Abstract
The gene Down syndrome cell adhesion molecule (Dscam) potentially encodes 38 016 distinct isoforms in Drosophila melanogaster via mutually exclusive splicing. Here we reveal a combinatorial mechanism of regulation of Dscam exon 17 mutually exclusive splicing through steric hindrance in combination with RNA secondary structure. This mutually exclusive behavior is enforced by steric hindrance, due to the close proximity of the exon 17.2 branch point to exon 17.1 in Diptera, and the interval size constraint in non-Dipteran species. Moreover, intron-exon RNA structures are evolutionarily conserved in 36 non-Drosophila species of six distantly related orders (Diptera, Lepidoptera, Coleoptera, Hymenoptera, Hemiptera, and Phthiraptera), which regulates the selection of exon 17 variants via masking the splice site. By contrast, a previously uncharacterized RNA structure specifically activated exon 17.1 by bringing splice sites closer together in Drosophila, while the other moderately suppressed exon 17.1 selection by hindering the accessibility of polypyrimidine sequences. Taken together, these data suggest a phylogeny of increased complexity in regulating alternative splicing of Dscam exon 17 spanning more than 300 million years of insect evolution. These results also provide models of the regulation of alternative splicing through steric hindrance in combination with dynamic structural codes.
Introduction
Alternative splicing is an important mechanism for the expansion of proteomic and functional diversity.Citation1-Citation3 Increasing numbers of novel alternative transcripts have been detected because of advances in high-throughput sequencing technology.Citation4 In humans, up to 95% of primary transcripts are estimated to undergo alternative splicing.Citation5,Citation6 Mutually exclusive splicing is a strictly regulated form of alternative splicing, in which the splicing machinery must select one of two or more candidate exons.Citation7 An exceedingly challenging case of mutually exclusive alternative splicing occurs in the Drosophila Down syndrome cell adhesion molecule (Dscam) gene, which encodes an axon guidance receptor of the immunoglobulin superfamily with numerous possible isoforms, which likely play key roles in the nervous and immune system of the fly.Citation8-Citation10 The Dscam gene, containing exons 4, 6, 9, and 17, selected from 12, 48, 33, and 2 alternative exons, respectively, could produce 38 016 distinct isoforms via mutually exclusive splicing in D. melanogaster.Citation11 Much evidence shows that a large number, or possibly the entire repertoire, of diverse Dscam isoforms is functionally required for the precision of complex axonal branching and connectivity in the central nervous system.Citation12,Citation13 Mutually exclusive splicing of the Dscam exon cluster 6 involves the formation of competing RNA secondary structures between a docking site and selector sequences.Citation14,Citation15 This long-range base pairing is essential for inclusion of exon 6 variants, as determined by deletion analysis.Citation16 A similar structural code has also been deciphered in exon clusters 4 and 9 of Dscam.Citation17 Recently, it was found that a locus control region (LCR) could activate the exon 6 cluster and allow for the selection of the most proximal exon variant when the docking site is paired with its selector sequence.Citation18
The transmembrane/juxtamembrane domain (TM1 or TM2) of Dscam is encoded by either alternatives exon 17.1 or 17.2. Alternative splicing could influence Dscam subcellular localization to axons or dendrites by producing different protein-targeting motifs within the transmembrane domain. Exon 17.1-containing Dscam isoforms were restricted to the cell body and dendrites, while exon 17.2-containing Dscam isoforms were localized to axons involved in governing axonal morphogenesis during the early development of the nervous system.Citation19 Recent studies reveal that Vap protein interacts specifically with TM2-containing Dscam isoforms, and loss-of-function genetics demonstrates that Vap is required for the localization of TM2-containing isoforms to axons, while dendritic localization of TM1-containing isoforms is not affected.Citation20 A model based on competing RNA secondary structures is proposed to explain the mutually exclusive selection of exon 17 of the Dscam gene.Citation15 However, this model requires experimental verification of the effects of structure-disrupting and compensatory structure-restoring mutations upon splicing. Moreover, although this model explained the mutually exclusive selection of exon 17, both isoforms can occur simultaneously as a pseudoknot structure, or each separately as a structured form. Furthermore, similar arrangements of RNA secondary structures have not been reported in Dscam exon 17 from other distantly related insect species.
In this study, we indicate that this type of mutually exclusive behavior is enforced by steric hindrances, due to the close proximity of the exon 17.2 branch point to exon 17.1 in Diptera, and to the interval size constraint between them in non-Dipteran species. Moreover, we show that taxon-specific RNA structures mediate the ratio of Dscam exon 17 variants during insect evolution. Intron-exon RNA secondary structures, which are evolutionarily conserved in 38 non-Drosophila species from six distantly related orders, regulate the selection of exon 17 variants via masking the splice site. In contrast, two long-range RNA secondary structures cooperate to regulate the ratio of exon 17 variants in Drosophila. Taken together, these data suggest a phylogeny of increased complexity in regulating mutually exclusive splicing of Dscam exon 17 spanning more than 300 million years of insect evolution. The results also suggest models of the regulation of alternative splicing through steric hindrance combined with the RNA structural and linear codes.
Results
Splicing of exons 17.1 and 17.2 is blocked by steric hindrance
Detailed genomic analysis reveals two alternative exons in the Dscam exon 17 cluster from all insect species investigated, while only one copy exists in waterflea species (Table S1). Thus, we were interested in the mechanisms underlying the mutually exclusive behavior of exons 17.1 and 17.2 splicing. Notably, the intervening intron between exons 17.1 and 17.2 is below 57 nt in all insect species, except for Diptera (; Fig. S1). The minimal intron requirement is around 50–60 nt for pre-mRNA splicing of mammalian and Drosophila species.Citation21,Citation22 Below this threshold distance, U1 and U2 cannot bind productively to the 5′-splice site and branch point.Citation7 Therefore, the small intron between exons 17.1 and 17.2 acts as hinders the splicing of the two exons in non-Dipteran species.
Figure 1. Splicing of exons 17.1 and 17.2 is blocked by the proximity of the branch point to the exon 17.1 splice-donor site in Drosophila. (A) Schematic showing the genomic region of Dscam exons 16–18. A schematic diagram of the partial pre-mRNA with constitutive exons depicted as black boxes, alternative exons as blue boxes, and introns as lines with the spacing indicated (means ± SD). (B) The size of the intervening intron between alternative exons 17.1 and 17.2 across insect species. Cladogram of taxa in this study: Diptera (Drosophila, Culicidae), Lepidoptera, Coleoptera, Hymenoptera, Hemiptera, and Phthiraptera. The correlation of intron size with evolutionary distance between D. melanogaster and other species is shown in the inset. (C) BP mapping in D. melanogaster. Diagram of the primer pair (P1 and P2) for PCR and sequencing. Two arrows represent the position and orientation of the primers used for PCR, with the PCR product shown on the right. The arrows are not drawn to scale. Electropherogram of the sequence containing the branch point (arrow), followed by the alternative 5′-splice site (arrow) in the lariat exons. (D) A sequence alignment of the PCR fragment (blue) and the Dscam gene (black) in D. melanogaster. The end of the alternative 5′-splice site (GT) is covalently linked to an A (the branch point) by a 5′-2' phosphodiester, which is located upstream from the alternative 3′-splice site (AG).
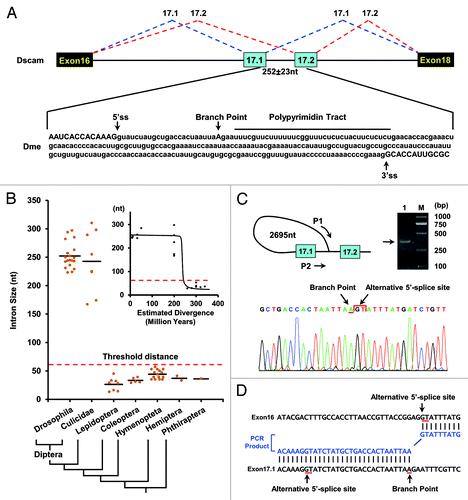
Although the intron between exons 17.1 and 17.2 is 150–350 nt long in Diptera (), interestingly, we discovered that a ~30 nt C/U-rich sequence was located just downstream of the exon 17.1 splice-donor site (; Fig. S1). Importantly, it was unique within the intervening intron sequence, and conserved among 27 species of Drosophila and mosquitoes. We hypothesized that if it functioned as a polypyrimidine tract, the distance between the lariat branch point and 5′-splice site was approximately 20 nt, which hinders the splicing of the two exons. To confirm this, we mapped the location of the lariat branch point in the intervening intron. As shown in , the lariat branch point, with an associated polypyrimidine tract, is in an unusual location, 228 nt upstream of the exon 17.2 acceptor in D. melanogaster. Importantly, it is located only 23 nt away from the exon 17.1 splice-donor site, which is below the minimum distance required for splicing. Similar results were obtained in mosquito species (Fig. S1). These observations are reminiscent of the mutually exclusive splicing of exons 2 and 3 in mammalian α-tropomyosin, in which the exon 3 branch point lies only 42 nt away from the 5′-splice site of exon 2, although the intron between exons 2 and 3 is 218 nt in length.Citation23 Thus, splicing of exons 17.1 and 17.2 is blocked by the proximity of the branch point to the exon 17.1 splice-donor site in these Dipteran species.
A conserved intron element is paired with the 3' ss of exon 17.1 in Hymenopterans
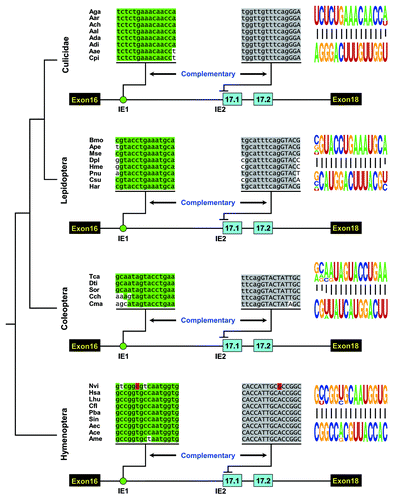
Next, we sought to elucidate how the ratio of exons 17.1 and 17.2 is regulated. Since the intron downstream of exon 16 is relatively small (80–500 bp) in Hymenopterans, contrary to the large intron in Drosophila (2–3 kb) or mosquitoes (2–13 kb) (; Fig. S1), we first analyzed the cis-elements involved in the regulation of exon 17 alternative splicing in Hymenopteran species. Evolutionary comparison identified a highly conserved element in an intron downstream of exon 16 among Hymenopteran species, spanning 10–150 million years ().Citation24 Importantly, this element could base pair with an intron-exon boundary sequence of exon 17.1 in each species (; Fig. S2). In contrast, the size of the loop is variable, ranging from 24 nt in A. mellifera to 409 nt in N. longicornis. The dsRNA stem in each species has a common core of 15 bp, which are highlighted in green (Fig. S2) and shown as a pictogram in . Within this core is an example of covariation maintaining the structural integrity of the dsRNA. The sixth base pair of the core is a U-A base pairing in the ant and honeybee species investigated, while C-G base pairs exist at this position in the Nasonia structures (; Fig. S2). Taken together, the high evolutionarily conservation of the dsRNA during over 150 million years strongly suggests that it is functionally relevant to the regulation of the splicing of exon 17 variants.
Figure 2. Phylogenetic arrangement of cis intronic elements within the alternative exon 17 cluster of insect Dscam. A schematic diagram of the partial pre-mRNA with constitutive exons depicted as black boxes, alternative exons as blue boxes, and introns as lines. These organisms represent species from four major orders of Insecta: Diptera (Culicidae), Lepidoptera, Coleoptera, and Hymenoptera. Above are sequences of taxonomically restricted consensus elements (IE) of various species (see Table S1 for abbreviations). The most identical nucleotides at each position are indicated by different colors. The conserved intronic elements could pair with an intron-exon boundary sequence of exon 17.1, although their primary sequences diverged. Nucleotide covariations that maintain the structural integrity of the dsRNA are shaded in red. Pictogram of the sequences of the dsRNA stem core in each order are shown on the right.
Conservation of the intron-exon duplex in non-Drosophila species
Next, we sought to determine whether such RNA secondary structures are conserved in other insect species. We discovered similar evolutionarily conserved RNA secondary structures in distantly related Lepidopteran and Coleopteran species, although their primary sequences were divergent (; Figs. S3 and S4). Similar secondary structures have been reported in the Hemipteran A. pisum, and the Phthirapteran P. humanus corporis (Fig. S5). These RNA structures contain several independent examples of compensatory double mutations that maintain the dsRNA structural integrity (Fig. S3).
Nonetheless, we failed to discover an element that could pair with an intron-exon boundary sequence of exon 17.1 in Drosophila species, which contain a relatively large intron 16. Interestingly, combining comparative genomics with structural predictions revealed an evolutionarily conserved intron-exon RNA structure in eight mosquito species (; Fig. S6). In each species, the size of the loop is strikingly variable, ranging from 1162 nt in Anopheles albimanus to 12 376 nt in Aedes aegypti (Fig. S6). This suggests that the divergence of the intron-exon duplex occurred before the radiation of Drosophila and the Culicidae. Collectively, the evolutionary conservation of intron-exon duplexes in non-Drosophila insect species suggests them to be the target of this splicing regulation.
The intron-exon duplex controls the switching of exons 17.1 and 17.2 in silkworms
Because an RNA stem structure masks an alternative acceptor splice site in exon 17.1, we speculated that these predicted intron-exon structures were involved in the mutually exclusive splicing of Dscam exon 17. To confirm this, we assessed disrupting and compensatory mutations in the RNA stem of the silkworm in transfection experiments (). Mutating the IE1 (M1) or the IE2 (M2) had little effect on exon 17 inclusion (). However, further sequencing and exon-specific restriction analysis indicated that exon 17.1 was almost exclusively selected in the presence of an IE1 or IE2 mutation, while the ratio of splicing of exon 17.1 to exon 17.2 is approximately 1:1 in the wild type (). In both cases, the mutations could relieve the acceptor site from the RNA stem structure, thereby facilitating efficient recognition and splicing. Importantly, compensatory double mutations by reestablishing a stem with a novel sequence (M12) led to a switch in the splicing pattern to approximately the wild-type level (). Thus, this RNA structure mediates the selection of alternative variants by dynamically suppressing the proximal acceptor site.
Figure 3. Intron-exon duplex controls the switching of mutually exclusive exons in the B. mori Dscam exon cluster 17. (A) Genomic organization of B. mori (Bmo) Dscam exon cluster 17. Constitutive exons (in black boxes), Alternative exons (in blue boxes), conserved elements IE1 and IE2 and intron (line) shown. IE1 could pair with IE2. (B) Predicted intron-exon RNA pairing of B. mori pre-mRNA. Disruptive mutations (M1, M2) and compensatory double mutations (M12) were designed to assess the effect of mutation on exon 17 inclusion. Mutations introduced into dsRNA are indicated above or below mutated sequences (M1, M2). (C) Effects of mutations on exon 17 inclusion are indicated for disruptive mutations (M1, M2) and compensatory double mutations (M12). WT, wild type. The RNA was used as an RT-PCR template, and isoform-specific restriction digestion was performed to evaluate the frequency of exon 17.1 utilization. (D) Effects of mutations on exon 17.1 and exon 17.2 selection. (E) Predicted RNA pairing for the wild type and a series of mutants (Mu1-Mu6, point mutations are shown in blue), with the estimated equilibrium of free energy (given in kcal/mol). (F) The strength of RNA pairing modulated exon 17 selection by mutated analysis. (G) Quantitation of the data in panel F. Data are expressed as percentages of the mean ± SD from three independent experiments. Exon 17.1 selection was negatively correlated with the strength of RNA pairing.
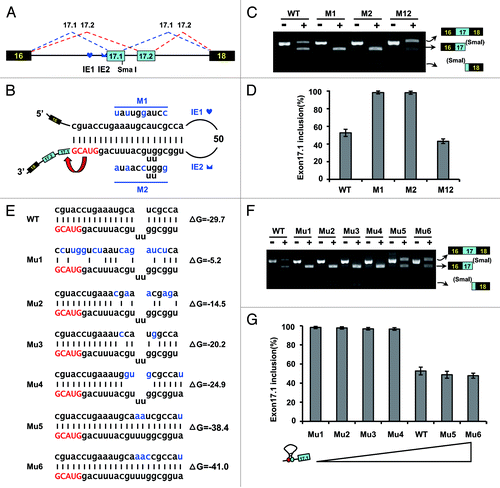
Since U2AF35 is known to bind to the 3′-splice site,Citation25 it is tempting to speculate that dynamic competition between the pairing of IE1 with the 3′-splice site and the binding of U2AF35 with the 3′-splice site might regulate splicing. To elucidate how the strength of RNA pairing modulates exon 17 selection, a series of mutations were performed to alter the thermodynamic stability (). Mutations that strengthened the pairing slightly reduced the exon 17.1 frequency (). In contrast, mutations that lowered the thermodynamic stability strikingly increased the exon 17.1 frequency, to a maximum of almost 100% (). Analysis of a series of mutations indicated that the strength of RNA pairing was negatively correlated with the frequency of exon 17.1 selection. However, complete suppression of exon 17.1 inclusion likely did not occur. The ratio of splicing of exon 17.1 to exon 17.2 was reduced to approximately 1:1, and the strength of RNA pairing was improved, implying that such RNA pairing is not sufficient to completely inhibit exon 17.1 inclusion.
Drosophila-specific RNA secondary structures within exon 17 clusters
As mentioned above, we failed to discover intron-exon secondary structures in exon 17.1 of Drosophila, as found in other insects. However, genomic comparison identified several small intronic elements (IE) within an intron upstream of exon 17.1, and predicted three pairs of complementary conserved motifs (). The latter two pairs of complementary conserved motifs were previously predicted by Anastassiou et al.,Citation15 and a model involving internal base pairing interactions between distal segments of the pre-mRNA could also account for the binary choice between exons 17.1 and 17.2 of the Drosophila Dscam gene, albeit without any experimental evidence.
Figure 4. Effect of predicted RNA structures on Dscam exon 17 splicing in Drosophila. (A) Conserved cis intronic elements within the Dscam exon 17 cluster. A schematic diagram of the partial pre-mRNA with constitutive exons depicted as black boxes, alternative exons as blue boxes, and introns as lines. Above are sequences of consensus intronic elements (for abbreviations, see Table S1). Sequences in an identical color indicate that upstream intronic elements could pair with downstream intronic sequences. (B) The predicted RNA pairings in D. melanogaster. Mutations introduced into dsRNA are indicated above or below mutated sequences (M1-M6). (C) Effect of predicted RNA structures in Dscam exon 17 splicing were tested using disruptive mutations (M1-M6) and compensatory double mutations (M12, M34, and M56). WT, wild-type. (D) Quantitation of the data in panel C. Data are expressed as percentages of the mean ± SD from three independent experiments.
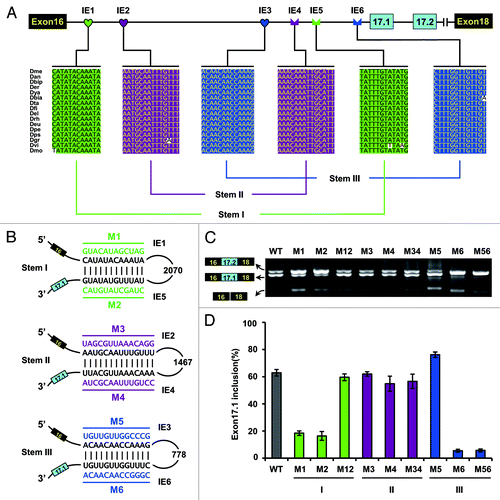
To confirm these predicted RNA structures, we disrupted and compensated the dsRNA structure to assess its effects on splicing in transfection experiments (). A portion of the Dscam gene containing exons 16–18 and intervening sequences was cloned into a Drosophila expression vector containing the inducible metallothionine promoter to generate a minigene (). As a result, inclusion of exon 17.1 was inhibited while exon 17.2 was selected almost exclusively in the presence of the IE1 or IE5 mutation (). This suggested that either the IE1 or IE5 mutation, concomitant with failure of IE1-to-IE5 RNA pairing, could specifically abolish exon 17.1 inclusion; in contrast, the selection of the alternative exon (exon 17.2) was enhanced. In both cases, the mutations led to a switch to almost exclusive selection of the exon 17.2 isoforms. Importantly, the reduced efficiency of the exon 17.1 inclusion caused by individual mutations could be reverted to almost wild-type levels by compensatory double mutations (). In this scenario, IE1-to-IE5 RNA pairing acts as a critical switch between the exon 17.1 and exon 17.2 isoforms.
By contrast, disrupting and compensatory mutations in the RNA stem II have little if any effect on exon 17 selections (). This led us to hypothesize that both elements may not function as an RNA stem in vivo. In addition, our results indicated that mutating IE6 destroyed exon 17.1 selection, while mutating IE3 had an only modest effect on exon 17 selection. However, compensatory mutations (M56) could not restore the selection of exon 17.1, whose splicing pattern was similar to that resulting from disruption of the IE6 mutant (M6). This suggests that IE6 does not function primarily as an RNA stem. Since we could not confirm stem II and stem III as predicted by Anastassiou et al.,Citation15 a model involving competition between stem II and stem III that accounts for the binary choice between exon 17.1 and 17.2 of the Drosophila Dscam gene could not be supported.
IE1-to-IE5 pairing functions to approximate splice elements
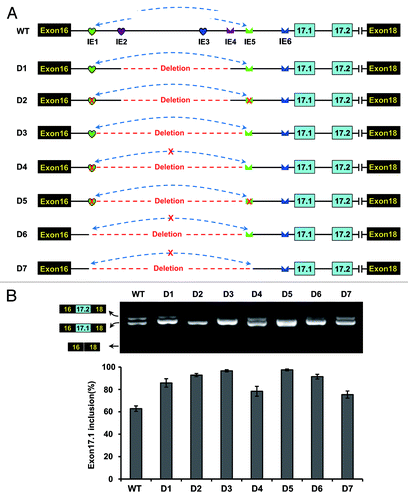
As described above, IE1-to-IE5 RNA pairing is important for exon 17.1 selection. Next, we determined how this non-sequence-specific RNA pairing mediates specific activation of exon 17.1. Two general scenarios may provide such information. In the first scenario, the RNA stem must be bound by regulatory proteins without sequence specificity, which controls the switch of the exon 17.1 and exon 17.2 variants. Alternatively, RNA pairing acts by approximation of upstream and downstream sequences, as has been reported previously.Citation17,Citation26,Citation27 To investigate this, we created a series of constructs in which was deleted IE1, the loop sequence, and IE5 (). These deletions were designed to mimic the approximation of sequences caused by the IE1-to-IE5 pairing. RT-PCR analysis revealed that predominantly exon 17.1 was included. Importantly, the inclusion of exon 17.1 was not significantly affected by deletion of IE1, IE5, or any RNA stem in these constructs (). Taken together, these findings suggest that such intronic RNA pairing may bring the necessary elements into proximity sufficient for activation of the selection of exon 17.1.
Figure 5. Intronic RNA pairing interaction functions through approximating splice sites in D. melanogaster. (A) Schematic diagrams of the minigene constructs used to test the importance of approximating. A red cross in IE1 or IE5 denotes a disrupting mutation. IE1–6 elements are indicated as in the legend to . (B) Effects of a series of deletions and mutations on exon 17.1 selection. Quantitative data are shown and expressed as percentages of the mean ± SD from three independent experiments.
IE6 functions in both structural- and sequence-dependent modes
The above results indicate that mutating IE6 destroys exon 17.1 selection, while mutating IE3 has only a modest effect on exon 17 selection. Moreover, compensatory mutations failed to restore the effect on exon 17 selection (), suggesting that the IE6 mutation could specifically silence exon 17.1 inclusion. This double-compensatory mutation failed to reactivate exon 17.1, suggesting that the effect of mutation on splicing was mediated via the primary sequence of IE6. Mapping of the bps indicated that the IE6 element was embedded in a polypyrimidine tract (PPT) (). The length of the polypyrimidine tract can increase the efficiency of splicing to a splice-acceptor site.Citation28 Therefore, the IE6 mutation prevented exon 17.1 inclusion by disturbing the polypyrimidine tract.
Figure 6. IE6 affects exon 17.1 selection in both structural- and sequence-dependent mode in D. melanogaster. (A) Schematic diagrams of minigene constructs used to assess the role of IE6 in splicing. The branch-point sequence (BPS), polypyrimidine tract (PPT), and the invariant AG at the 3′ ss are shown. M1, mutating IE3 abolished the IE3-IE6 pairing (indicated by the red cross); M2 and M3, mutating IE3 increased the IE3-IE6 pairing strength (indicated by the green arrow), whereas pairing strength was stronger in M3 than in M2. (B) Effects of mutations on the exon 17 selection. Data are expressed as percentages of the mean ± SD from three independent experiments. (C) Exon 17.1 frequency was negatively correlated with the strength of RNA pairing. (D) A refined model for alternative splicing regulation of Dscam exon 17 in Drosophila. The green and red arrows indicate the activation and suppression of exon 17.1.
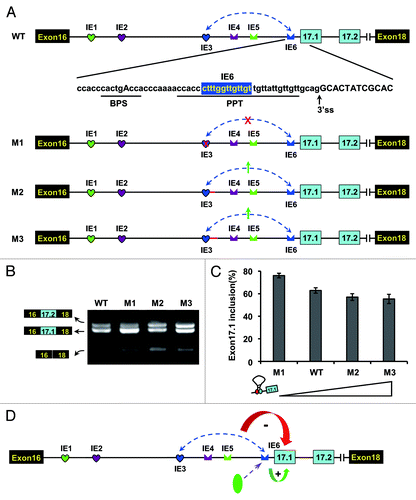
To determine whether IE3-to-IE6 pairing also contributed to the selection of alternative exon 17s, a series of mutations were introduced into the IE3 sequence without changing the IE6 sequence. The effects of these mutations were consistent with a negative relationship between the calculated stability of RNA pairing and the inclusion frequency of alternative exon 17.1 (). This result indicated that IE3-to-IE6 RNA pairing moderately inhibited exon 17.1 selection. Considering that IE6 was embedded in the polypyrimidine tract (PPT), it is tempting to suggest that IE3-to-IE6 RNA pairing functions to hinder the accessibility of nucleotide sequences by splicing factors (i.e., U2AF65). It would be interesting to elucidate how IE6 competitively pairs with IE3 or binds to the polypyrimidine-tract-binding protein (PTB) ().
Thus, two RNA secondary structures may cooperate to mediate exon 17 selection. Stem I could activate exon 17.1, while stem III could moderately inhibit exon 17.1. Based on the efficiency of exon 17 selection in the fly tissues investigated and the above mutational results, stem I largely overrode the inhibitory role of stem III. Although it is possible for both to occur simultaneously through the formation of a pseudoknot, this might be uneconomical if two contradictory activation/inhibition stems are located in the same structure.
Discussion
Model of Dscam exon 17 mutually exclusive splicing
Our data indicated that mutually exclusive splicing of exons 17.1 and 17.2 could be regulated by a combinatorial mechanism through steric hindrance in combination with the dynamic RNA structure. First, the mutually exclusive behavior of alternative exons 17.1 and 17.2 was controlled by steric hindrance, due to the close proximity of the exon 17.2 branch point to exon 17.1 in Diptera, and due to the size of the intervening intron in other insect species. In the former case, although the intron between exons 17.1 and 17.2 is 150–350 nt long in Drosophila and mosquitoes, the exon 17.2 branch point to the splice-donor site hinders the splicing of the two exons, through an analogous mechanism first reported for the mutually exclusive splicing of exons 2 and 3 in mammalian α-tropomyosin.Citation23 We also found more examples of mutually exclusive splicing in Drosophila genes, such as mtacp1 (CG9160), slgA(CG1417), TrpA1 (CG5751), which are controlled by such a steric-hindrance mechanism. Steric hindrance, therefore, is an important mechanism of the control of the mutually exclusive behavior of two alternative exons.
Furthermore, our data indicated that RNA secondary structures regulate the exon 17.1 to 17.2 ratio via multiple mechanisms. The intron/exon of the RNA stem-loop is conserved among the 38 non-Drosophila species from the six orders investigated. Such RNA structures mask the splice site, thereby inhibiting the selection of exon 17.1, as has been reported previously.Citation29 Thus, dynamic RNA structure could efficiently mediate the exon 17.1 and 17.2 ratio by altering the pairing strength through evolving one or a few nucleotides. Interestingly, different mechanisms evolved for Dscam exon 17 alternative splicing in Drosophila, whereas intronic RNA secondary structures evolved as new controllers by competing with the binding proteins ( and ). Indeed, another Drosophila-specific RNA secondary structure, which is involved in an approximation-activation mechanism, has evolved, similar to previous studies.Citation17,Citation26,Citation27,Citation30 Thus, the alternative splicing of the exon 17 cluster is regulated by two sets of RNA secondary structure that function in concert.
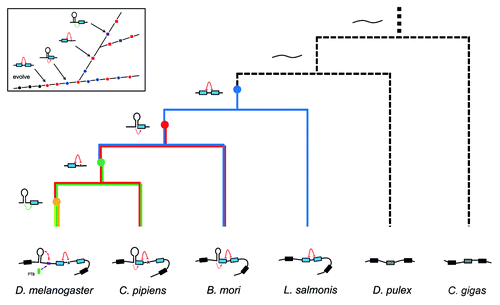
Figure 7. Regulatory phylogeny of Dscam exon 17 alternative splicing by steric hindrance in combination with the dynamic RNA structure. Partial pre-mRNA structures and proposed ancestor molecules are shown associated with a cladogram of the phylogenetic relationships determined in this study.Citation38 RNA structures in D. melanogaster (Dme) were confirmed in Drosophila S2 cells ( and ), and RNA structures in B. mori (Bmo) were confirmed in BmN cells (). Nodes denoting the ancestral origins of particular exon duplication events are indicated by solid circles. Constitutive Dscam mRNAs are depicted as unstructured (black). Inset shows the emergence of control elements relative to the ancestor.
Finally, Dscam exon 17 splicing is influenced by splicing factors. Several trans-acting splicing regulators have been identified by RNAi-knockdown to affect Dscam exon 17 splicing in Drosophila cells.Citation31 In these RNAi experiments, the knockdown of these regulators could increase exon 17 skipping (i.e., CG10279 and CG7269), and also markedly change the ratio of exon 17.1 and 17.2 isoforms. Nonetheless, none of the splicing regulators led to the splicing of exon 17.1 and 17.2 isoforms, as did hrp36 on the exon 6 cluster,Citation32 nor could they act as a dynamic switch between exon 17.1 and 17.2, as could stem I. Notably, some conservation of a nucleotide sequence within the RNA duplex is present in the various insect orders (5′ UUUCAGGUAC 3′) as is its partner sequence (Figs. S3–S6). This might correspond to the binding site for splicing factors that mediate the selection of exon 17 isoforms.
In summary, this study, together with a previous report,Citation31 indicates that Dscam exon 17 alternative splicing is controlled by steric hindrance, the dynamic RNA structure, and multi-linear cis-elements together with trans-acting factors. These combinatorial codes and their interactions add an additional layer to the higher-order regulation of splicing. In this manner, the number of protein regulators required to govern the developmental- or tissue-specific selection of variable exons may be reduced. This multi-layered control system may increase the efficiency and flexibility of developmental- or tissue-specific splicing regulation.
Our data suggest that Dscam exon 17 mutually exclusive splicing is mechanistically distinct from that of exons 4, 6, and 9. In these latter cases, one-to-many competing RNA secondary structures between the docking site and selector sequence direct the mutually exclusive splicing.Citation14-Citation18 Although steric hindrance of intron size could prevent adjacent alternative exons from being spliced together, it could not do so for non-adjacent exons. Therefore, the steric hindrance mechanism is suitable for two-duplicate-exon clusters (i.e., exon 17), but not for three-or-more-duplicate-exon clusters. (i.e., exons 4, 6, and 9). In addition, the latter mechanism would be advantageous when considering the species-specific evolutionary expansion from two-duplicate-exon to multiple-exon clusters.
Evolution of increased complexity in splicing regulation
By integrating the genetic and molecular data from 61 arthropod species, we suggest a credible phylogeny of regulation of Dscam exon 17 alternative splicing (). First, the ancestor gene underwent tandem exon duplication before the divergence of the Pancrustacea and Myriapoda, over 420 million years ago. The small intron between exons 17.1 and 17.2 acted as a steric hindrance against the splicing of the two exons in these ancestors. Indeed, we discovered an evolutionary intermediate with a small intron that lacked the RNA duplex in the Crustacean Lepeophtheirus salmonis. During subsequent evolution, one intronic element was purifying-selected by pairing with the 3′-splice site of exon 17.1. Such an RNA pairing interaction suppressed the inclusion of exon 17.1, and simultaneously increased the selection of exon 17.2. Thus, a combinatorial system for alternative splicing regulation through the RNA duplex in combination with the steric hindrance emerged. Furthermore, the structural system could be maintained heritably during subsequent evolution, although the sequences of purifying-selected elements have changed globally (i.e., B. mori, A. mellifera vs. T. castaneum). During Dipteran speciation, the intervening intron was enlarged, and the splicing of exons 17.1 and 17.2 is blocked by the proximity of the branch point to the exon 17.1 splice-donor site.
During subsequent evolution, intron-exon RNA secondary structures were shifted into intronic RNA secondary structures in Drosophila, while the intron-exon RNA structures were maintained in mosquitoes (). Mechanistically, the inhibitory role of masking of the splice site by RNA pairing has shifted to hindrance of the accessibility of the PPT motif in Drosophila. Another RNA secondary structure also evolved, which may bring splice sites closer together. These two pairs of RNA secondary structure act in concert to regulate splicing, in addition to the steric hindrance. Due to the evolutionarily conserved proximity of the RNA secondary structure to exon 16 and the long loop sequence in mosquitoes, it seems likely that RNA secondary structure modulates the activity of the 5′-splice sites, in addition to masking the 3′-splice site.
Traditionally, single-stranded RNAs generally adopt local secondary structures that are confined to small windows.Citation33 However, there is increasing evidence of long-range RNA interactions in higher eukaryotes. More potential long-range RNA secondary structures were associated with splicing in Drosophila and mammals.Citation30,Citation34 Our results reveal conserved stem-loop structures in 38 non-Drosophila species, with a variable loop sequence, ranging from 24 nt in A. mellifera to more than 12 knt in A. aegypti. This shapes the dynamic evolution of an increasing complexity from simple local RNA structures in the ancestor gene to complex long-distance structures. Thus, the present findings provide a deeper understanding of molecular regulatory complexity.
Materials and Methods
Materials
The insects and other species used in this study are presented in Table S1. Fruitflies (D. melanogaster), silkworms (B. mori), and mosquito (C. pipiens) were obtained as reported previously.Citation17,Citation35
Sequence alignments and RNA pairing predictions
The alignments of conserved regions among species were performed using the ClustalW2 software (http://www.ebi.ac.uk/Tools/msa/clustalw2/). The RNA pairings were predicted using the Mfold software.Citation36 The consensus sequences of RNA pairing were derived using WebLogos (http://weblogo.berkeley.edu/logo.cgi).Citation37
RT-PCR
Total RNA was isolated using the RNeasy Mini Kit (Qiagen). Total RNA was reverse transcribed using SuperScript III RTase (Invitrogen) with an oligo(dT)15 primer, and the resulting single-stranded cDNA product was treated with DNase I at 37 °C for 30 min. Specific primers flanking the alternative exons were designed (Table S2). PCR was performed with denaturing at 95 °C for 3 min, 35 cycles of denaturing at 95 °C for 45 s, annealing at 55 °C for 50 s, and extension at 72 °C for 2 min and 10 s, followed by extension at 72 °C for 10 min. The products of PCR and RT-PCR were purified and cloned into the pGEM-T Easy vector (Promega), then transformed into TG1 competent cells.
Determination of branch points
The spliced RNA products were reverse-transcribed by SuperScript III RTase (Invitrogen) using random primers. The resulting cDNAs were amplified by Taq DNA polymerase using external primers, followed by a second amplification using internal primers. The products were subcloned in pGEM-T Easy vector (Promega). DNA sequencing was then performed.
Quantification of mRNA splice isoforms
We assayed the RNA splice isoform ratio using RT-PCR followed by exon-specific restriction digestion. Total RNA was isolated from different development stages and tissue samples. RT-PCR was performed under the following cycling conditions with an initial denaturation of 2 min at 94 °C followed by 30–35 cycles of denaturation at 94 °C for 30 s, annealing at 60–65 °C for 30 s, and extension at 72 °C for 15 s, with a final extension at 72 °C for 10 min. PCR products were then digested by exon-specific restriction enzymes. Images were captured through a CCD camera, and the quantification of mRNA isoforms was performed by comparison of the integrated optical density of detected bands measured by the GIS 1D Gel Image System, ver. 3.73 (Tanon). Data were expressed as percentages of the mean and SD. The error bars throughout this study were calculated from the average of three independent experiments.
Minigene construction, mutagenesis, and transfection
Genomic DNAs isolated from B. mori and D. melanogaster were used as templates to amplify the corresponding DNA segments encompassing the upstream constitutive exon, the downstream constitutive exon, and the intervening sequence by PCR. Wild-type minigene DNA was cloned into the pGEM-T Easy vector (Promega). Disrupting and compensatory mutagenesis was performed to restore RNA secondary structure based on the schematic diagrams of minigene constructs. All constructs were confirmed by sequencing. All protocols were as reported previously.Citation17
Additional material
Download Zip (720.7 KB)Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
This work was partly supported by research grants from the National Natural Science Foundation of China (31125011, 31071148, 31270844), the Natural Science Foundation of Zhejiang Province (No. R3090177), the National Science and Technology Project (2012ZX09102301-009), and the Doctoral Foundation of Ministry of Education (20110101130012).
Accession Numbers
The insect Dscam gene sequences were deposited into GenBank with accession numbers KC733353-KC733360.
Supplemental Materials
Supplemental materials may be found here: www.landesbioscience.com/journals/rnabiology/article/27176/
References
- Black DL. Mechanisms of alternative pre-messenger RNA splicing. Annu Rev Biochem 2003; 72:291 - 336; http://dx.doi.org/10.1146/annurev.biochem.72.121801.161720; PMID: 12626338
- Blencowe BJ. Alternative splicing: new insights from global analyses. Cell 2006; 126:37 - 47; http://dx.doi.org/10.1016/j.cell.2006.06.023; PMID: 16839875
- Nilsen TW, Graveley BR. Expansion of the eukaryotic proteome by alternative splicing. Nature 2010; 463:457 - 63; http://dx.doi.org/10.1038/nature08909; PMID: 20110989
- Ozsolak F, Milos PM. RNA sequencing: advances, challenges and opportunities. Nat Rev Genet 2011; 12:87 - 98; http://dx.doi.org/10.1038/nrg2934; PMID: 21191423
- Pan Q, Shai O, Lee LJ, Frey BJ, Blencowe BJ. Deep surveying of alternative splicing complexity in the human transcriptome by high-throughput sequencing. Nat Genet 2008; 40:1413 - 5; http://dx.doi.org/10.1038/ng.259; PMID: 18978789
- Sultan M, Schulz MH, Richard H, Magen A, Klingenhoff A, Scherf M, Seifert M, Borodina T, Soldatov A, Parkhomchuk D, et al. A global view of gene activity and alternative splicing by deep sequencing of the human transcriptome. Science 2008; 321:956 - 60; http://dx.doi.org/10.1126/science.1160342; PMID: 18599741
- Smith CW. Alternative splicing--when two’s a crowd. Cell 2005; 123:1 - 3; http://dx.doi.org/10.1016/j.cell.2005.09.010; PMID: 16213205
- Wojtowicz WM, Flanagan JJ, Millard SS, Zipursky SL, Clemens JC. Alternative splicing of Drosophila Dscam generates axon guidance receptors that exhibit isoform-specific homophilic binding. Cell 2004; 118:619 - 33; http://dx.doi.org/10.1016/j.cell.2004.08.021; PMID: 15339666
- Zhan XL, Clemens JC, Neves G, Hattori D, Flanagan JJ, Hummel T, Vasconcelos ML, Chess A, Zipursky SL. Analysis of Dscam diversity in regulating axon guidance in Drosophila mushroom bodies. Neuron 2004; 43:673 - 86; http://dx.doi.org/10.1016/j.neuron.2004.07.020; PMID: 15339649
- Watson FL, Püttmann-Holgado R, Thomas F, Lamar DL, Hughes M, Kondo M, Rebel VI, Schmucker D. Extensive diversity of Ig-superfamily proteins in the immune system of insects. Science 2005; 309:1874 - 8; http://dx.doi.org/10.1126/science.1116887; PMID: 16109846
- Schmucker D, Clemens JC, Shu H, Worby CA, Xiao J, Muda M, Dixon JE, Zipursky SL. Drosophila Dscam is an axon guidance receptor exhibiting extraordinary molecular diversity. Cell 2000; 101:671 - 84; http://dx.doi.org/10.1016/S0092-8674(00)80878-8; PMID: 10892653
- Chen BE, Kondo M, Garnier A, Watson FL, Püettmann-Holgado R, Lamar DR, Schmucker D. The molecular diversity of Dscam is functionally required for neuronal wiring specificity in Drosophila. Cell 2006; 125:607 - 20; http://dx.doi.org/10.1016/j.cell.2006.03.034; PMID: 16678102
- Hattori D, Chen Y, Matthews BJ, Salwinski L, Sabatti C, Grueber WB, Zipursky SL. Robust discrimination between self and non-self neurites requires thousands of Dscam1 isoforms. Nature 2009; 461:644 - 8; http://dx.doi.org/10.1038/nature08431; PMID: 19794492
- Graveley BR. Mutually exclusive splicing of the insect Dscam pre-mRNA directed by competing intronic RNA secondary structures. Cell 2005; 123:65 - 73; http://dx.doi.org/10.1016/j.cell.2005.07.028; PMID: 16213213
- Anastassiou D, Liu H, Varadan V. Variable window binding for mutually exclusive alternative splicing. Genome Biol 2006; 7:R2; http://dx.doi.org/10.1186/gb-2006-7-1-r2; PMID: 16507134
- May GE, Olson S, McManus CJ, Graveley BR. Competing RNA secondary structures are required for mutually exclusive splicing of the Dscam exon 6 cluster. RNA 2011; 17:222 - 9; http://dx.doi.org/10.1261/rna.2521311; PMID: 21159795
- Yang Y, Zhan L, Zhang W, Sun F, Wang W, Tian N, Bi J, Wang H, Shi D, Jiang Y, et al. RNA secondary structure in mutually exclusive splicing. Nat Struct Mol Biol 2011; 18:159 - 68; http://dx.doi.org/10.1038/nsmb.1959; PMID: 21217700
- Wang X, Li G, Yang Y, Wang W, Zhang W, Pan H, Zhang P, Yue Y, Lin H, Liu B, et al. An RNA architectural locus control region involved in Dscam mutually exclusive splicing. Nat Commun 2012; 3:1255; http://dx.doi.org/10.1038/ncomms2269; PMID: 23212384
- Wang J, Ma X, Yang JS, Zheng X, Zugates CT, Lee CH, Lee T. Transmembrane/juxtamembrane domain-dependent Dscam distribution and function during mushroom body neuronal morphogenesis. Neuron 2004; 43:663 - 72; http://dx.doi.org/10.1016/j.neuron.2004.06.033; PMID: 15339648
- Yang Z, Huh SU, Drennan JM, Kathuria H, Martinez JS, Tsuda H, Hall MC, Clemens JC. Drosophila Vap-33 is required for axonal localization of Dscam isoforms. J Neurosci 2012; 32:17241 - 50; http://dx.doi.org/10.1523/JNEUROSCI.2834-12.2012; PMID: 23197716
- Ruskin B, Greene JM, Green MR. Cryptic branch point activation allows accurate in vitro splicing of human beta-globin intron mutants. Cell 1985; 41:833 - 44; http://dx.doi.org/10.1016/S0092-8674(85)80064-7; PMID: 3879973
- Kennedy CF, Berget SM. Pyrimidine tracts between the 5′ splice site and branch point facilitate splicing and recognition of a small Drosophila intron. Mol Cell Biol 1997; 17:2774 - 80; PMID: 9111348
- Smith CW, Nadal-Ginard B. Mutually exclusive splicing of alpha-tropomyosin exons enforced by an unusual lariat branch point location: implications for constitutive splicing. Cell 1989; 56:749 - 58; http://dx.doi.org/10.1016/0092-8674(89)90678-8; PMID: 2924347
- Gadau J, Helmkampf M, Nygaard S, Roux J, Simola DF, Smith CR, Suen G, Wurm Y, Smith CD. The genomic impact of 100 million years of social evolution in seven ant species. Trends Genet 2012; 28:14 - 21; http://dx.doi.org/10.1016/j.tig.2011.08.005; PMID: 21982512
- Wahl MC, Will CL, Lührmann R. The spliceosome: design principles of a dynamic RNP machine. Cell 2009; 136:701 - 18; http://dx.doi.org/10.1016/j.cell.2009.02.009; PMID: 19239890
- Muh SJ, Hovhannisyan RH, Carstens RP. A Non-sequence-specific double-stranded RNA structural element regulates splicing of two mutually exclusive exons of fibroblast growth factor receptor 2 (FGFR2). J Biol Chem 2002; 277:50143 - 54; http://dx.doi.org/10.1074/jbc.M207409200; PMID: 12393912
- Baraniak AP, Lasda EL, Wagner EJ, Garcia-Blanco MA. A stem structure in fibroblast growth factor receptor 2 transcripts mediates cell-type-specific splicing by approximating intronic control elements. Mol Cell Biol 2003; 23:9327 - 37; http://dx.doi.org/10.1128/MCB.23.24.9327-9337.2003; PMID: 14645542
- Coolidge CJ, Seely RJ, Patton JG. Functional analysis of the polypyrimidine tract in pre-mRNA splicing. Nucleic Acids Res 1997; 25:888 - 96; http://dx.doi.org/10.1093/nar/25.4.888; PMID: 9016643
- Libri D, Piseri A, Fiszman MY. Tissue-specific splicing in vivo of the beta-tropomyosin gene: dependence on an RNA secondary structure. Science 1991; 252:1842 - 5; http://dx.doi.org/10.1126/science.2063196; PMID: 2063196
- Raker VA, Mironov AA, Gelfand MS, Pervouchine DD. Modulation of alternative splicing by long-range RNA structures in Drosophila.. Nucleic Acids Res 2009; 37:4533 - 44; http://dx.doi.org/10.1093/nar/gkp407; PMID: 19465384
- Park JW, Parisky K, Celotto AM, Reenan RA, Graveley BR. Identification of alternative splicing regulators by RNA interference in Drosophila. Proc Natl Acad Sci U S A 2004; 101:15974 - 9; http://dx.doi.org/10.1073/pnas.0407004101; PMID: 15492211
- Olson S, Blanchette M, Park J, Savva Y, Yeo GW, Yeakley JM, Rio DC, Graveley BR. A regulator of Dscam mutually exclusive splicing fidelity. Nat Struct Mol Biol 2007; 14:1134 - 40; http://dx.doi.org/10.1038/nsmb1339; PMID: 21188797
- Eperon LP, Graham IR, Griffiths AD, Eperon IC. Effects of RNA secondary structure on alternative splicing of pre-mRNA: is folding limited to a region behind the transcribing RNA polymerase?. Cell 1988; 54:393 - 401; http://dx.doi.org/10.1016/0092-8674(88)90202-4; PMID: 2840206
- Pervouchine DD, Khrameeva EE, Pichugina MY, Nikolaienko OV, Gelfand MS, Rubtsov PM, Mironov AA. Evidence for widespread association of mammalian splicing and conserved long-range RNA structures. RNA 2012; 18:1 - 15; http://dx.doi.org/10.1261/rna.029249.111; PMID: 22128342
- Yang Y, Lv J, Gui B, Yin H, Wu X, Zhang Y, Jin Y. A-to-I RNA editing alters less-conserved residues of highly conserved coding regions: implications for dual functions in evolution. RNA 2008; 14:1516 - 25; http://dx.doi.org/10.1261/rna.1063708; PMID: 18567816
- Zuker M. Mfold web server for nucleic acid folding and hybridization prediction. Nucleic Acids Res 2003; 31:3406 - 15; http://dx.doi.org/10.1093/nar/gkg595; PMID: 12824337
- Crooks GE, Hon G, Chandonia JM, Brenner SE. WebLogo: a sequence logo generator. Genome Res 2004; 14:1188 - 90; http://dx.doi.org/10.1101/gr.849004; PMID: 15173120
- Regier JC, Shultz JW, Zwick A, Hussey A, Ball B, Wetzer R, Martin JW, Cunningham CW. Arthropod relationships revealed by phylogenomic analysis of nuclear protein-coding sequences. Nature 2010; 463:1079 - 83; http://dx.doi.org/10.1038/nature08742; PMID: 20147900