Abstract
RNAs have many important functional properties, including that they are independently controllable and highly tunable. As a result of these advantageous properties, their use in a myriad of sophisticated devices has been widely explored. Yet, the exploitation of RNAs for synthetic applications is highly dependent on the ability to characterize the many new molecules that continue to be discovered by large-scale sequencing and high-throughput screening techniques. In this review, we present an exhaustive survey of the most recent synthetic bacterial riboswitches and small RNAs while emphasizing their virtues in gene expression management. We also explore the use of these RNA components as building blocks in the RNA synthetic biology toolbox and discuss examples of synthetic RNA components used to rewire bacterial regulatory circuitry. We anticipate that this field will expand its catalog of smart devices by mimicking and manipulating natural RNA mechanisms and functions.
Glossary
Composability: refers to the property of a system to break down in units (parts) due to the system modularity and ability to recombine in different configurations to satisfy specific human requirements.Citation1
Biological “part”: a genetically encoded functional unit that can be composed into a more complex assembly (e.g., a transcription promoter, an RNA aptamer, or a gene reporter).Citation2
Genetic device: is an assembly of biological “parts” designed to execute a human-defined biological function.Citation2
Regulator: biological part embedded in a regulatory-network and exerts constitutive control over gene expression. It determines the gene expression dynamic range.Citation2
Sensor: biological part that detects a signal and transforms it into a gene-regulatory action or a structural reconfiguration of another biological part.Citation2
Adaptor: non-coding nucleotide sequences or unstructured peptides connecting parts that isolate function by providing physical separation.Citation2
Actuators: reporter genes that provide an output signal either as a particular phenotype or measurable outcome.Citation2
Stabilizers: we define stabilizers as biological parts (e.g., non-coding sequences of nucleotides) that prevent decay of another part or device by providing physical protection and/or being docking domains for other helpers (e.g., Hfq domain in trans-encoded antisense sRNAs).
Introduction
The RNA world has been shaken by the recent recognition that small transcripts, once thought as “junk,”Citation3 can serve critical roles as cellular regulators. This period of intense discovery has led to the realization of the ubiquitous presence of these short non-coding RNAs throughout bacterial genomes, and of their value as controllers.Citation4-Citation6 Furthermore, regulatory RNAs are involved in important sensing functions and regulatory responses to environmental cues.Citation7
As these sophisticated regulators are explored and their mechanisms and functions unveiled, Synthetic Biology has continued to arise as a field. Scientists now describe it as a discipline that pursues the engineering of life. As such, the ultimate goal of this field is to synthesize biological entities that can perform non-natural functions, in rational, standard, and systematic ways. Importantly, the discipline of Synthetic Biology currently spans from the biomolecular level to the macroscopic level of whole cells, tissues, and even organs.Citation8 Some early examples include the genetic toggle switchCitation9 and the repressilator.Citation10 In this earlier stage promoters, ribosome binding sites, and repressors were at the core of the artificial manipulation work. Later on, these genetic control elements were combined to generate a diversity of devices with specific responses (for a review, see refs.Citation11 and Citation12).
Synthetic Biology has been empowered by a wide variety of technologies that include the ability to combine existing techniques that allow import and export of natural capabilities across different organisms. These technologies include traditional cloning, cloning of entire biological machines,Citation13 fabrication of metabolic pathways,Citation14,Citation15 alteration of the amino acid sequence of a protein,Citation11,Citation16 design of tailored RNA molecules,Citation17 codon-optimization,Citation18 and chemical modification of biological entities to build upon natural regulatory mechanisms.Citation19
The case for using RNA in Synthetic Biology is based on the ability of RNA elements to exert: independent control (particularly cis-acting molecules) by acting on a single molecule in a specific way, high tunability since their structures can be easily manipulated, orthogonality given the ability to prevent undesired interactions between variants, scalability due to their intervention in all levels of gene expression, composability given their relative potential to be assembled in a modular way, and portability since they can be more readily transferred to other organisms relative to their protein counterparts.Citation20 Another advantage of using RNA-based sensing approaches is that these molecules exhibit more compact genetic footprints than their protein counterparts and, as such, are more “economical” since they generally place less of an energetic load on the cell by not requiring translation.Citation21 Furthermore, RNA-based post-transcriptional responses generally act at a faster time scale than transcription-based strategiesCitation22 and RNA-based mechanisms can be evolved more rapidly than protein-based mechanisms.Citation23
Since 1982, when the Nobel Prize committee recognized the discovery of RNA catalytic propertiesCitation24 as groundbreaking,Citation25 several “big” catalytic RNAs have been exploited for various biotechnological and medical applications. The catalytic RNAs that have made exciting headlines include group II introns,Citation26 RNase P,Citation27 the group I ribozyme,Citation28 and the Hammerhead catalytic moduleCitation29 (also known as the Hammerhead ribozyme-HHR). These applications represent some of the first developments that preceded small regulatory RNAs in the Synthetic Biology field. Our improved understanding of the design rules that govern these highly specialized regulatory molecules enables the visions of replicating their unique attributes and of further enhancing their roles. We also begin to see how these regulatory units could be transferred across living and non-living systems for the bottom-up synthesis of completely novel and highly controllable cellular phenotypes. is a general overview that illustrates the universe of RNA devices and parts, and the different levels of complexity that scientists have been able to reach by engineering RNAs in Synthetic Biology.
Figure 1. Universe of synthetic RNA devices and parts. An overview of the universe of RNA devices and parts that have been engineered in all three kingdoms of life. The red square delimits the space of RNA devices that this review focuses on. The blue arrow to the left of the picture represents how the complexity increases from bottom to top. The classification of RNA devices has been made consistent to previous work.Citation119
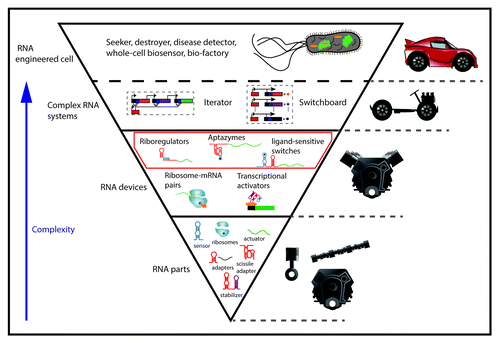
In the remainder of this article, we survey recently reported and some pioneering mechanisms and applications that have been associated with riboswitches (a cis-acting RNA molecule that can control expression of its neighboring messenger RNA-mRNA) and trans-encoded small RNAs (sRNA) (a trans-acting molecule that regulates mRNAs by antisense mechanisms). We aim to present a comprehensive introductory review for the non-expert reader. We focus on these two types of RNAs because they are two of the most understood classes of regulatory RNAs. We also compare their natural mechanisms and molecular properties. And finally we offer insights into the types of opportunities that exist in this field to enable full exploitation of regulatory RNAs in Synthetic Biology as directed toward biotechnological applications.
Natural Riboswitches and sRNAs
Riboswitches: Single gene expression controllers
Riboswitches () are natural genetic control elements that are found in cis at the 5′ (or 3′) untranslated regions of mRNAs. These regulatory RNA elements typically regulate expression of downstream genes by binding to cellular metabolites (e.g., small molecules) that cause a “switch” in their structural conformation. As a result, this structural switch leads to changes in expression levels of their partner gene.Citation30 Likewise, several riboswitch molecular architectures have been characterized;Citation31 although these exhibit a certain degree of similarity in their local motifs, they show a diverse array of secondary and tertiary structures. For a recent review of riboswitches, see reference Citation32. The basic functional mechanisms for three of the main classes of riboswitches are illustrated in .
Figure 2. Gene expression control mechanisms of ligand-binding riboswitches. (A) Transcriptional elongation control by a ligand-activated riboswitch. In the absence of ligand, an anti-terminator stem is present allowing completion of transcription by a polymerase. A ligand binds a specific site in the aptamer and causes the formation of a terminator stem that arrests transcription and prevents the synthesis of a complete transcript (dashed line). The opposite mechanism in which transcription turns on upon binding has been identified in nature to a lesser extent (not shown in figure). (B) Translational initiation control by a ligand-activated riboswitch. In the presence of the ligand, a riboswitch undergoes a conformational change and sequesters the Ribosome-Binding Site (RBS) to prevent the ribosome from binding the mRNA. In the same way as with transcriptional controllers, there are examples of riboswitches that turn on translation upon binding (not shown in figure). (C) Translational control by an allosteric ribozyme. The glmS glucosamine-6-phosphate (GlcN6P)-activated ribozyme (aptazyme) is shown. In the absence of GlcN6P, translation is ON and the transcript is protected against degradation. In the presence of the ligand binding to the aptamer, the transcript is cleaved in the 5′ UTR by RNaseJ1 and is subject to decay.Citation37
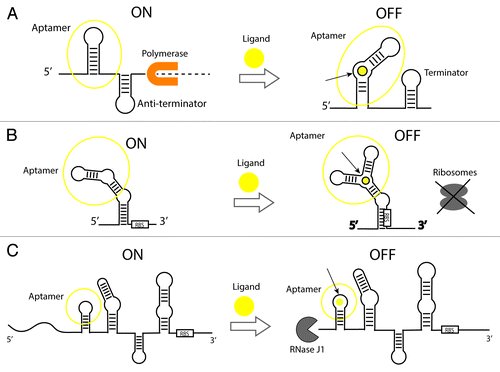
A variety of mechanisms have been uncovered for riboswitch regulation (reviewed in ref. Citation33). An interesting distinction is the one-domain (e.g., exhibited by thermosensors that respond to temperature changes) vs. two-domain molecular organization of riboswitches.Citation34 Most widely studied two-domain switches are RNAs composed of a ligand-binding aptamer and an “expression platform,” where the expression platform regulates gene expression in response to conformational changes in the aptamer region (). Importantly, the aptamer region could be highly tuned for specificity in the types of molecules that it can recognize. One of the most powerful examples of this has been the ability to tune riboswitch recognition to discriminate between subtle structural differences in small molecules that differ only by the presence of an H and a CH3 groups (e.g., theophylline vs. caffeine) with nM affinity.Citation21 Indeed, it is the strong sensitivity intrinsic to these RNA switches that is highly exploitable in the detection of small molecules, macromolecules, and external stimuli. This extreme specificity is attributed to a modular component of riboswitches called the aptamer (). Aptamers are RNA molecules capable of binding a broad variety of small effector molecules and undergoing a structural reconfiguration. In fact, aptamers can be considered synthetic predecessors of natural riboswitches since they were evolved into conditional expression controllers in 1998,Citation35 several years before the first natural riboswitch was identified in the early 2000s.Citation36
A unique class of riboswitches is the so-called aptazymes. As shown in , aptazymes are allosterically controlled ribozymes. One example of this is the allosteric glucosamine-6-phospate (GlcN6P)-responsive ribozyme discovered by Winkler et al.Citation37 This aptazyme that is located at the 5′ UTR of B. subtilis glmS mRNA, cleaves and induces RNase J1 degradation of the message. The interest on this class of regulators is spotlighted by the fact that several aptazymes were engineered in vitro even before they were discovered in 2004.Citation38
An interesting one-domain type of riboswitch is the temperature and pH stimuli-responsive class, where RNA undergoes structural rearrangement in direct response to temperature or pH.Citation33,Citation39 Although these RNA switches could be equally valuable for sensing technologies, they are far less studied and less exploited than metabolite-responsive elements. This is due in part to the paucity of riboswitches that have been uncovered in this category and to the lack of mechanistic insights and molecular principles of their function and design. Yet, depending on the application, these sensors can offer advantages over metabolite sensing elements since they can function without the need of an intermediate activator or an additional structural domain.Citation40 These features make these RNA switches more portable for certain synthetic constructions and are particularly attractive for applications in which using small effector molecules to trigger gene regulation is prohibitive.
One of the few reports geared toward engineering artificial thermosensors consisted of a structural model-based approach to design synthetic functional RNA thermometersCitation41 capable of controlling translation initiation as efficiently as natural thermosensors. These temperature-sensitive riboswitches were engineered in E. coli using β-galactosidase as a gene reporter. In another example, Wieland et al.Citation42 successfully engineered a series of temperature-sensitive riboswitches by a dual approach: GC-rich four-stranded-constructs (quadruplexes), suspected to be of relevance to the control of gene expression, were rationally designed and experimentally tested in vivo for suppression of a reporter gene. Quadruplexes of moderate secondary structure stability, as determined by circular dichroism spectroscopy, show temperature sensitivity in close resemblance to RNA thermosensors. Interestingly, in recent years, thermosensors have been treated in the literature almost as a separate class of regulatory RNAs. This is partly due to the far less intense exploitation of one-domain riboswitches for artificial gene management applications in contrast with other classes of riboswitches.Citation40 Therefore further discussion of riboswitches will focus on ligand-binding riboswitches (including aptazymes) and discuss the coupling of riboswitch-sensing ability with response circuits to generate new synthetic functionalities.
sRNAs: Multiple gene expression controllers
Small RNAs (sRNAs), ranging from 21~400 nucleotides, are an intriguing class of RNAs that (like riboswitches) do not encode proteins but have intrinsic roles as cellular regulators.Citation43 A key property of sRNAs is their ability to simultaneously turn on and off a variety of metabolic pathways in response to environmental changes (e.g., nutrient availability, osmolarity, pH, and temperature).Citation44 To exert their function, they can (1) base-pair with mRNAs (mRNAs) to enhance or attenuate transcription (), (2) bind mRNAs to directly block (, i), or indirectly enhance or inhibit translation (, ii), (3) sequester proteins into ribonucleoprotein complexes to prevent their activity (not shown in ), or (4) directly catalyze mRNA and protein degradation (, ii).Citation45 The importance of sRNA regulatory mechanisms in bacterial survival and adaptation is evident by their ubiquitous presence in microbes.Citation46 Indeed, it is known that sRNAs can be essential for organism survival under stress.Citation43,Citation44,Citation46 Recent reviews on this topic highlight the diversity of sRNAs that continues to be evident with discovery of sRNAs in a wide range of bacterial species.Citation4,Citation47
Figure 3. Gene expression control mechanisms by bacterial sRNAs.Citation45 (A) Transcription attenuation/enhancement. (A) sRNA binds to its target mRNA and causes a structural reconfiguration upon base-pairing, ultimately enhancing or attenuating transcription by the polymerase. (B) Translational control. Translational control is imparted by sRNAs in various ways: (1) A sRNA base-pairs to its target mRNA sequestering the Ribosome-Binding Site (RBS) and directly prevents translation initiation by the ribosomes. (2) A sRNA binds to the target mRNA at a distance from the RBS and the target mRNA suffers a structural change that indirectly affects ribosome binding. sRNA binding to its target can also enhance or inhibit mRNA decay by changing interactions with exonucleases and/or endonucleases.
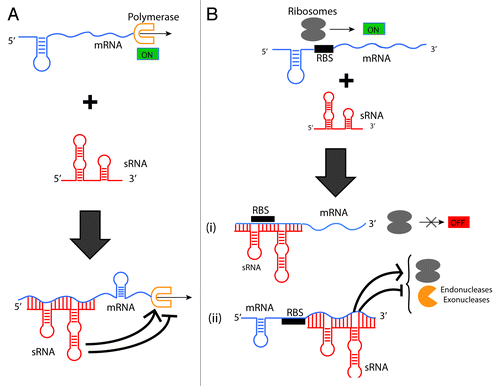
Remarkably, sRNAs are also extremely precise in their recognition of RNAs and proteins; this is evidenced by the observation by the minimal basepairing requirement (8-9 basepairs) to bind a specific target.Citation43 The structural robustness and plasticity of small RNAs has recently been discussed in the context of these attributes’ evolutionary advantage. As it has been noted, these molecules can tolerate a large degree of “functional innovation” via acquired mutations without compromising their structures.Citation48
The fact that small RNA molecules can control major metabolic transformations has attracted much scientific interest. One of the most intriguing aspects of sRNAs is their ability to bind (and even sequester) multiple proteins (in addition to mRNAs), posing a rather powerful and unique regulatory mechanism. Two examples of regulatory protein-binding sRNAs that have been extensively studied are the CsrB/C (carbon storage sRNA regulatory system)Citation49 and 6S RNA.Citation50 Although regulatory RNA–protein interactions are only one form of global post-transcriptional gene expression control, the topology of their networks, and their mechanism of action are unique relative to other forms of post-transcriptional regulation (e.g., sigma transcription factors, repressor networks, and operon regulation by ligand-responsive riboswitches). The uniqueness of this form of regulation lies in the fact that these regulatory RNAs are not encoded close to their mRNA (or protein) targets, can recognize RNA at the sequence and structural level, can work by combining several binding domains in a modular way, and can directly control RNA stability by protection mechanisms.Citation51
Bacterial small RNAs can be categorized as cis-encoded and trans-encoded. The former class refers to those encoded in a partial overlap with their target genes, whereas the latter are located in the genome apart from their targets. Since trans-encoded sRNAs account for the great majority of bacterial sRNAs, we will focus hereafter on trans-encoded sRNAs and will refer to them as sRNAs. Many trans-encoded sRNAs interact with multiple target mRNAs.Citation52 In addition to these interactions with mRNAs, associations with the cellular chaperone Hfq have been widely observed, particularly in gram-negative bacteria. Hfq’s complexation with sRNAs is especially important in times of cellular and environmental stress.Citation47,Citation52 Hfq is a major RNA chaperone that has been shown to: (1) increase the stability of small RNAs in vivo and in vitro; (2) bind several mRNAs and sRNAs to assist their pairing in global stress responses, (3) stabilize mRNA-small RNA interactions,Citation43 and (4) facilitate negative regulation by delivering the sRNA-mRNA pair to the degradosome (multi-ribonuclease complex).Citation52 The cellular importance of Hfq is further underscored by the fact that it is a well-conserved protein in bacteria with homologs in eukaryotes. i.e. Sm proteins that form part of the spliceosome and play important roles in RNA metabolism.Citation53 It is, however, important to note that Hfq is not present in all bacterial organisms where sRNAs have been discovered.Citation54 In general, these findings suggest at least two possibilities: (1) that additional mechanisms (currently unknown) exist in these cells for sRNA stability purposes or (2) that not all bacterial sRNAs require protein chaperones for stabilization or assistance with target binding. Increased knowledge on natural protection mechanisms that have evolved in living systems could be highly useful in mimicking stability strategies for RNAs, particularly in the context of synthetic drug release vehicles. For recent reviews on Hfq function, structure, and its relationship with small regulatory RNAs, see references Citation7, Citation52, and Citation55.
Interactions with Hfq are intriguing from a Synthetic Biology perspective, given that their stability is highly relevant to making regulatory RNAs portable for applications outside the natural cellular environment. Although not much work has been done in this area, two general strategies for stabilizing RNAs have naturally evolved: special secondary structural motifs (e.g., hairpins and loops) and partnerships with proteins, such as Hfq.Citation56 For a recent review on this topic, see reference Citation57.
In summary, important mechanistic differences exist between small RNAs and riboswitches (discussed above) that make them ideal candidates for different sets of applications. A gross description of these two “styles” of gene regulation can be captured by the thought of riboswitches being “local” managers, in stark contrast to the more “global” gene management style of sRNAs. Regarding applications, it is easy to imagine several cases where manipulation to gene expression could be beneficial at a highly localized level (without affecting other genes) for which synthetic riboswitches might be ideal candidates. However, sRNA’s ability to co-manage entire gene clusters and have molecules that could be engineered to be more promiscuous in their interactions also poses significant advantages in a context where broader molecular targeting is desired. summarizes comparisons between natural features (with potential for Synthetic Biology applications) exhibited by riboswitches and sRNAs.
Table 1. Comparison of natural features exhibited by ligand-activated riboswitches and trans-encoded sRNAs
Recent Synthetic Regulatory RNAs for Engineering of Novel Cellular Capabilities
A considerable number of successes indicate the potential for using regulatory RNAs to expand our current Synthetic Biology toolbox and to help solve important problems in metabolic engineering, environmental remediation, agriculture, and molecular biology (, , , ). Next, we review some of the most recent examples of these successes, with a focus on engineered sRNAs and riboswitches and their specific attributes.
Table 2. Recent synthetic riboswitches and aptazymes and their (potential) applications
Table 3. Recent synthetic sRNAs and their (potential) applications (basic devices)
Table 4. Dual and chimeric riboregulators
Table 5. Recent examples of complex genetic circuits constructed by composing RNA regulators
Riboswitches: Important parts in the plug-and-play approach to synthetic biology
Most recently, remarkable scientific achievements have been accomplished in cellular and molecular engineering by our ability to program “intelligent” responses using synthetically designed artificial transcription–translation circuits.Citation12 In this context, riboswitches have played an important role as inspiring models for the engineering of living systems. They have been exploited as predictable, robust, and precise devices that can act as sensors of various environmental signals,Citation58,Citation59 as metabolic engineering tools for gene expression control,Citation60 and even as biocomputers (logic operations and band pass filters).Citation61,Citation62 General strategies to synthesize artificial riboswitches are shown in , where we illustrate that these regulatory elements are made of decomposable elements.
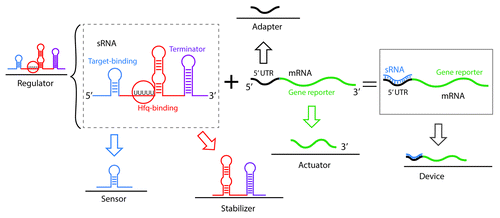
Figure 4. Composability of riboswitches as a strategy for the synthesis of artificial RNA devices. Composability refers to the ability of a system to break down in units (parts) due to the system modularity and recombine in different configurations to satisfy specific human requirements. (A) Composability of riboswitches. Riboswitches can be decomposed and recombined for the synthesis of new devices with high modularity. An artificial riboswitch-based device is composed of a regulator (riboswitch), a signal (ligand), and an actuator (gene reporter). The regulator (inside the solid square) can be further decomposed into a sensor (aptamer) and an adaptor (expression platform that usually contains a terminator stem). (B) Composability of aptazymes (allosteric ribozymes). A synthetic catalytic device is composed of a catalytic regulator (aptazyme), a signal (ligand), and an actuator (gene reporter). The catalytic regulator (inside the solid box) can be further disassembled in a sensor (aptamer) and a scissile adaptor (ribozyme).
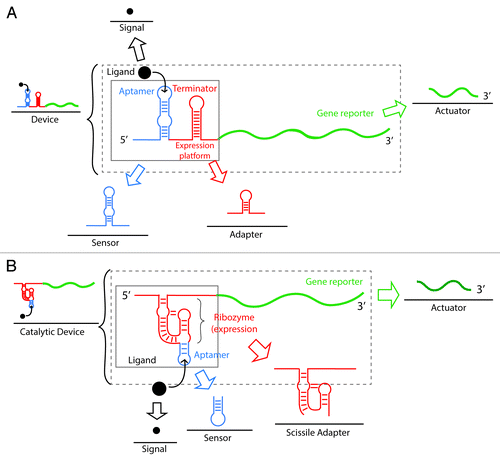
As aforementioned, aptamers have contributed to pioneer the field of engineered riboswitches since the idea of an RNA switch had already been devised even before natural riboswitches were discovered in the early 2000s.Citation63 Although aptamers have been obtained through combinations of in vivo and in vitro selections, they are typically developed by in vitro selection schemes such as Systematic Evolution of Ligands by EXponential enrichmentCitation64 (SELEX). To date, this remains one of the most widely used and effective methods. Many different aptamers have now been developed and used as basic riboswitches in higher organisms (e.g., yeast and mammalian cells) or have been linked to more sophisticated engineered expression platforms (modular component that interprets and exerts the gene expression control action) ().Citation65 In addition, aptamers have been used as tools in a myriad of biotechnological applications both in vitro and in vivo; this further asserts their role as predecessors of riboswitches. Biotechnological aptamer applications include functional molecular biology studies, gene expression control, antibiotic research, diagnostics, biosensors, therapeutic agents, and drug targeting. For reviews of these systems, see references Citation66 and Citation67.
As for some riboswitch-based applications, specific eye-opening examples include the ligand-responsive riboswitch aimed for the search and destruction of the widely used agricultural herbicide atrazine.Citation58 In this example, artificial chemotaxis have been engineered in E. coli cells to navigate toward high concentrations of atrazine by the action of an in vitro- and in vivo-evolved atrazine-responsive riboswitch (). Specifically, an atrazine catabolic pathway was introduced for these artificial bacteria to destroy the herbicide. The RNA device was designed by taking advantage of the high modularity and composability of riboswitches (). This is one of the most developed applications and it reveals the potential of a synthetic riboswitch in the field of bioremediation and agriculture (). This same approach shows potential for rewiring of bacteria to find disease sites.Citation59 lists a comprehensive survey of synthetic riboswitches in the field.
Exploiting sRNA regulation in metabolic engineering
Although many examples of designer sRNAs are being developed for a single target (), the natural ability of small RNAs to target multiple mRNAs can be highly exploited to tune a higher number of functionally interdependent genes and pathways for strain optimization.Citation68 In addition, sRNAs show high modularity due to their molecular makeup. These properties of sRNAs have empowered synthetic biologists to decompose and recombine sRNA parts to engineer artificial riboregulators with different functions. A diversity of strategies have been used to design and engineer the different sRNA parts for various applications that include rational design, model-driven computational design, in vivo and in vitro molecular evolution and selection and, even harvesting of natural parts. In general, the trend observed in a number of studies is the preservation of Hfq-binding domain and of a natural sRNA transcription terminator (both domains constitute the stabilizer) as a scaffold combined with the synthesis of remaining parts to be assembled on top of this template. shows a schematic representation of sRNA composability as a strategy for the synthesis of artificial sRNA devices.
Figure 5. Composability of sRNAs as a strategy for the synthesis of artificial RNA devices. Composability, as for the case of riboswitches () is the ability of a system to break down in units (parts) due to the system modularity and recombine in different configurations to satisfy specific human requirements. sRNAs are regulators of high modularity. An sRNA-based regulator can be broken down in two main parts: a sensor (target binding domain) and a stabilizer (that can include an Hfq-binding site and the transcriptional termination domain). In the context of a genetic device, the sRNA binds an mRNA target. In this case, the 5′ UTR of the target mRNA acts as an adaptor that transmits the signal to the gene reporter actuator. The combination of the sRNA and mRNA target comprises a functional synthetic device.
The potential use of the capability to manipulate entire RNA-driven stress response circuits for cellular engineering has been previously exemplified by introducing gene knockouts within the carbon storage regulator (Csr) system. The Csr is a sRNA-driven system involved in bacterial responses to carbon depletion.Citation49,Citation69,Citation70 Specifically, a major sRNA component of this system (CsrB) has been targeted to increase intracellular levels of the phosphoenolpyruvate (PEP) metabolite, by diverting carbon flux from glycolysis to gluconeogenesis.Citation71 Most recently, the implementation of synthetic small regulatory RNAs in metabolic engineering has been reported in the development of a sRNA-based system repressing translation of DsRed2 (red fluorescent protein) mRNA at various levels and confirming tunability by the modulation of lacZ (mRNA encoding for β-galactosidase) expression.Citation68 In this study, the construction of three different sRNAs was shown for the mRNAs LuxR, AraC, and KanR without cross-activity. This strategy enabled the isolation of E. coli strains optimized for the production of tyrosine and cadaverine. These results suggest that specific gene expression can be fine-tuned by the design of artificial sRNAs as an alternative strategy to gene knockouts. An advantage of this strategy is that synthetic RNAs alleviate the need to generate strain libraries and provide a wider range of gene expression.
Numerous factors likely influence sRNA efficacy when used in artificial regulatory schemes. Some of these include the types of mRNA targets that it can bind, kinetics of binding, and the extent of the sequence area that should be involved in binding. As a result, recent work has shown the notion of complementing rational sRNA design with high-throughput screening strategies that can efficiently identify synthetic RNAs that are capable of regulating genes in trans. A specific recent example is the strategy proposed by Yokobayashi and collaborators in 2011 for the design of artificial sRNAs.Citation72 A high-throughput screening method was developed for the identification of synthetic sRNAs that efficiently represses endogenous genes in E. coli by arbitrarily selecting the well-characterized sRNAs (DsrA, GcvB, MicF, and Spot42) to test their methodology. In this work, the antisense domain was randomized and fused to sRNA scaffolds, preserving the Hfq binding domain unmodified. A library of random artificial sRNAs was generated and tested against a native 5′ mRNA leader sequence fused to Green Fluorescent Protein (GFP) as a reporter, leading to the finding of sRNA synthetic candidates that successfully repress two mRNAs (ompF and fliC). Interestingly, they observed key characteristics in those artificial constructs repressing ompF of high similarity to the features shown by MicF (known to regulate the same mRNA).Citation72 In general, the strategy of maintaining a stabilizer (e.g., sRNA scaffold containing the Hfq-binding domain and a transcription terminator sequence) and reengineering the sensor (i.e., a target-binding domain) through a combination of rational and computational model-driven approaches seems to be commonly observed across recent works in this area. The same pattern is observed with the common application of in vitro and in vivo screening technologies in the field. These features are illustrated in . We present in an extensive survey of the synthetic sRNAs and of some of their potential applications.
As suggested by and , the types of strategies used to build synthetic devices and their applications are significantly different when using riboswitch and sRNA-like features. Riboswitches have been used in general for tighter local gene expression control, typically relying less on rational design and more on in vitro and in vivo evolution techniques, and mostly aiming for bio-sensing applications. On the other hand, sRNAs have been used mostly to build global artificial regulators for metabolic engineering applications. Engineered sRNAs seem to be more recent than their riboswitch counterparts and have in general relied more on rational criteria for their design and construction. Moreover, several sRNA-related studies have addressed the necessity of using modeling and in silico approaches to aid the synthesis of artificial sRNA-like regulators.
Engineering dual and chimeric riboregulators
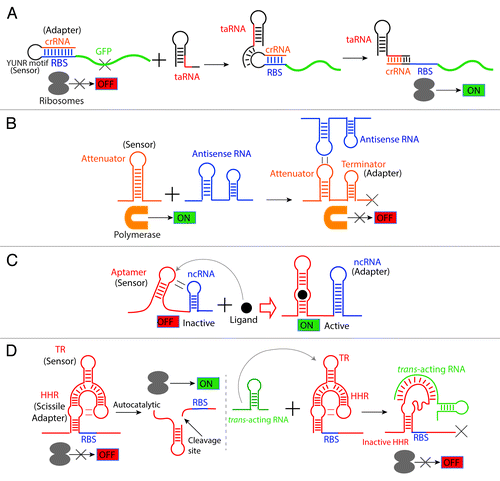
In another class of applications, some groups have used a combined approach by engineering the cis-acting region of the target mRNA as well as the trans-acting antisense RNA (sRNA). Through this more holistic approach, de novo RNA devices have been created. For instance, Isaacs et al. have used this approach to construct a riboregulator that controls GFP expression with a high dynamic range. A cis-repressive element (crRNA) was designed to act as an anti-RBS that blocks ribosome binding. A trans-acting element (taRNA) transcribed from another promoter interacts with the crRNA-RBS stem-loop disrupting the structure and exposing the RBS to generate fluorescence by GFP expression (Fig. 6A).Citation73 This approach showcases the high composability of regulatory RNAs, further exemplified in . For instance, the use of an antisense small trans-acting RNA to induce transcription termination via the exposure of an intrinsic terminator stem in the transcription attenuator (from the plasmid pT181) has been illustrated in .Citation74 A similar creative way to combine the cis-acting capabilities of an aptamer to activate a small RNA is shown in .Citation75 This chimeric device boldly combines riboswitch-like features with sRNA-like features emphasizing the high composability of RNA devices. Finally, one of the most holistic examples is the engineering of an allosteric ribozyme susceptible to activation by a small antisense RNA that ultimately turns translation off ().Citation76
Figure 6. Dual and chimeric riboregulators. Based on the composability of these regulatory molecules, they can be recombined and assembled into a new regulator that exerts a completely different function. (A) Translational control by a small trans-acting RNA-responsive switch. Isaacs and collaborators designed a riboregulator combining a riboswitch-like sensor (YUNR motif), an adaptor that contains an anti-RBS sequence (crRNA) that can pair with the RBS and an sRNA-like effector molecule (taRNA). Translation is OFF (left) when the taRNA absent since crRNA sequesters the Ribosomal Binding Site (RBS). Translation turns ON (right) in the presence of the taRNA since it releases the RBS by recognizing the YUNR motifCitation120 that leads to disruption of the CrRNA-RBS stem-loop.Citation73 (B) Transcriptional control by a small trans-acting RNA-responsive switch. A transcriptional attenuator (from plasmid pT181) was engineered to be RNA sensitive. The sensor loop in the attenuator was evolved to detect a short antisense RNA (that acts as an effector molecule) via a kissing-loop interaction. This interaction exposes an intrinsic transcriptional terminator (the adaptor) to turn transcription OFF.Citation74 (C) A ligand-responsive riboswitch activating an ncRNA. A chimeric regulator was engineered by using a natural aptamer as sensor and a non-coding antisense RNA (ncRNA) as the adaptor. By means of a kissing-loop interaction in the absence of the ligand, the aptamer interacts with the ncRNA hairpin inactivating its antisense function. In the presence of the ligand, the hairpin interaction is disrupted and the ncRNA recovers its regulatory functions.Citation75 (D) An sRNA-responsive aptazyme. A trans-acting RNA-sensitive hallosteric ribozyme was engineered by designing a sensor (TR: trans-acting RNA responsive element) that detects the trans-acting RNA and coupling it with a Hammerhead ribozyme (HHR). In the absence of the trans-acting RNA (left), the aptazyme undergoes cleavage exposing the (initially occluded) ribosomal binding site and allowing translation. In contrast, upon binding of the trans-acting RNA to the TR element, the Hammerhead ribozyme undergoes a structural change that renders it catalytically inactive; this masks the RBS and prevents translation initiation.Citation76
The construction of more complex, chimeric, and dual devices can be understood as the combination of properties exhibited naturally by riboswitches and sRNAs. This represents strong evidence of the RNA modularity. As illustrated in and , RNA parts include riboswitches and sRNAs (regulators) that can be further decomposed into aptamers and sRNA target-binding domains (sensors), riboswitch expression platforms and the 5′ UTR’s of sRNA target mRNAs (adaptors), Hfq-binding, and transcription terminator domains (stabilizers), among other parts.
Composing RNA regulators into complex genetic circuits
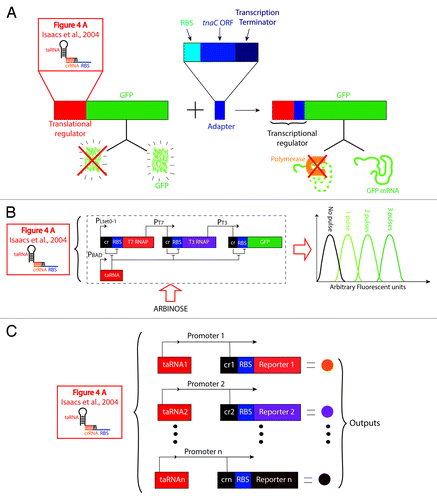
It is fascinating and almost surreal that decomposing and recombining RNA parts to construct advanced devices with novel functions is now a reality; some of the recent examples that represent this level of advancement in synthetic constructions are listed in . A particularly interesting example is the design of complex genetic circuits to perform Boolean logic operations based on the premise that the cell is by itself a biocomputer with the ability to perform complex closed-loop control over its own operations.Citation77 One research group for example, has arranged RNA-sensitive switches (crRNA-taRNACitation73 ) in a complex network for elegant translational control with a high and robust dynamic range.Citation78 In addition, the design of Friedland and collaborators (illustrated in ) has the ability to count up to three events based on a system of three genes regulated by the crRNA-taRNA translational control system arranged in series. These three genes (T7 RNA polymerase, T3 RNA polymerase, and the gene reporter GFP) are controlled by promoters in a transcriptional cascade array. The taRNA is under the PBAD promoter control, which is induced by arabinose. In the absence of arabinose, the system generates baseline levels of fluorescence. However, when pulses of arabinose are fed into the system, the fluorescence response highly correlates with the number of these arabinose injections. In another recent example, the same crRNA-taRNA riboregulation systemCitation73 () was composed into a genetic circuit of high complexity, resembling a switchboard.Citation79 This circuit consists of an orthogonal parallel array of riboregulators that can be applied to multi-sensing tasks. When arranging the orthogonal pairs of crRNA and taRNA under the same promoter, the system gains the ability to monitor and regulate several metabolic pathways simultaneously (). Liu et al.Citation1 developed an adaptor () that converts a translational regulator into a transcriptional regulator. To test their system, the researchers used the crRNA-taRNA riboregulatorCitation73 and the IS10 antisense regulator.Citation80 The adaptor is a regulatory element based on the tnaC, a leader peptide from the tna operon in E. coli (for more details about the mechanism by which tnaC acts, refer to ref. Citation81). Finally, in a series of attempts to mimic natural systems, small-molecule riboswitches have been arrayed in tandem to constitute logic gates () (e.g., NOR, NAND, AND, etc.).Citation61,Citation62,Citation82,Citation83 This is another way by which the power of turning simple riboswitch-like RNA devices into complex translational and transcriptional control systems has been showcased.
Figure 7. Application of a translational regulator to construct complex genetic circuits. The following three examples use the crRNA-taRNA, a riboregulator that controls translation initiation (, Isaacs et al.,Citation73 2004), to engineer different synthetic systems. (A) An adaptor that converts regulators from translational to transcriptional. By fusing an adaptor to a translational regulator, the translational regulator becomes transcriptional regulator. The adaptor consists of a Ribosomal Binding Site (RBS), an Open Reading Frame (ORF) of a short leader peptide (tnaC), and a transcription termination region. By fusing this element to the translational regulator (e.g., crRNA-taRNA), translation of the adaptor is controllable (not shown). In the absence of the adaptor, regulation of GFP is at the translation initiation level by RBS sequestration. In contrast, by merging the adaptor, regulation of GFP is at the transcriptional elongation level; in this case, when the upstream translational regulator turns off synthesis of the adaptor, the transcription terminator in this element is exposed and arrests transcription of GFP (at the mRNA level). Likewise, when the translational regulator turns on synthesis of the adaptor, translation of GFP is activated at the protein level.Citation1 (B) Riboregulated Transcription Cascade (RTC) with the ability to count. By arranging 3 genes controlled by the translational regulator (crRNA-taRNA) into a transcriptional cascade a genetic circuit is built with the ability to count. When PBAD promoter is not induced, the crRNA element present in each gene blocks translation of the T7 and T3 RNA Polymerase (RNAP), and GFP. In contrast, when PBAD is induced with arabinose, the level of fluorescence generated correlates with the number of pulses of arabinose fed into the system.Citation78 (C) Switchboard for multi-sensing and metabolic pathway control. A series of rationally designed orthogonal variants of the crRNA-taRNA system (cr1, cr2…crn; taRNA1, taRNA2…taRNAn) are fused to multiple gene reporters (Reporter 1, Reporter 2…Reporter n) for simultaneous and independent regulation. The complex genetic circuit is arranged in parallel (each pair of crRNA-taRNA variants is under the control of a different promoter) to be able to sense multiple inputs and convert them into a measurable output (e.g., enzymatic and/or fluorescence reads).Citation79
Current Challenges and Opportunities for Synthetic Biology of Bacterial Regulatory RNAs
There is a need for additional strategies to assay natural capabilities of newly discovered RNA regulators to allow full sorting of the “hidden” wealth of information within all newly identified sequences. While recent high-throughput technologiesCitation84-Citation86 have enabled the fast discovery of a vast number of potential RNA regulators in bacteria, our ability to characterize their targets and structures lags far behind. This illustrates the gap between regulatory RNA discovery and mechanistic analysis. Below, we summarize current techniques that are typically involved in the functional characterization of regulatory RNAs. Although several have been successful, there is a need to develop efficient high-throughput strategies to catalog newly identified regulatory RNAs given the large volumes of data that are being produced in this area on a daily basis. In fact, up to recently, there was still a lack of consistency in the nomenclature used for newly discovered regulatory RNAs, making it difficult to keep track of the ones that were already identified (and perhaps even characterized) in the literature across research groups.
Experimental and bioinformatic strategies for target identification
Identification and complete validation of the cellular targets affected by regulatory RNAs is key to trace their mechanistic roles. Currently, experimental methods for sRNA target identification are numerous and of great variety as they are tailored to a regulatory RNA in question. Consequently, Vogel et al. have classified these strategies as genetic-based, chromosomal reporter gene fusions, affinity based assays, co-immunoprecipitation, proteomics, and translational fusion on plasmid.Citation87 Given the proximity of riboswitches to their targets, identification of their gene target is less challenging than in the case of sRNAs where the target can be encoded from anywhere in the genome. It is important to note that relying solely on individual strategies often favor identification of either protein or mRNA target, but not both. The challenge of functionally characterizing regulatory RNAs has been extensively reviewed in references Citation87 and Citation88.
Given the lack of uniformity in experimental techniques and the labor intensity of these methods, computational-assisted algorithms have become instrumental in this field. The use of bioinformatic approaches has been particularly helpful to define an initial pool of target candidates that can then be experimentally validated.Citation89,Citation90 A successful example of the use of bioinformatic approaches for target prediction has been the identification of the sRNA OxyS target, the ybaY mRNA.Citation91 Computational algorithms for these purposes have also been reviewed in references Citation91 and Citation92. In addition, recent innovative ways of characterizing sRNA have relied on a biology network approach to infer regulatory relationships between sRNAs and mRNAs.Citation93
A creative approach to bridge the gap between discovery of bacterial regulatory RNAs and functional characterization is to use a holistic whole cell approach. To this end, Modi et al.Citation93 proposed a network biology approach in which a computational algorithm was applied to the study of sRNAs. They fed extensive gene expression profiles into the algorithm to infer regulatory relationships between IsrA, GlmZ, GcvB, and their mRNA targets that were experimentally confirmed. The study provided strong support to the applicability of a regulator network approach to perform functional analysis on bacterial small RNAs while surpassing the accuracy and ease of current target prediction methods. Further advancement in our ability to place the function of a regulatory RNA within the context of the rest of the cellular environment is critical for the design of de novo RNA structures. This is particularly relevant to Synthetic Biology given that target binding specificity is another important metric in the functional evaluation of synthetic regulatory molecules that can fully exhibit the functional range of their natural counterparts.
Experimental and computational strategies for structural characterization
Successful design of desired synthetic functions into natural RNAs or de novo scaffolds is highly dependent on the ability to engineer targeted structural changes into these molecules. As such, structural characterization is an important step in the design of synthetic RNA performance. To date, X-ray crystallography and NMR are some of the most widely used techniques for determination of RNA structure. There are several examples of riboswitch structure and riboswitch–ligand complexes that have been studied using X-ray crystallography techniques.Citation94,Citation95 However, these methods are limited mainly in terms of the molecular size ranges that they can handle. As a result, small-Angle X-ray Scattering (SAXS) and some adaptations of NMR techniques have been applied to resolve non-coding short RNA structures. Thus far, their applications have been mainly aimed at capturing dynamic structural reconfigurations in riboswitches and small RNA–Hfq interactions.Citation95-Citation97
A diversity of chemical, biochemical, and enzymatic structural probing techniques has also been applied.Citation98-Citation100 These techniques have been applied, either alone or in combination, to resolve structural features, conformations, and interactions in sRNAs and riboswitches. For instance, the binding of the RybB sRNA to the outer membrane protein OmpN mRNA has been resolved at the nucleotide level using enzymatic and chemical structural probing approaches.Citation101 RybB is the sRNA that controls expression of outer membrane proteins (OMPs) in enterobacteria. There is a large body of published work reporting success with these techniques to characterize changes in sRNAs and riboswitches upon target binding. Recently, interactions of the glmS aptazyme with its effector molecules in B. subtilis were further investigated using enzymatic protection assays, leading to striking evidence that suggested a differential modulation of this riboswitch by multiple molecular cues.Citation102 Another fascinating example is the recent development that couples enzymatic assays with transcriptome-wide sequencing to reveal the secondary structure of regulatory RNAs in a high-throughput manner.Citation89
As in the case of target identification, computational structural prediction methods have been important to complement structural probing data. Among the most accurate algorithms are the stochastic methods that can utilize structural data in their algorithms,Citation90 the homology/phylogenetic comparative analysisCitation99 and thermodynamics-based algorithms.Citation103
The need to move toward structure–function relations
An important aspect of being able to fully predict how a synthetic RNA will function is the ability to correlate structural trends to functional capabilities. An interesting recent example of a structure–function study for gene silencing by sRNAs demonstrated a quantitative correlation between gene repression and the predicted free energy of the sRNA–gene target complex.Citation104
Although there is an arsenal of structure prediction tools available for non-coding RNA computational analysis that have been specifically designed to analyze short non-coding RNAs (reviewed in ref. Citation105), they have been almost exclusively applied to eukaryotic short non-coding RNAs (e.g., micro RNAs). To the best of our knowledge, there are neither structural prediction algorithms evaluated in their performance exclusively on bacterial regulatory RNAs nor methods specifically tailored to predict bacterial regulatory RNA structures. We can only reasonably expect the current available algorithms, to predict short RNA structure with improved accuracy. In addition, despite the prevalence of hairpins in close proximity in bacterial small regulatory RNAs, to the best of our knowledge, the role of pseudoknots has not yet been explored in these molecules. Pseudoknots are RNA structural motifs in which two stem loops base pair with each other and are currently a challenge for prediction algorithms.
Direct implementation of computational and predictive tools into the creation of synthetic elements
A fascinating recent example of the integration of mechanistic modeling and kinetic RNA modeling simulations to design regulatory RNA elements with desired characteristics has been the dynamic control of glmS riboswitch-like gene expression regulators.Citation60 Using a rational design approach, a method to construct RNA-regulated genetic devices was designed based on both biochemical and biophysical predictions. Specifically, static ribozyme- and dynamic aptazyme- regulated genetic devices with quantitatively predictable functions were engineered with the aid of coupled coarse-grained mechanistic and molecular folding simulations. The success of this innovative approach was confirmed by the extraordinary statistical fit that was observed between theoretical predictions and experimental observations. The framework developed in this work represents an important advancement towards applications of synthetic RNA device design. The development of computational methods represents an enormous opportunity in the field of RNA Synthetic Biology.
Unexplored diversity of natural riboswitches in living systems
Identification and characterization of additional RNA scaffolds would largely expand the current repertoire of tools to design novel riboswitches or to further engineer natural ones for new functionalities.Citation23 However, our understanding of these molecules is based on a small fraction of all the hypothesized naturally existing ones.Citation106 Several strategies have been used to diversify the catalog of existing riboswitches. For instance, covariance search methods have been applied to predict the presence of riboswitches in a wide range of organisms. Although these studies have been instructive in revealing mechanistic variations in gene control between microbial groups (as predicted by different structural and molecular organizational featuresCitation106), the identification of putative riboswitches is based on the limited set of known riboswitch characteristics in a few organisms like E. coli and B. subtilis.
Other creative approaches have attempted to assess the abundance and diversity of riboswitches in microbial communities by performing large-scale metagenomic searches via massive computational and experimental explorations;Citation107 these studies have also projected the existence of hundreds of different riboswitches (~300) that await validation and characterization. However, a major drawback in the analysis of large-scale metagenomic sequence data sets is the isolation of functional elements, given that these RNAs tend to: (1) be short (often mistaken for chemically degraded RNAs), (2) lack open reading frames, and (3) sometimes evolve rapidly at the sequence levels while still conserving structure that is integral to their function.Citation107,Citation108
Need for novel in vitro RNA stabilization methods
Although new sensing capabilities of natural and unnatural ligands have been successfully engineered into RNAs via in vitro and in vivo molecular evolution approaches,Citation109-Citation111 the ability to use these molecules by incorporating them into more complex systems and devices represents a bigger practical challenge.Citation23 All potential RNA Synthetic Biology applications require reproducible and controlled molecular stability, especially when activity is required outside the native cellular host and in the context of other biological and inorganic materials. Examples of these stringent applications are the targeted delivery of therapeutic RNAs (e.g., siRNAs) from a polymeric matrix;Citation112 these same delivery tools are now being explored for introduction of other RNA synthetic elements in cells. Currently, we lack knowledge on how to mimic the stabilizing effects of protein surfaces since mechanistic aspects of the use of proteins to protect RNAs in biological systems remain largely unexplored. Several strategies have been used to address RNA instability. For instance, molecular inhibitors of cellular nucleases (e.g., RNase E) have been designed to prevent molecular degradation.Citation113 Still, other creative methods have focused on optimizing intrinsic RNA stability by engineering special secondary motifs and nucleotides to change the chemistry and structure of RNAs themselves in a way that mimics natural post-transcriptional modifications.Citation114 However, one major drawback of directly manipulating RNAs is that only a very small region of the molecule can be targeted (only a few angstroms in size) without loss of desired RNA function. As such, efficient methods for improving RNA stability are another area that awaits further development.
The future of artificial bacterial RNA regulators and their applications
Given the myriad of possible modular combinations using the known RNA parts, and the ones still to be discovered, the possibilities of constructing de novo RNA devices based on regulatory RNAs depend only on our imagination. As we continue to gather fundamental understanding on the principles underlying RNA structure, interactions, and function, the expectations are that we would be able to rationally engineer RNA devices at the smallest possible resolution: single nucleotide (or even single atom). We are already starting to take the first steps toward this as different research groups have already rationally designed riboregulators based on RNA motifs e.g., YUNR consensus sequenceCitation73 and kissing–loop interactionsCitation74,Citation75 ().
Overall, the main challenge in the field of synthetic RNA biology is to turn all the special tricks that RNAs naturally perform into useful applications. A further challenge is to be able to synthesize de novo RNA molecules at the atomic level. Although the field has been rapidly moving in this direction, critics still await for major breakthroughs that will yield “useful things” that can directly impact human lives. So far, a variety of applications that stem from “lower hanging fruits” to truly spectacular innovations have been envisioned. Recent work also indicates that additional potential applications could be on their way. Specifically, it seems highly plausible to see an explosion of direct applications of RNA-based Synthetic Biology in bacteria and other organisms traditionally used for production of biochemical compounds. For instance, in enzyme evolution for metabolic engineering applications, Michener et al. have developed an approach to use metabolite-sensing ribozymes linked to gene reporters to evolve and improve enzymatic activity in yeast in a high-throughput way.Citation115 This could have direct application to the bio manufacturing of a variety of compounds that are already produced in E. coli. In addition, in medicine, naturally occurring E. coli could be further explored as a living sensor seeking disease sites and as a therapeutic agent to treat disease-specific sites. The futuristic and almost surreal concept of a therapeutic bacterium is supported by recent works, including Sinha et al. who engineered E. coli to seek and destroy a herbicideCitation58 and by the use of non-RNA-based strategies to search for and control cancer cells using Vibrio fischeri.Citation116
Finally, bacteria show potential for use in the development of RNA devices that can be transferred to other cellular platforms for more relevant applications since many RNA functions are considered portable and as such can be transferred from one kingdom to the other with no considerable additional adaptation.Citation117,Citation118 Additionally, the highly valuable ability of bacterial sRNAs to modulate multiple pathways in metabolic engineering applications could strengthen the potential of using bacteria as a bio-factory.
Other potential fields of application for synthetic regulatory RNAs in a bacterial context that have been explored using either higher organisms or other non-RNA-based technologies include clinical applications like cell therapy and regenerative medicine, microbiome engineering, functional genomics, drug testing, etc.Citation22 In addition, we predict that other non-clinical applications in fields like biosafety and anti-biofouling (e.g., pipes, boats, implantable devices, surgical instruments) will be in the pipeline soon.
Final Thoughts
In a way that parallels how the discovery of large catalytic RNAs revolutionized our perceived limits of biotechnology and bioengineering, the more recent realization of smaller RNA transcripts as powerful regulators has continued to shake our imagination as to the many potential applications of these regulatory molecules in Synthetic Biology. Yet, one of the main challenges faced by this field is the continual fundamental understanding and characterization of the wealth of mechanistic information contained within these molecules. How can we find new RNA scaffolds that display entirely new functional mechanisms in a high-throughput way? Are there more efficient and creative ways to identify cellular targets and pathways that are directly affected by a particular non-coding RNA? What can evolutionary studies tell us about the need for these mechanisms across species? How can we best capture ways by which these molecules are stabilized inside living systems and transfer that knowledge to inorganic surfaces? How can we design rules to predict optimal combinations of multiple RNA units in tandem to tune sensing capabilities? Or, yet, how do we arrange several RNA motifs (biological parts) to increase the functional complexity of RNA devices? How do we accurately predict the behavior of such complex RNA devices from individual parts? How do we develop reliable techniques to profoundly understand underlying principles of complex RNA devices and their ultimate phenotypic impact? So far, we have only touched the surface of all these questions by focusing on a handful of riboswitches and small RNAs via different approaches: rational design (sometimes computationally aided), molecular evolution, and the use of natural elements found in nature.Citation21,Citation34 Increased fundamental understanding in this area will continue to broaden our creativity in exploiting the many different types of non-coding RNAs that we suspect are present in nature in a much wider range of impactful applications.
Similarly, more insights into the questions we pose will lead to being able to “catch up” applications of RNA biology to those being currently executed by proteins as these are, overall, much better understood. While other molecules (like proteins) might pose advantageous properties over RNAs in different contexts, the addition of RNA to the toolkit will add one more avenue that by itself (or in combination with other types of molecules) will highly contribute to the ongoing creativity of the synthetic biology field.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
We are grateful to funding to Contreras LM from the Welch Foundation (Grant NO. F-1756), Defense Threat Reduction Agency (DTRA) Young Investigator Program (HDTRA1-12-0016), Air Force Office of Scientific Research (AFOSR) Young Investigator program (FA9550-13-1-0160), and the NSF CAREER program (CBET-1254754). We want to acknowledge as well the Consejo Nacional de Ciencia y Tecnología (CONACYT, Mexico) for the graduate fellowship granted to Vazquez-Anderson J (CONACYT-194638). We also thank Steven Sowa, Kevin Baldridge, and Kevin Vasquez for their valuable comments and edition contributions toward the successful completion of this manuscript.
References
- Liu CC, Qi L, Lucks JB, Segall-Shapiro TH, Wang D, Mutalik VK, Arkin AP. An adaptor from translational to transcriptional control enables predictable assembly of complex regulation. Nat Methods 2012; 9:1088 - 94; http://dx.doi.org/10.1038/nmeth.2184; PMID: 23023598
- Wang Y-H, Wei KY, Smolke CD. Synthetic biology: advancing the design of diverse genetic systems. Annu Rev Chem Biomol Eng 2013; 4:69 - 102; http://dx.doi.org/10.1146/annurev-chembioeng-061312-103351; PMID: 23413816
- Nowak R. Mining treasures from ‘junk DNA’. Science 1994; 263:608 - 10; http://dx.doi.org/10.1126/science.7508142; PMID: 7508142
- Gottesman S, Storz G. Bacterial small RNA regulators: versatile roles and rapidly evolving variations. Cold Spring Harb Perspect Biol 2011; 3:1 - 16; http://dx.doi.org/10.1101/cshperspect.a003798; PMID: 20980440
- Li L, Huang D, Cheung MK, Nong W, Huang Q, Kwan HS. BSRD: a repository for bacterial small regulatory RNA. Nucleic Acids Res 2013; 41:D233 - 8; http://dx.doi.org/10.1093/nar/gks1264; PMID: 23203879
- Mandal M, Boese B, Barrick JE, Winkler WC, Breaker RR. Riboswitches control fundamental biochemical pathways in Bacillus subtilis and other bacteria. Cell 2003; 113:577 - 86; http://dx.doi.org/10.1016/S0092-8674(03)00391-X; PMID: 12787499
- Waters LS, Storz G. Regulatory RNAs in bacteria. Cell 2009; 136:615 - 28; http://dx.doi.org/10.1016/j.cell.2009.01.043; PMID: 19239884
- Serrano L. Synthetic biology: promises and challenges. Mol Syst Biol 2007; 3:158; http://dx.doi.org/10.1038/msb4100202; PMID: 18091727
- Gardner TS, Cantor CR, Collins JJ. Construction of a genetic toggle switch in Escherichia coli. Nature 2000; 403:339 - 42; http://dx.doi.org/10.1038/35002131; PMID: 10659857
- Elowitz MB, Leibler S. A synthetic oscillatory network of transcriptional regulators. Nature 2000; 403:335 - 8; http://dx.doi.org/10.1038/35002125; PMID: 10659856
- Purnick PE, Weiss R. The second wave of synthetic biology: from modules to systems. Nat Rev Mol Cell Biol 2009; 10:410 - 22; http://dx.doi.org/10.1038/nrm2698; PMID: 19461664
- Khalil AS, Collins JJ. Synthetic biology: applications come of age. Nat Rev Genet 2010; 11:367 - 79; http://dx.doi.org/10.1038/nrg2775; PMID: 20395970
- Bloom JD, Meyer MM, Meinhold P, Otey CR, MacMillan D, Arnold FH. Evolving strategies for enzyme engineering. Curr Opin Struct Biol 2005; 15:447 - 52; http://dx.doi.org/10.1016/j.sbi.2005.06.004; PMID: 16006119
- Tyo KE, Alper HS, Stephanopoulos GN. Expanding the metabolic engineering toolbox: more options to engineer cells. Trends Biotechnol 2007; 25:132 - 7; http://dx.doi.org/10.1016/j.tibtech.2007.01.003; PMID: 17254656
- Pickens LB, Tang Y, Chooi YH. Metabolic engineering for the production of natural products. Annu Rev Chem Biomol Eng 2011; 2:211 - 36; http://dx.doi.org/10.1146/annurev-chembioeng-061010-114209; PMID: 22432617
- Zhang WH, Otting G, Jackson CJ. Protein engineering with unnatural amino acids. Curr Opin Struct Biol 2013; 23:581 - 7; http://dx.doi.org/10.1016/j.sbi.2013.06.009; PMID: 23835227
- Richard IH, Brea M, Alexandre L. Current Challenges in Nucleic Acid Synthesis. Isr J Chem 2013; 53:326 - 49; http://dx.doi.org/10.1002/ijch.201300032
- Gustafsson C, Govindarajan S, Minshull J. Codon bias and heterologous protein expression. Trends Biotechnol 2004; 22:346 - 53; http://dx.doi.org/10.1016/j.tibtech.2004.04.006; PMID: 15245907
- Hurtado-Guerrero R, Davies GJ. Recent structural and mechanistic insights into post-translational enzymatic glycosylation. Curr Opin Chem Biol 2012; 16:479 - 87; http://dx.doi.org/10.1016/j.cbpa.2012.10.013; PMID: 23142486
- Liu CC, Arkin AP. Cell biology. The case for RNA. Science 2010; 330:1185 - 6; http://dx.doi.org/10.1126/science.1199495; PMID: 21109657
- Liang JC, Bloom RJ, Smolke CD. Engineering biological systems with synthetic RNA molecules. Mol Cell 2011; 43:915 - 26; http://dx.doi.org/10.1016/j.molcel.2011.08.023; PMID: 21925380
- Ruder WC, Lu T, Collins JJ. Synthetic biology moving into the clinic. Science 2011; 333:1248 - 52; http://dx.doi.org/10.1126/science.1206843; PMID: 21885773
- Kaempfer R. RNA sensors: novel regulators of gene expression. EMBO Rep 2003; 4:1043 - 7; http://dx.doi.org/10.1038/sj.embor.7400005; PMID: 14593443
- Cech TR, Zaug AJ, Grabowski PJ. In vitro splicing of the ribosomal RNA precursor of Tetrahymena: involvement of a guanosine nucleotide in the excision of the intervening sequence. Cell 1981; 27:487 - 96; http://dx.doi.org/10.1016/0092-8674(81)90390-1; PMID: 6101203
- Gold L, Polisky B, Uhlenbeck O, Yarus M. Diversity of oligonucleotide functions. Annu Rev Biochem 1995; 64:763 - 97; http://dx.doi.org/10.1146/annurev.bi.64.070195.003555; PMID: 7574500
- Cui X, Davis G. Mobile group II intron targeting: applications in prokaryotes and perspectives in eukaryotes. Front Biosci 2007; 12:4972 - 85; http://dx.doi.org/10.2741/2442; PMID: 17569624
- Lundblad EW, Altman S. Inhibition of gene expression by RNase P. N Biotechnol 2010; 27:212 - 21; http://dx.doi.org/10.1016/j.nbt.2010.03.003; PMID: 20211282
- Fiskaa T, Birgisdottir AB. RNA reprogramming and repair based on trans-splicing group I ribozymes. N Biotechnol 2010; 27:194 - 203; http://dx.doi.org/10.1016/j.nbt.2010.02.013; PMID: 20219714
- Tedeschi L, Lande C, Cecchettini A, Citti L. Hammerhead ribozymes in therapeutic target discovery and validation. Drug Discov Today 2009; 14:776 - 83; http://dx.doi.org/10.1016/j.drudis.2009.05.003; PMID: 19477286
- Mandal M, Breaker RR. Gene regulation by riboswitches. Nat Rev Mol Cell Biol 2004; 5:451 - 63; http://dx.doi.org/10.1038/nrm1403; PMID: 15173824
- Serganov A. Determination of riboswitch structures: light at the end of the tunnel?. RNA Biol 2010; 7:98 - 103; http://dx.doi.org/10.4161/rna.7.1.10756; PMID: 20061809
- Serganov A, Nudler E. A decade of riboswitches. Cell 2013; 152:17 - 24; http://dx.doi.org/10.1016/j.cell.2012.12.024; PMID: 23332744
- Henkin TM. Riboswitch RNAs: using RNA to sense cellular metabolism. Genes Dev 2008; 22:3383 - 90; http://dx.doi.org/10.1101/gad.1747308; PMID: 19141470
- Lioliou E, Romilly C, Romby P, Fechter P. RNA-mediated regulation in bacteria: from natural to artificial systems. N Biotechnol 2010; 27:222 - 35; http://dx.doi.org/10.1016/j.nbt.2010.03.002; PMID: 20211281
- Werstuck G, Green MR. Controlling gene expression in living cells through small molecule-RNA interactions. Science 1998; 282:296 - 8; http://dx.doi.org/10.1126/science.282.5387.296; PMID: 9765156
- Winkler W, Nahvi A, Breaker RR. Thiamine derivatives bind messenger RNAs directly to regulate bacterial gene expression. Nature 2002; 419:952 - 6; http://dx.doi.org/10.1038/nature01145; PMID: 12410317
- Winkler WC, Nahvi A, Roth A, Collins JA, Breaker RR. Control of gene expression by a natural metabolite-responsive ribozyme. Nature 2004; 428:281 - 6; http://dx.doi.org/10.1038/nature02362; PMID: 15029187
- Thompson KM, Syrett HA, Knudsen SM, Ellington AD. Group I aptazymes as genetic regulatory switches. BMC Biotechnol 2002; 2:21; http://dx.doi.org/10.1186/1472-6750-2-21; PMID: 12466025
- Nechooshtan G, Elgrably-Weiss M, Sheaffer A, Westhof E, Altuvia S. A pH-responsive riboregulator. Genes Dev 2009; 23:2650 - 62; http://dx.doi.org/10.1101/gad.552209; PMID: 19933154
- Kortmann J, Narberhaus F. Bacterial RNA thermometers: molecular zippers and switches. Nat Rev Microbiol 2012; 10:255 - 65; http://dx.doi.org/10.1038/nrmicro2730; PMID: 22421878
- Waldminghaus T, Kortmann J, Gesing S, Narberhaus F. Generation of synthetic RNA-based thermosensors. Biol Chem 2008; 389:1319 - 26; http://dx.doi.org/10.1515/BC.2008.150; PMID: 18713019
- Wieland M, Hartig JS. RNA quadruplex-based modulation of gene expression. Chem Biol 2007; 14:757 - 63; http://dx.doi.org/10.1016/j.chembiol.2007.06.005; PMID: 17656312
- Gottesman S. The small RNA regulators of Escherichia coli: roles and mechanisms*. Annu Rev Microbiol 2004; 58:303 - 28; http://dx.doi.org/10.1146/annurev.micro.58.030603.123841; PMID: 15487940
- Wassarman KM. Small RNAs in bacteria: diverse regulators of gene expression in response to environmental changes. Cell 2002; 109:141 - 4; http://dx.doi.org/10.1016/S0092-8674(02)00717-1; PMID: 12007399
- Thomason MK, Storz G. Bacterial antisense RNAs: how many are there, and what are they doing?. Annu Rev Genet 2010; 44:167 - 88; http://dx.doi.org/10.1146/annurev-genet-102209-163523; PMID: 20707673
- Gottesman S. Micros for microbes: non-coding regulatory RNAs in bacteria. Trends Genet 2005; 21:399 - 404; http://dx.doi.org/10.1016/j.tig.2005.05.008; PMID: 15913835
- Storz G, Vogel J, Wassarman KM. Regulation by small RNAs in bacteria: expanding frontiers. Mol Cell 2011; 43:880 - 91; http://dx.doi.org/10.1016/j.molcel.2011.08.022; PMID: 21925377
- Rodrigo G, Fares MA. Describing the structural robustness landscape of bacterial small RNAs. BMC Evol Biol 2012; 12:52; http://dx.doi.org/10.1186/1471-2148-12-52; PMID: 22500888
- Babitzke P, Romeo T. CsrB sRNA family: sequestration of RNA-binding regulatory proteins. Curr Opin Microbiol 2007; 10:156 - 63; http://dx.doi.org/10.1016/j.mib.2007.03.007; PMID: 17383221
- Wassarman KM. 6S RNA: a regulator of transcription. Mol Microbiol 2007; 65:1425 - 31; http://dx.doi.org/10.1111/j.1365-2958.2007.05894.x; PMID: 17714443
- Lunde BM, Moore C, Varani G. RNA-binding proteins: modular design for efficient function. Nat Rev Mol Cell Biol 2007; 8:479 - 90; http://dx.doi.org/10.1038/nrm2178; PMID: 17473849
- De Lay N, Schu DJ, Gottesman S. Bacterial small RNA-based negative regulation: Hfq and its accomplices. J Biol Chem 2013; 288:7996 - 8003; http://dx.doi.org/10.1074/jbc.R112.441386; PMID: 23362267
- Møller T, Franch T, Højrup P, Keene DR, Bächinger HP, Brennan RG, Valentin-Hansen P. Hfq: a bacterial Sm-like protein that mediates RNA-RNA interaction. Mol Cell 2002; 9:23 - 30; http://dx.doi.org/10.1016/S1097-2765(01)00436-1; PMID: 11804583
- Romby P, Charpentier E. An overview of RNAs with regulatory functions in gram-positive bacteria. Cell Mol Life Sci 2010; 67:217 - 37; http://dx.doi.org/10.1007/s00018-009-0162-8; PMID: 19859665
- Brennan RG, Link TM. Hfq structure, function and ligand binding. Curr Opin Microbiol 2007; 10:125 - 33; http://dx.doi.org/10.1016/j.mib.2007.03.015; PMID: 17395525
- Russell R. RNA misfolding and the action of chaperones. Front Biosci 2008; 13:1 - 20; http://dx.doi.org/10.2741/2557; PMID: 17981525
- Andrade JM, Pobre V, Arraiano CM. Small RNA modules confer different stabilities and interact differently with multiple targets. PLoS One 2013; 8:e52866; http://dx.doi.org/10.1371/journal.pone.0052866; PMID: 23349691
- Sinha J, Reyes SJ, Gallivan JP. Reprogramming bacteria to seek and destroy an herbicide. Nat Chem Biol 2010; 6:464 - 70; http://dx.doi.org/10.1038/nchembio.369; PMID: 20453864
- Topp S, Gallivan JP. Guiding bacteria with small molecules and RNA. J Am Chem Soc 2007; 129:6807 - 11; http://dx.doi.org/10.1021/ja0692480; PMID: 17480075
- Carothers JM, Goler JA, Juminaga D, Keasling JD. Model-driven engineering of RNA devices to quantitatively program gene expression. Science 2011; 334:1716 - 9; http://dx.doi.org/10.1126/science.1212209; PMID: 22194579
- Sharma V, Nomura Y, Yokobayashi Y. Engineering complex riboswitch regulation by dual genetic selection. J Am Chem Soc 2008; 130:16310 - 5; http://dx.doi.org/10.1021/ja805203w; PMID: 18998646
- Muranaka N, Yokobayashi Y. Posttranscriptional signal integration of engineered riboswitches yields band-pass output. Angew Chem Int Ed Engl 2010; 49:4653 - 5; http://dx.doi.org/10.1002/anie.201001482; PMID: 20480477
- Winkler WC, Breaker RR. Regulation of bacterial gene expression by riboswitches. Annu Rev Microbiol 2005; 59:487 - 517; http://dx.doi.org/10.1146/annurev.micro.59.030804.121336; PMID: 16153177
- Ellington AD, Szostak JW. In vitro selection of RNA molecules that bind specific ligands. Nature 1990; 346:818 - 22; http://dx.doi.org/10.1038/346818a0; PMID: 1697402
- Suess B, Weigand JE. Engineered riboswitches: overview, problems and trends. RNA Biol 2008; 5:24 - 9; http://dx.doi.org/10.4161/rna.5.1.5955; PMID: 18388492
- Rimmele M. Nucleic acid aptamers as tools and drugs: recent developments. Chembiochem 2003; 4:963 - 71; http://dx.doi.org/10.1002/cbic.200300648; PMID: 14523912
- Famulok M, Hartig JS, Mayer G. Functional aptamers and aptazymes in biotechnology, diagnostics, and therapy. Chem Rev 2007; 107:3715 - 43; http://dx.doi.org/10.1021/cr0306743; PMID: 17715981
- Na D, Yoo SM, Chung H, Park H, Park JH, Lee SY. Metabolic engineering of Escherichia coli using synthetic small regulatory RNAs. Nat Biotechnol 2013; 31:170 - 4; http://dx.doi.org/10.1038/nbt.2461; PMID: 23334451
- Edwards AN, Patterson-Fortin LM, Vakulskas CA, Mercante JW, Potrykus K, Vinella D, Camacho MI, Fields JA, Thompson SA, Georgellis D, et al. Circuitry linking the Csr and stringent response global regulatory systems. Mol Microbiol 2011; 80:1561 - 80; http://dx.doi.org/10.1111/j.1365-2958.2011.07663.x; PMID: 21488981
- Timmermans J, Van Melderen L. Post-transcriptional global regulation by CsrA in bacteria. Cell Mol Life Sci 2010; 67:2897 - 908; http://dx.doi.org/10.1007/s00018-010-0381-z; PMID: 20446015
- Tatarko M, Romeo T. Disruption of a global regulatory gene to enhance central carbon flux into phenylalanine biosynthesis in Escherichia coli. Curr Microbiol 2001; 43:26 - 32; http://dx.doi.org/10.1007/s002840010255; PMID: 11375660
- Sharma V, Yamamura A, Yokobayashi Y. Engineering artificial small RNAs for conditional gene silencing in Escherichia coli. ACS Synth Biol 2012; 1:6 - 13; http://dx.doi.org/10.1021/sb200001q; PMID: 23651005
- Isaacs FJ, Dwyer DJ, Ding C, Pervouchine DD, Cantor CR, Collins JJ. Engineered riboregulators enable post-transcriptional control of gene expression. Nat Biotechnol 2004; 22:841 - 7; http://dx.doi.org/10.1038/nbt986; PMID: 15208640
- Lucks JB, Qi L, Mutalik VK, Wang D, Arkin AP. Versatile RNA-sensing transcriptional regulators for engineering genetic networks. Proc Natl Acad Sci U S A 2011; 108:8617 - 22; http://dx.doi.org/10.1073/pnas.1015741108; PMID: 21555549
- Qi L, Lucks JB, Liu CC, Mutalik VK, Arkin AP. Engineering naturally occurring trans-acting non-coding RNAs to sense molecular signals. Nucleic Acids Res 2012; 40:5775 - 86; http://dx.doi.org/10.1093/nar/gks168; PMID: 22383579
- Klauser B, Hartig JS. An engineered small RNA-mediated genetic switch based on a ribozyme expression platform. Nucleic Acids Res 2013; 41:5542 - 52; http://dx.doi.org/10.1093/nar/gkt253; PMID: 23585277
- Benenson Y. RNA-based computation in live cells. Curr Opin Biotechnol 2009; 20:471 - 8; http://dx.doi.org/10.1016/j.copbio.2009.08.002; PMID: 19720518
- Friedland AE, Lu TK, Wang X, Shi D, Church G, Collins JJ. Synthetic gene networks that count. Science 2009; 324:1199 - 202; http://dx.doi.org/10.1126/science.1172005; PMID: 19478183
- Callura JM, Cantor CR, Collins JJ. Genetic switchboard for synthetic biology applications. Proc Natl Acad Sci U S A 2012; 109:5850 - 5; http://dx.doi.org/10.1073/pnas.1203808109; PMID: 22454498
- Simons RW, Kleckner N. Translational control of IS10 transposition. Cell 1983; 34:683 - 91; http://dx.doi.org/10.1016/0092-8674(83)90401-4; PMID: 6311438
- Seidelt B, Innis CA, Wilson DN, Gartmann M, Armache J-P, Villa E, Trabuco LG, Becker T, Mielke T, Schulten K, et al. Structural insight into nascent polypeptide chain-mediated translational stalling. Science 2009; 326:1412 - 5; http://dx.doi.org/10.1126/science.1177662; PMID: 19933110
- Muranaka N, Yokobayashi Y. A synthetic riboswitch with chemical band-pass response. Chem Commun (Camb) 2010; 46:6825 - 7; http://dx.doi.org/10.1039/c0cc01438a; PMID: 20721392
- Phan TT, Schumann W. Development of a glycine-inducible expression system for Bacillus subtilis. J Biotechnol 2007; 128:486 - 99; http://dx.doi.org/10.1016/j.jbiotec.2006.12.007; PMID: 17208325
- Metzker ML. Sequencing technologies - the next generation. Nat Rev Genet 2010; 11:31 - 46; http://dx.doi.org/10.1038/nrg2626; PMID: 19997069
- Lynch SA, Gallivan JP. A flow cytometry-based screen for synthetic riboswitches. Nucleic Acids Res 2009; 37:184 - 92; http://dx.doi.org/10.1093/nar/gkn924; PMID: 19033367
- Gelderman G, Contreras LM. Discovery of posttranscriptional regulatory RNAs using next generation sequencing technologies. Methods Mol Biol 2013; 985:269 - 95; http://dx.doi.org/10.1007/978-1-62703-299-5_14; PMID: 23417809
- Vogel J, Wagner EG. Target identification of small noncoding RNAs in bacteria. Curr Opin Microbiol 2007; 10:262 - 70; http://dx.doi.org/10.1016/j.mib.2007.06.001; PMID: 17574901
- Sharma CM, Vogel J. Experimental approaches for the discovery and characterization of regulatory small RNA. Curr Opin Microbiol 2009; 12:536 - 46; http://dx.doi.org/10.1016/j.mib.2009.07.006; PMID: 19758836
- Kertesz M, Wan Y, Mazor E, Rinn JL, Nutter RC, Chang HY, Segal E. Genome-wide measurement of RNA secondary structure in yeast. Nature 2010; 467:103 - 7; http://dx.doi.org/10.1038/nature09322; PMID: 20811459
- Mikulík K. Structure and functional properties of prokaryotic small noncoding RNAs. Folia Microbiol (Praha) 2003; 48:443 - 68; http://dx.doi.org/10.1007/BF02931326; PMID: 14533476
- Tjaden B, Goodwin SS, Opdyke JA, Guillier M, Fu DX, Gottesman S, Storz G. Target prediction for small, noncoding RNAs in bacteria. Nucleic Acids Res 2006; 34:2791 - 802; http://dx.doi.org/10.1093/nar/gkl356; PMID: 16717284
- Tjaden B. Computational Identification of sRNA Targets. In: Keiler KC, ed. Bacterial Regulatory RNA: Humana Press, 2012:227-34.
- Modi SR, Camacho DM, Kohanski MA, Walker GC, Collins JJ. Functional characterization of bacterial sRNAs using a network biology approach. Proc Natl Acad Sci U S A 2011; 108:15522 - 7; http://dx.doi.org/10.1073/pnas.1104318108; PMID: 21876160
- Pikovskaya O, Serganov AA, Polonskaia A, Serganov A, Patel DJ. Preparation and crystallization of riboswitch-ligand complexes. Methods Mol Biol 2009; 540:115 - 28; http://dx.doi.org/10.1007/978-1-59745-558-9_9; PMID: 19381556
- Schwalbe H, Buck J, Fürtig B, Noeske J, Wöhnert J. Structures of RNA switches: insight into molecular recognition and tertiary structure. Angew Chem Int Ed Engl 2007; 46:1212 - 9; http://dx.doi.org/10.1002/anie.200604163; PMID: 17226886
- Lipfert J, Herschlag D, Doniach S. Riboswitch conformations revealed by small-angle X-ray scattering. Methods Mol Biol 2009; 540:141 - 59; http://dx.doi.org/10.1007/978-1-59745-558-9_11; PMID: 19381558
- Buck J, Fürtig B, Noeske J, Wöhnert J, Schwalbe H. Time-resolved NMR methods resolving ligand-induced RNA folding at atomic resolution. Proc Natl Acad Sci U S A 2007; 104:15699 - 704; http://dx.doi.org/10.1073/pnas.0703182104; PMID: 17895388
- Serganov A. Riboswitches: Methods and Protocols. Humana Press, 2009.
- Wassarman KM, Zhang A, Storz G. Small RNAs in Escherichia coli. Trends Microbiol 1999; 7:37 - 45; http://dx.doi.org/10.1016/S0966-842X(98)01379-1; PMID: 10068996
- Durand S, Storz G. Reprogramming of anaerobic metabolism by the FnrS small RNA. Mol Microbiol 2010; 75:1215 - 31; http://dx.doi.org/10.1111/j.1365-2958.2010.07044.x; PMID: 20070527
- Bouvier M, Sharma CM, Mika F, Nierhaus KH, Vogel J. Small RNA binding to 5′ mRNA coding region inhibits translational initiation. Mol Cell 2008; 32:827 - 37; http://dx.doi.org/10.1016/j.molcel.2008.10.027; PMID: 19111662
- Watson PY, Fedor MJ. The glmS riboswitch integrates signals from activating and inhibitory metabolites in vivo. Nat Struct Mol Biol 2011; 18:359 - 63; http://dx.doi.org/10.1038/nsmb.1989; PMID: 21317896
- Mathews D, Disney M. Childs… J. Incorporating chemical modification constraints into a dynamic programming algorithm for prediction of RNA secondary structure. Proceedings of the … 2004.
- Hao Y, Zhang ZJ, Erickson DW, Huang M, Huang Y, Li J, Hwa T, Shi H. Quantifying the sequence-function relation in gene silencing by bacterial small RNAs. Proc Natl Acad Sci U S A 2011; 108:12473 - 8; http://dx.doi.org/10.1073/pnas.1100432108; PMID: 21742981
- Washietl S, Will S, Hendrix DA, Goff LA, Rinn JL, Berger B, Kellis M. Computational analysis of noncoding RNAs. Wiley Interdiscip Rev RNA 2012; 3:759 - 78; http://dx.doi.org/10.1002/wrna.1134; PMID: 22991327
- Barrick JE, Breaker RR. The distributions, mechanisms, and structures of metabolite-binding riboswitches. Genome Biol 2007; 8:R239; http://dx.doi.org/10.1186/gb-2007-8-11-r239; PMID: 17997835
- Hammann C, Westhof E. Searching genomes for ribozymes and riboswitches. Genome Biol 2007; 8:210; http://dx.doi.org/10.1186/gb-2007-8-4-210; PMID: 17472738
- Machado-Lima A, del Portillo HA, Durham AM. Computational methods in noncoding RNA research. J Math Biol 2008; 56:15 - 49; http://dx.doi.org/10.1007/s00285-007-0122-6; PMID: 17786447
- Wittmann A, Suess B. Engineered riboswitches: Expanding researchers’ toolbox with synthetic RNA regulators. FEBS Lett 2012; 586:2076 - 83; http://dx.doi.org/10.1016/j.febslet.2012.02.038; PMID: 22710175
- Weigand JE, Sanchez M, Gunnesch EB, Zeiher S, Schroeder R, Suess B. Screening for engineered neomycin riboswitches that control translation initiation. RNA 2008; 14:89 - 97; http://dx.doi.org/10.1261/rna.772408; PMID: 18000033
- Wieland M, Hartig JS. Improved aptazyme design and in vivo screening enable riboswitching in bacteria. Angew Chem Int Ed Engl 2008; 47:2604 - 7; http://dx.doi.org/10.1002/anie.200703700; PMID: 18270990
- Knipe JM, Peters JT, Peppas NA. Theranostic agents for intracellular gene delivery with spatiotemporal imaging. Nano Today 2013; 8:21 - 38; http://dx.doi.org/10.1016/j.nantod.2012.12.004; PMID: 23606894
- Carrier TA, Keasling JD. Controlling messenger RNA stability in bacteria: strategies for engineering gene expression. Biotechnol Prog 1997; 13:699 - 708; http://dx.doi.org/10.1021/bp970095h; PMID: 9413129
- Cantara WA, Crain PF, Rozenski J, McCloskey JA, Harris KA, Zhang X, Vendeix FA, Fabris D, Agris PF. The RNA Modification Database, RNAMDB: 2011 update. Nucleic Acids Res 2011; 39:D195 - 201; http://dx.doi.org/10.1093/nar/gkq1028; PMID: 21071406
- Michener JK, Smolke CD. High-throughput enzyme evolution in Saccharomyces cerevisiae using a synthetic RNA switch. Metab Eng 2012; 14:306 - 16; http://dx.doi.org/10.1016/j.ymben.2012.04.004; PMID: 22554528
- Anderson JC, Clarke EJ, Arkin AP, Voigt CA. Environmentally controlled invasion of cancer cells by engineered bacteria. J Mol Biol 2006; 355:619 - 27; http://dx.doi.org/10.1016/j.jmb.2005.10.076; PMID: 16330045
- Burnett JC, Rossi JJ. RNA-based therapeutics: current progress and future prospects. Chem Biol 2012; 19:60 - 71; http://dx.doi.org/10.1016/j.chembiol.2011.12.008; PMID: 22284355
- Liang JC, Bloom RJ, Smolke CD. Engineering biological systems with synthetic RNA molecules. Mol Cell 2011; 43:915 - 26; http://dx.doi.org/10.1016/j.molcel.2011.08.023; PMID: 21925380
- Isaacs FJ, Dwyer DJ, Collins JJ. RNA synthetic biology. Nat Biotechnol 2006; 24:545 - 54; http://dx.doi.org/10.1038/nbt1208; PMID: 16680139
- Franch T, Petersen M, Wagner EG, Jacobsen JP, Gerdes K. Antisense RNA regulation in prokaryotes: rapid RNA/RNA interaction facilitated by a general U-turn loop structure. J Mol Biol 1999; 294:1115 - 25; http://dx.doi.org/10.1006/jmbi.1999.3306; PMID: 10600370
- Suess B, Fink B, Berens C, Stentz R, Hillen W. A theophylline responsive riboswitch based on helix slipping controls gene expression in vivo. Nucleic Acids Res 2004; 32:1610 - 4; http://dx.doi.org/10.1093/nar/gkh321; PMID: 15004248
- Rudolph MM, Vockenhuber M-P, Suess B. Synthetic riboswitches for the conditional control of gene expression in Streptomyces coelicolor. Microbiology 2013; 159:1416 - 22; http://dx.doi.org/10.1099/mic.0.067322-0; PMID: 23676435
- Fowler CC, Brown ED, Li Y. Using a riboswitch sensor to examine coenzyme B(12) metabolism and transport in E. coli. Chem Biol 2010; 17:756 - 65; http://dx.doi.org/10.1016/j.chembiol.2010.05.025; PMID: 20659688
- Nomura Y, Yokobayashi Y. Reengineering a natural riboswitch by dual genetic selection. J Am Chem Soc 2007; 129:13814 - 5; http://dx.doi.org/10.1021/ja076298b; PMID: 17944473
- Topp S, Reynoso CM, Seeliger JC, Goldlust IS, Desai SK, Murat D, Shen A, Puri AW, Komeili A, Bertozzi CR, et al. Synthetic riboswitches that induce gene expression in diverse bacterial species. Appl Environ Microbiol 2010; 76:7881 - 4; http://dx.doi.org/10.1128/AEM.01537-10; PMID: 20935124
- Jin Y, Watt RM, Danchin A, Huang JD. Use of a riboswitch-controlled conditional hypomorphic mutation to uncover a role for the essential csrA gene in bacterial autoaggregation. J Biol Chem 2009; 284:28738 - 45; http://dx.doi.org/10.1074/jbc.M109.028076; PMID: 19706608
- Seeliger JC, Topp S, Sogi KM, Previti ML, Gallivan JP, Bertozzi CR. A riboswitch-based inducible gene expression system for mycobacteria. PLoS One 2012; 7:e29266; http://dx.doi.org/10.1371/journal.pone.0029266; PMID: 22279533
- Ogawa A, Maeda M. Aptazyme-based riboswitches as label-free and detector-free sensors for cofactors. Bioorg Med Chem Lett 2007; 17:3156 - 60; http://dx.doi.org/10.1016/j.bmcl.2007.03.033; PMID: 17391960
- Ogawa A, Maeda M. An artificial aptazyme-based riboswitch and its cascading system in E. coli. Chembiochem 2008; 9:206 - 9; http://dx.doi.org/10.1002/cbic.200700478; PMID: 18098257
- Desai SK, Gallivan JP. Genetic screens and selections for small molecules based on a synthetic riboswitch that activates protein translation. J Am Chem Soc 2004; 126:13247 - 54; http://dx.doi.org/10.1021/ja048634j; PMID: 15479078
- Goodman HM, Abelson J, Landy A, Brenner S, Smith JD. Amber suppression: a nucleotide change in the anticodon of a tyrosine transfer RNA. Nature 1968; 217:1019 - 24; http://dx.doi.org/10.1038/2171019a0; PMID: 5643523
- Hunsicker A, Steber M, Mayer G, Meitert J, Klotzsche M, Blind M, Hillen W, Berens C, Suess B. An RNA aptamer that induces transcription. Chem Biol 2009; 16:173 - 80; http://dx.doi.org/10.1016/j.chembiol.2008.12.008; PMID: 19246008
- Sharma V, Sakai Y, Smythe KA, Yokobayashi Y. Knockdown of recA gene expression by artificial small RNAs in Escherichia coli. Biochem Biophys Res Commun 2013; 430:256 - 9; http://dx.doi.org/10.1016/j.bbrc.2012.10.141; PMID: 23178465
- Rodrigo G, Landrain TE, Majer E, Daròs J-A, Jaramillo A. Full design automation of multi-state RNA devices to program gene expression using energy-based optimization. PLoS Comput Biol 2013; 9:e1003172; http://dx.doi.org/10.1371/journal.pcbi.1003172; PMID: 23935479
- Park H, Bak G, Kim SC, Lee Y. Exploring sRNA-mediated gene silencing mechanisms using artificial small RNAs derived from a natural RNA scaffold in Escherichia coli. Nucleic Acids Res 2013; 41:3787 - 804; http://dx.doi.org/10.1093/nar/gkt061; PMID: 23393193
- Stefan A, Schwarz F, Bressanin D, Hochkoeppler A. Shine-Dalgarno sequence enhances the efficiency of lacZ repression by artificial anti-lac antisense RNAs in Escherichia coli. J Biosci Bioeng 2010; 110:523 - 8; http://dx.doi.org/10.1016/j.jbiosc.2010.05.012; PMID: 20646957
- Man S, Cheng R, Miao C, Gong Q, Gu Y, Lu X, Han F, Yu W. Artificial trans-encoded small non-coding RNAs specifically silence the selected gene expression in bacteria. Nucleic Acids Res 2011; 39:e50; http://dx.doi.org/10.1093/nar/gkr034; PMID: 21296758
- Mutalik VK, Qi L, Guimaraes JC, Lucks JB, Arkin AP. Rationally designed families of orthogonal RNA regulators of translation. Nat Chem Biol 2012; 8:447 - 54; http://dx.doi.org/10.1038/nchembio.919; PMID: 22446835
- Callura JM, Dwyer DJ, Isaacs FJ, Cantor CR, Collins JJ. Tracking, tuning, and terminating microbial physiology using synthetic riboregulators. Proc Natl Acad Sci U S A 2010; 107:15898 - 903; http://dx.doi.org/10.1073/pnas.1009747107; PMID: 20713708
- Klauser B, Saragliadis A, Ausländer S, Wieland M, Berthold MR, Hartig JS. Post-transcriptional Boolean computation by combining aptazymes controlling mRNA translation initiation and tRNA activation. Mol Biosyst 2012; 8:2242 - 8; http://dx.doi.org/10.1039/c2mb25091h; PMID: 22777205