RNA active site: interactions between a Diels-Alderase ribozyme and its substrates and products. J Am Chem Soc 2002; 124:3238 - 44; http://dx.doi.org/10.1021/ja0167405; PMID: 11916406
- Page MI, Jencks WP. Entropic contributions to rate accelerations in enzymic and intramolecular reactions and the chelate effect. Proc Natl Acad Sci U S A 1971; 68:1678 - 83; http://dx.doi.org/10.1073/pnas.68.8.1678; PMID: 5288752
- McConnell TS, Cech TR. A positive entropy change for guanosine binding and for the chemical step in the Tetrahymena ribozyme reaction. Biochemistry 1995; 34:4056 - 67; http://dx.doi.org/10.1021/bi00012a024; PMID: 7696271
- Lu B, Wong CF. Direct estimation of entropy loss due to reduced translational and rotational motions upon molecular binding. Biopolymers 2005; 79:277 - 85; http://dx.doi.org/10.1002/bip.20344; PMID: 16078192
- Bereźniak T, Zahran M, Imhof P, Jäschke A, Smith JC. Magnesium-dependent active-site conformational selection in the Diels-Alderase ribozyme. J Am Chem Soc 2010; 132:12587 - 96; http://dx.doi.org/10.1021/ja101370e; PMID: 20722413
- Bereźniak T, Jäschke A, Smith JC, Imhof P. Stereoselection in the Diels-Alderase ribozyme: a molecular dynamics study. J Comput Chem 2012; 33:1603 - 14; http://dx.doi.org/10.1002/jcc.22993; PMID: 22549366
- Keller D, Swigon D, Bustamante C. Relating single-molecule measurements to thermodynamics. Biophys J 2003; 84:733 - 8; http://dx.doi.org/10.1016/S0006-3495(03)74892-9; PMID: 12547757
- Nierth A, Singer M, Jäschke A. Efficient photoactivation of a Diels-Alderase ribozyme. Chem Commun (Camb) 2010; 46:7975 - 7; http://dx.doi.org/10.1039/c0cc03162c; PMID: 20871890