Abstract
mRNA localization and localized translation is a common mechanism that contributes to cell polarity and cellular asymmetry. In metazoan, mRNA transport participates in embryonic axis determination and neuronal plasticity. Since the mRNA localization process and its molecular machinery are rather complex in higher eukaryotes, the unicellular yeast Saccharomyces cerevisiae has become an attractive model to study mRNA localization. Although the focus has so far been on the mechanism of ASH1 mRNA transport, it has become evident that mRNA localization also assists in protein sorting to organelles, as well as in polarity establishment and maintenance. A diversity of different pathways has been identified that targets mRNA to their destination site, ranging from motor protein-dependent trafficking of translationally silenced mRNAs to co-translational targeting, in which mRNAs hitch-hike to organelles on ribosomes during nascent polypeptide chain elongation. The presence of these diverse pathways in yeast allows a systemic analysis of the contribution of mRNA localization to the physiology of a cell.
Introduction
Localized mRNAs as source for targeted protein synthesis have been known for more than 25 y and were first described in oocytes of fruit flies, claw frogs, and marine invertebrates where their localization is key to developmental processes such as tissue differentiation (for review see refs. Citation1 and Citation2). However, mRNA localization also occurs in various somatic cell types, including fibroblasts and neurons.Citation3,4 Localized mRNAs are characterized by specific signals, so called localization elements (LEs) or zipcodes. These are usually located in the 3′ untranslated region (3′-UTR) of the mRNA but can also be found in the coding region.Citation5 They can dramatically vary in sequence, size (from around ten to several hundred nucleotides) and structure. This diversity in cis-acting elements is mirrored in the variety of different protein binding partners that usually contain well-known RNA-binding domains. As suggested by the complexity of most LEs, they are likely bound by several protein partners, generating large ribonucleoprotein (RNP) complexes.Citation1 The prevailing mechanism to localize these mRNPs is active transport by motor proteins. Whereas mRNA transport in oocytes and neurons involves microtubule-dependent motor proteins, myosin-driven transport along actin filaments has so far been reported for few cases only, including the transport of β-actin mRNA in fibroblasts and of ASH1 mRNA in budding yeast.Citation6
The budding yeast Saccharomyces cerevisiae has provided a pivotal model system to study mRNA localization and many mechanistic insights into the molecular transport machinery of a single mRNA, ASH1, have been gained using this organism.Citation7-11 However, budding yeast has also proven a valid system to address mRNAs localization to specific organelles, where localized transcripts appear to improve protein translocation by providing a local source of the protein.Citation12 Despite being a unicellular organism, budding yeast expresses a large number of localized mRNAs. More than 30 mRNAs are currently known to localize to specific subcellular regions and several hundred are associated with the cytoplasmic face of endoplasmic reticulum (ER) or mitochondrial membranes. Since budding yeast grows and divides in a polarized manner, polarized distribution of mRNAs is frequently observed. Recent observations indicate that mRNA localization can even support the establishment of polarity in budding yeast as it has been described for Drosophila oocytes.Citation37 As polarized growth requires the directional segregation of organelles into the growing bud and several localized mRNAs require translation at these membrane compartments, organelle distribution and mRNA localization must be coordinated. Budding yeast with its impressive number of localization processes despite the limited number of expressed genes might give us a chance to understand the overall impact of mRNA localization on cell physiology. This review is thus targeted to provide an overview on the different mRNA localization processes and their influence on biological activities. We will also discuss the evidence for co-translational and translation-independent mRNA localization and dwell on the question if mRNA localization drives protein distribution or if it merely occurs as consequence of hitch-hiking due to signal peptide-mediated targeting of proteins during their translation.
One to Find Them All: ASH1, the SHE Genes, and Their Roles in Mating Type Switching
In haploid yeast cells, the mating type is determined by the MAT locus. The ability to switch between MATa and MATα mating types in homothallic strains is confined to the mother cell, where the process is initiated by a double-stranded DNA break at the MAT locus, catalyzed by the HO endonuclease. Restriction of this enzyme to mother cells is due to repression of the gene in daughter cells, which is performed by the transcription repressor Ash1p (Asymmetric HO expression; see ref. 13). The question how asymmetric distribution of Ash1p is accomplished was finally resolved by a genetic approach and revealed the genes termed SHE1 – SHE5 (for SWI5-dependent HO expression), which constitute the major mRNA localization machinery in yeast.Citation14 Consistent with the observed loss of Ash1p asymmetry in she mutants, the SHE genes were shown to be required for the localization of ASH1 mRNA to the incipient daughter cell.Citation7,14,15 The detailed analysis of ASH1 trafficking revealed the key elements that are critical for polarized mRNA localization. These can be grouped as follows: the actin cytoskeleton, including the actin polymerizing factor She5p/Bni1p, the actin-based myosin V motor She1/Myo4p, and the myosin-specific chaperone She4p; an RNA-binding complex, composed of the proteins She2p and She3p, that links ASH1 mRNA to Myo4p; cis-acting recognition elements within the ASH1 transcript that mediate recognition by She2p and She3p; the proteins Khd1p and Puf6p that mediate translational repression of ASH1 during transport; additional factors like the protein Loc1p that recognize and support packaging of ASH1 mRNA in the nucleus; and finally, factors that are responsible for anchoring the ASH1 transcript at the bud tip and promote subsequent translation.Citation16-18 We will briefly delineate the key aspects of the molecular events as they occur from transcription of the ASH1 gene within the nucleus to the translation of Ash1p at the bud tip. A more detailed discussion can be found in the accompanying review by Niedner and co-authors (this issue).
ASH1 mRNP Assembly, Transport, and Anchoring
ASH1 mRNA localization depends on cis-acting sequences (localization elements or zipcodes) in the coding and 3′ untranslated region (3′-UTR) of the transcript. Each of four elements within ASH1 is able to target the reporter mRNA properly in a SHE-dependent manner.Citation19 However, in vivo and in vitro the elements cooperate, as demonstrated in recent reconstitution studies, where multiple elements were shown to be advantageous for the efficiency of transcript movement along actin cables.Citation11,20 The zipcodes generate RNA-specific secondary structures with a loop-stem-loop and bulges that are specifically recognized by the RNA-binding protein She2p.Citation21-23 Similar zipcode elements can also be found in other bud-localized mRNAs, like IST2 and EAR1/YMR171c, confirming the importance of this recognition element for the proper sorting of mRNAs.Citation21
Recognition of these elements and assembly of the ASH1 localization complex already starts in the nucleus. She2p is recruited co-transcriptionally to the nascent RNA and this binding seems to be important for the cytoplasmic localization since exclusion of She2p from the nucleus results in defective localization of the ASH1 transcript and protein product.Citation24,25 In the nucleus, She2p may also recruit Loc1p and Puf6p to the ASH1 transcript, which is important for assembling a translationally silenced and stable mRNP (for details see accompanying review by Niedner et al.).Citation24,26
During or shortly after export from the nucleus, Loc1p is replaced by She3p.Citation26 Since She3p tightly interacts with the myosin Myo4p, its integration into the mRNP allows the mRNP's interaction with the actin cytoskeleton.Citation9,27,28 Early work has already demonstrated the critical role of actin filaments in localizing and moving ASH1-particles to the distal bud tip during late anaphase. Impairment of actin dynamics by the drug latrunculin A or by mutating the genes encoding Myo4/She1p, tropomyosin, actin, or profilin abolishes ASH1 mRNA localization to the bud.Citation7,29 Their direct impact on mRNP movement was finally proven by elegant imaging experiments. In order to directly observe ASH1-mRNP dynamics by live imaging, Bertrand et al. employed a chimeric mRNA composed of a reporter, the 3′-UTR of ASH1 and several copies of the RNA stem loops that can bind to the RNA-phage capsid protein MS2-CP with nanomolar affinity.Citation8 When MS2-CP was fused to GFP and expressed in cells harboring the chimeric ASH1 transcript, ASH1 mRNPs were observed to move in a Myo4p and actin dependent manner from the mother to the daughter cell.
The two remaining She proteins, She4p and She5p play roles in actin-myosin dynamics as well: She4/Dim1p is a member of the UNC-45/CRO1/She4 (UCS) domain containing family that binds to myosin motor domains, thus explaining its implication in myosin-requiring events such as mRNA localization and endocytosis.Citation17,30 She5/Bni1p belongs to the formin family.Citation16 Members of this class of proteins act as nucleators of unbranched actin filaments that serve as running tracks for the myosin and therefore indirectly facilitate actin dependent motile processes.
Several observations suggest that ASH1 mRNPs are eventually anchored once they have reached their final destination. Localized ASH1 transcripts form a well-defined cap-like structure at the bud tip in vegetative cells and at the shmoo tip in mating cells.Citation31 In mutant cells lacking She5/Bni1 or its interacting partner Bud6/Aip3, asymmetric transport to the incipient bud can be observed. However, the distinct accumulation of the ASH1 transcript within the cap structure is not apparent.Citation31 Consistent with a role of these two proteins in actin organization during budding and mating, actin structures formed as a result of polarization may function as cortical docking sites in regions of polarized cell growth.Citation32 Anchoring of ASH1 mRNPs also requires a remodeling of the She2p-She3p-Myo4p-ASH1 complex and potentially its dissociation since artificial tethering of ASH1 mRNA to a She2p-MS2-CP fusion protein allows its transport but not anchoring at the bud tip.Citation33 Finally, translation of ASH1 mRNA must occur for proper attachment to the bud tip, which is consistent with its translational activation close to the bud cell cortex by phosphorylating and inactivating the translational repressor proteins Puf6p and Khd1p.Citation18,34
Links Between Polarity Establishment and mRNA Localization
The initiation of the budding process in S. cerevisiae requires specialization of a small patch (of about 0.5 μm) of the mother cell cortex and involves a virtually parallel polarization of many cellular components toward that site, thereby promoting the emergence of the future daughter cell. Cell polarization is the result of a hierarchical program connecting e.g., cell division axis selection, assembly of a polarized actin cytoskeleton, and recruitment of cytoskeletal motor proteins for the delivery of secretory vesicles, organelles, and mRNPs. Docking and fusion of secretory vesicles with the plasma membrane as well as local mRNA translation provide the differences in protein composition ultimately determining distinct fates of mother and daughter cell.
The key player in polarity establishment is the highly conserved small GTPase Cdc42p, a member of the Rho family of small GTPases.Citation35 It clusters at sites of polarized growth, the incipient bud site, the tip of small buds, and the mother-bud neck in large-budded cells. Importantly, Cdc42p is likely to arrive at the future bud site independently of the integrity of the actin cytoskeleton, as its localization is not affected upon de-polymerization of actin filaments and its asymmetric clustering is sufficient to induce actin polymerization and secondary polarization events.Citation35 Cdc42p is also critical for membrane trafficking within the late secretory pathway, which also involves a multitude of evolutionarily conserved components, including small GTPases of the Rho (e.g., Rho1p) and Rab (e.g., Sec4p) families, Rab effectors like Sro7p (see below), a multi-subunit complex known as the exocyst, exocytic SNARE proteins, and the type V myosin Myo2p. Like Cdc42p, these proteins accumulate at the presumptive bud site and incipient bud, and collectively they have been termed polarity and secretion factors (POLs).Citation36 Several mechanisms have been suggested to explain Cdc42/POL cap formation.Citation35 Local translation of POL mRNAs might contribute to polarity establishment as well.
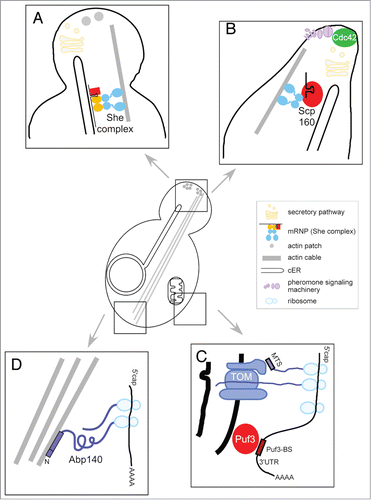
Using fluorescent in situ hybridization (FISH) and live cell imaging, Gerst and colleagues found that endogenous CDC42 and SEC4 mRNPs localize to a spot near the cell surface in unbudded G1-phase cells.Citation37 Early in G2/M and prior to nuclear division, nearly all of them are found in the bud and after mitosis they are symmetrically distributed in mother and daughter cell.Citation37 A similar bud tip-enrichment was observed for eight other POL mRNAs, including transcripts encoding the exocyst components Sec3p and Exo84p, and the Sec4p effector and SNARE regulator Sro7p.Citation37 By simultaneously following GFP-labeled mRNA and RFP-marked protein localization in vivo, SRO7 and CDC42 mRNA granules were found to appear at the presumptive bud site before their RFP-labeled protein product, which suggests that mRNA targeting and possibly local translation contributes to POL protein deposition at presumptive polarization sites. Similar to ASH1 targeting, CDC42, SEC4, and SRO7 mRNAs localization depends on SHE1–5, an intact late secretory pathway, and a functional actin cytoskeleton ().Citation37 Moreover, it relies on sequences in the 3′UTR and coding region of the corresponding mRNAs. This is consistent with the existence of a known consensus motif for She2p binding in the coding sequence of SRO7, similar to that found in ASH1 and IST2 transcripts.Citation21,37 Notably, immuno-precipitation of She2p leads to co-enrichment of asymmetrically localized POL transcripts but not of non-localized mRNAs encoding other polarization factors. However, mRNA localization is not necessarily a prerequisite for polarized deposition of proteins: in myo4Δ cells, Cdc42p and Sec4p can become polarized even when mRNA localization is lost. Since both small GTPases bear C-terminal prenylation that permits membrane anchoring, these POL proteins can reach their destination via an alternate route, the secretory pathway. In the absence of Myo4p, this is not possible for Sro7p, as this protein lacks a membrane-anchor.
Figure 1. (A) Bud-directed mRNA transport occurs simultaneously with ER inheritance. During polarized growth, POL mRNAs targeting and local translation contribute to POL protein deposition within the emerging bud. This mRNP targeting correlates with cER inheritance and depends on SHE1–5, the secretory pathway and a polarized actin cytoskeleton. (B) Shmoo-directed mRNPs use Scp160p and Myo4p for asymmetric targeting. During mating, targeted mRNA transport is mediated by Scp160p, a 14 KH-domain protein that associates with ER and Myo4p. The pheromone-induced RNA-binding of Scp160p is relevant for cell polarization, chemotropism and mating efficiency. Polarized localization of some POL factors, like Cdc42p, likely occurs via secretion. (C) Pre- and co-translational targeting of mRNAs encoding mitochondrial proteins (mMPs). Targeting of mMPs to the outer mitochondrial membrane and the TOM complex likely combines at least two mechanisms: recognition of a signal within the proximal region of the 3′-UTR recognized by the RNA-binding protein Puf3p and active translation of the peptide sequence including the mitochondrial targeting sequence (MTS). (D) Co-translational targeting of ABP140 RNA to the distal pole of the mother cell via actin retrograde flow. Asymmetric ABP140 mRNA localization requires active translation of Abp140p and tethering the nascent chain of Abp140p to actin cables via its N-terminal actin-binding domain (ABD) and a subsequent amino acid stretch.
Polarized mRNA Transport During Mating
Polarized growth is also observed during the mating process when haploid a and α cells respond to pheromones secreted by cells of the opposite mating type. The pheromones are recognized by specific G-protein coupled receptors. Upon receptor stimulation, GDP to GTP exchange occurs on the α subunit of a trimeric G proteins, Gα (Gpa1), which allows the released Gβγ dimer (Ste4p/Ste18p) to activate a downstream signaling cascade, ultimately inducing cell cycle arrest and polarized growth in the direction of the partner cell. Activated Gα also fulfills a signaling function in the mating response pathway meditated e.g., via effectors like mitogen-activated kinases or phosphatidylinositol 3-kinase or the RNA binding protein Scp160p.Citation38-40 The generation of mating projections, pear-shaped “shmoos,” is important for cell-cell recognition. In mating mixtures, cells find and contact the closest potential mating partner by determining the direction of the most potent pheromone source and growing toward it. This ability to detect and respond to a chemical gradient is called chemotropism. The mechanism is extremely sensitive and requires the continual regulation and deposition of mating components and POL factors, in particular the small Rho GTPase Cdc42p, at the tip of the mating projection.Citation39
During shmooing, several mRNAs are placed in a polarized fashion within the mating projection (), a mechanism partially similar to that of POL mRNA deposition during budding. Among the described POL factors, SRO7 and SEC3 transcripts reside in close proximity to tubular ER and accumulate within the shmoo tip concomitantly with local protein synthesis.Citation41 In contrast, polarized localization of Cdc42p and Sec4p to the mating projection takes place via another mechanism (most likely via the secretory pathway), as the corresponding mRNAs reside in the cell body. Thus, during budding and mating both common and distinct modes of mRNA localization are employed. This concerns in particular the RNA-binding protein (RBP) that mediates mRNA targeting. Instead of She2p and She3p, Scp160p is necessary for localization of various transcripts. Scp160p contains 14 hnRNP K homology (KH) domains, associates with ER-bound polysomes, and interacts directly or indirectly with Myo4p ().Citation41,42 Consistent with its function as a Gpa1 effector during pheromone signaling, Scp160p can interact with numerous pheromone-induced transcripts (such as FUS3, STE7, and KAR3 mRNAs), whose encoded proteins have roles in signaling downstream of receptor stimulation.Citation41 The pheromone-induced RNA-binding function of Scp160p is relevant for cell polarization, chemotropism, and mating efficiency, as scp160Δ mutants and cells expressing a truncated protein (Scp160p-ΔKH14) display aberrant mating projections and a reduced efficiency in the response to pheromone gradients. Thus, targeted mRNA transport via Scp160p appears to be critical for the local assembly of a functional cellular domain, the shmoo, as a result of pheromone signaling (). It will be interesting to discover further details concerning the mechanism Scp160p employs within this Myo4- and cER-dependent mRNA localization pathway.
Yeast mRNP Localization and ER Inheritance are Linked
Cortical endoplasmic reticulum (cER), also called plasma membrane-attached ER, is a specialized form of the ER, which consists of tubular ER structures present in close contact to the plasma membrane. The contact sites appear to play an important role in lipid biogenesis.Citation43,44 During the G1/S phase of the cell cycle, the segregation of cER from the mother to the daughter cell occurs via multiple steps: after orienting and extending along the mother-bud axis, the cytoplasmic tubules migrate into the emerging bud by an actin-dependent mechanism. This phase is followed by anchoring of the tubules at the bud tip and spreading along the bud plasma membrane.Citation43,45 Deletion of two genes essential for yeast mRNA localization, MYO4 and SHE3, also impairs delivery of cER to the daughter cell.Citation46 In both mutants a strong delay in the transport of tubular ER structures from the mother cell was observed, consistent with the idea of a functional Myo4-She3 motor complex that actively drives the ER tubules along actin cables. Although initial experiments suggested that cER delivery and mRNP localization are uncoupled, later studies showed that localized mRNAs can co-fractionate with ER and co-migrate with tubular ER structures toward the bud.Citation37,46,47 This was originally demonstrated for ASH1, SRO7, and CDC42 mRNAs and later for six localized mRNAs encoding secreted or membrane proteins.Citation48,49 However, later it became clear that ASH1 mRNA localization occurs independently of ER segregation.Citation48 This is consistent with its expression and localization occurring exclusively during anaphase, whereas cER inheritance happens during G1/S-phase. Only when ASH1 is artificially expressed simultaneously with cER inheritance, the transcript indeed co-migrates and co-fractionates with ER tubules into the emerging daughter cell.Citation37,47
Several studies support the view of a concerted mechanism of cER inheritance and mRNA localization. Genome-wide screens to discover novel She-associated transcripts revealed a large fraction of bud-localized transcripts that code for membrane and secreted proteins destined e.g., to the plasma membrane, the ER, vacuoles, or the cell wall.Citation50,51 Indeed, co-trafficking of such transcripts with ER tubules could spatially coordinate subcellular targeting and subsequent translation and minimize diffusion away from the site of exocytosis. Consistent with that, a correlation between ER inheritance and asymmetric localization of mRNAs encoding POL factors, ER and plasma membrane proteins was observed. In a sec3Δ mutant, in which docking of ER tubules at the bud cortex is severely impaired, the bud-enrichment of numerous transcripts (e.g., POL mRNAs, WSC2, EAR1, SRL1) is strongly diminished.Citation37,48 Similar observations were made in other mutants with impaired cER inheritance (myo4Δ, she3Δ, sec5Δ, scs2Δ, srp101–47, aux1Δ), in particular in strains that are defective in cER movement, tubule formation, and anchoring.Citation47,48
Evidence for physical association of localized mRNPs with ER membranes stems from complementary approaches, including microscopic and cell fractionation studies. ASH1 and POL transcripts co-fractionate with ER marker proteins upon subcellular fractionation.Citation37,47 This co-isolation depends on She2p, which also co-fractionates with ER membranes, even in the absence of Myo4p and She3p or ongoing translation. Recent in vitro reconstitution studies provide further evidence in support of the ability of She2p to target selected mRNPs to ER membranes.Citation52 In floatation gradients a significant fraction of recombinant She2p is able to co-migrate with membranes derived from the ER but not mitochondria. This binding is saturable and affected by prior treatment of ER microsomes with protease. However, binding is not abolished and recombinant She2p can also interact with protein-free liposomes. Intriguingly, while a selectivity of She2p toward individual phospholipids has not yet been uncovered, a preferential association with liposomes of small diameter (30–40 nm) that resemble in diameter yeast ER tubules was observed.Citation43,52 The underlying mechanism for of this possible curvature recognition remains to be revealed.
SRP- and Translation-Independent Targeting of mRNPs
According to the classical and widely-accepted concept, mRNAs encoding secreted and membrane proteins (designated mSMPs) are directed to the ER via a co-translational mechanism: following ribosome-mediated synthesis of the signal sequence, the emerging peptide is recognized by the signal recognition particle (SRP), which targets the nascent polypeptide chain to the SRP-receptor and a protein-conducting channel, the translocon, where protein elongation and translocation into the ER lumen take place.Citation53 This model implicates at least partial translation of the mRNA prior to anchoring, because the ER-targeting device is the signal sequence. However, there is growing support for additional mRNA targeting mechanisms.Citation54-56 For instance, sequence analysis of mSMPs from various species revealed a unique nucleotide composition that distinguishes them from the typical pool of mRNAs encoding cytoplasmic proteins: nucleotide stretches encoding signal sequences exhibit decreased usage of adenines (“no-A stretches”) and transcripts of membrane proteins are highly biased for uracil.Citation57,58 Furthermore, several yeast RNA-binding proteins like She2p, Whi3p and Scp160p have been shown to associate with ER membranes.Citation54 Last but not least, 11 endogenous mSMPs and two mRNAs encoding soluble proteins reveal a large extent of co-localization with ER, either the nuclear peripheral or the cER.Citation49 The same was observed for non-translatable, artificial mRNAs with high uracil content. Similar to POL transcripts, mSMP targeting to ER can occur translation-independently and is not affected by mutation in genes encoding the SRP or the translational machinery.Citation49 However, none of these mSMPs show a polarized mRNA or protein localization, indicating that factors other than She2p mediate ER association.
mRNA Localization to Mitochondria
Most mitochondrial proteins are encoded by the nuclear genome and therefore need to be imported into mitochondria. The majority of those proteins requires an N-terminal signal peptide (MTS), which is recognized by the translocase of the outer membrane (TOM) complex in the outer mitochondrial membrane, which then mediates the translocation of the polypeptide into the intermembrane space.Citation59 The common view that mitochondrial import occurs post-translationally mainly stems from observations that in vitro synthesized precursor proteins are able to function as substrates for import into purified mitochondria (for review see ref. 59). However, more than 40 y ago Butow and coworkers detected polysomes bound to the cytoplasmic face of the outer mitochondrial membrane and hypothesized the existence of a co-translational import pathway involving simultaneous translation of proteins.Citation60,61 Obviously, translation of mRNAs in proximity of the target organelle would enrich the encoded proteins at this site and facilitate subsequent import. Such a mechanism might be of particular relevance for proteins that, upon full translation in the cytoplasm, are prone to aggregate.Citation62 Evidence in favor of these early findings was obtained by high throughput analyses, in which the enrichment of mRNAs was followed upon isolation of mitochondrium-associated and cytosolic polysomes. Since mitochondrium-associated polysomes contain preferentially mRNAs encoding mitochondrial proteins (mMPs), this supports the idea that they are translated in proximity of mitochondria.Citation63 Intriguingly, mitochondrium-bound mMPs share the feature of being derived from genes of bacterial origin, whereas most of the mRNAs bound to free polysomes are of eukaryotic origin.Citation63 This might indicate the need for a tight coupling of translation and translocation for mRNAs of prokaryotic origin, as it has been shown for fumarase that is encoded by a former prokaryotic gene.Citation12 However, the basis for this possible link is still a mystery.
The enrichment of a large fraction of mRNAs at the mitochondrial surface raises questions like, what is the biological function and mechanism of such mRNA targeting. Studies of three mMPs, ATM1 (encoding an ABC transporter), ATP2 (coding for the β subunit of the F1-ATPase), and OXA1 (encoding a membrane insertase) originally suggested that the 3′-UTR is required, either for association of the respective mRNAs with mitochondria-bound polysomes or for their association with mitochondria.Citation64-66 The 3′-UTRs of ATM1 and ATP2 were found to be essential for mitochondrial co-localization and sufficient to target a reporter mRNA to the vicinity of this organelle.Citation65 Replacement of the 3′-UTR of ATP2 by that of the cytoplasmic ADH1 not only abolished mitochondrial association, but also resulted in a respiratory defect and reduced Atp2p import.Citation65 Consistent with the idea of mitochondrial targeting via cis-acting sequences in the 3′-UTR, a 100 nucleotide large cis element was identified in the proximal region of the 3′-UTR of ATM1 and ATP2.Citation64,65 However, subsequent studies using the MS2 imaging system to follow genomically tagged mRNAs or an improved FISH protocol combined with an automated analysis attenuated the importance of the 3′-UTR in ATP2 targeting, as in the absence of this region only moderate defects in mRNA targeting were apparent.Citation67,68
Localization via signals within the 3′-UTR generally requires recognition by RNA-binding proteins (RBPs). In case of mMP localization, the best candidate is Puf3p, one of the six yeast members of the Pumilio protein family.Citation69 Puf3p binds to the cytoplasmic face of the mitochondrial outer membrane.Citation70 In transcriptome binding studies, Gerber and colleagues identified more than 200 target mRNAs of Puf3p, with a large fraction representing mMPs.Citation71 An independent survey of 480 nuclear-encoded mRNAs for mitochondrial proteins allowed their grouping into two classes: class I mRNAs were either shown to bind to Puf3p or contain a Puf3p binding site (UGUA[U/A]AUA), whereas class II mRNAs lack these features, even though they are enriched in mitochondria-associated polysomes.Citation72 class II mRNAs code for subunits of the respiratory complexes or mitochondrial metabolic enzymes, while class I mRNAs encode assembly factors or components of the mitochondrial translation machinery. Deletion of PUF3 reduces transcript binding to mitochondria-bound polysomes and for one example, BCS1 mRNA, deletion of the Puf3p binding site (Puf3p-BS) resulted in loss of BCS1 mitochondrial localization.Citation72 Consistent with the model of Puf3p-dependent mRNA targeting to mitochondria is also the observation that deletion of PUF3 affects localization of Puf3-BS containing mMPs in 75% of the examined mRNAs.Citation68 However, another study could not entirely second these findings, but rather suggested an assisting role for Puf3p in mitochondrial targeting.Citation73 Here, addition of a Puf3p-BS to a GFP reporter only led to partial mitochondrial localization. These observations are consistent with the idea that individual mMPs vary in their dependence on Puf3p and imply additional targeting mechanism(s) co-operating with Puf3p. Several studies indicate that one of such mechanisms involves the translation of the MTS.Citation67,68,73 Upon emergence from the ribosome, binding of the MTS by the TOM complex would subsequently target the complex of nascent chain, ribosome and mRNA to the outer mitochondrial membrane (). This model is in agreement with other findings that both the MTS coding region and its translation participate in mRNA targeting to mitochondria.Citation64,67 Impairing translation with puromycin decreases the amount of mitochondria-associated mMPs.Citation72,73 Furthermore, deletion of the TOM components Tom70p, Tom7p, or Tom20p reduce mitochondrial association, although at varying levels in different studies.Citation68,73 Finally, an additional peptide sequence distal from the MTS needs to be translated to maintain a stable association between the mMP and the outer membrane.Citation67
In summary, it appears that mMP localization relies on various mechanisms and likely combines co-translational as well as RBP-dependent targeting (). Especially the function of Puf3p needs to be clarified, as this RBP seems to fulfill multiple roles regarding mitochondria: in addition to its role in localizing mMPs, it regulates target stability and functions in mitochondrial segregation.Citation70,74
mRNA Localization to Peroxisomes
Peroxisomes are organelles surrounded by a single lipid bilayer that are found in all eukaryotic cells, where they facilitate functions including β-oxidation of fatty acids or synthesis of cholesterol and bile acids.Citation75 Their importance is exemplified by the existence of multiple genetic disorders related to peroxisomal dysfunction. Like with mitochondria, the classical concept of protein import into peroxisomes is that of a post-translational system. Proteins destined for the peroxisomal lumen contain peroxisomal targeting signals that are recognized by a specific import machinery.Citation75 Targeting of mRNAs encoding peroxisomal proteins to the vicinity where translocation takes place might facilitate this step, probably by increasing the local protein concentration and by preventing aggregation upon translation. Evidence for peroxisomal mRNA targeting stems from one study, in which the subcellular distribution of each of the 50 mRNAs encoding yeast peroxisomal proteins was investigated by imaging and cell fractionation.Citation76 In contrast to mitochondrial mRNA colocalization, a large variation in colocalization of transcripts encoding peroxisomal proteins was observed. Basically, three patterns were revealed: a non-peroxisomal pattern (seen for the majority of transcripts), an ER or mitochondrial localization (five mRNAs), and a peroxisomal pattern (with a subgroup of 12 mRNAs showing a high degree of colocalization). Even in the latter case, only a subset of peroxisomes in a cell colocalized with mRNPs, suggesting the existence of distinct peroxisomal “states” (e.g., in regard to maturation), of which some may not associate with mRNPs. More detailed studies on one mRNA, the PEX14 transcript, revealed that its 3′-UTR as well as translation are important for peroxisomal association. Although the mechanism for targeting of PEX14 and other peroxisomal transcripts still awaits uncovering, the pumilio-type RBP Puf5p might be, at least partly, involved. Puf5p can bind to PEX14 mRNA, and its absence reduces, however does not block, PEX14 mRNA association with peroxisomes.Citation71
Association of mRNAs with Cytoskeletal Elements
In higher eukaryotic cells, one of the best-studied localized mRNAs is β-actin.Citation3,77 Its localization enhances steering of movement and directional growth of fibroblasts and neurons. Other transcripts encoding actin-related factors like the highly conserved Arp2/3 complex are also specifically associated with migrating cell edges.Citation78 Local enrichment of such transcripts in regions with enhanced actin polymerization can drive cell extension by rapid increases in actin concentration. Thus, cells might reinforce their polarization by transporting localized mRNAs encoding the cytoskeletal elements or modifying factors on the cytoskeleton itself.
Since most mRNA localization processes require motor proteins, Brown and colleagues employed a systematic approach in S. cerevisiae to identify mRNAs specifically associated with such motors.Citation79 Using DNA microarrays after affinity-purification of yeast motor proteins (five myosins, five kinesin-like proteins, and the dynein Dyn1p), specific sets of functionally distinct mRNAs were found to associate with mainly myosin motors, with a total of around 1000 identified transcripts. Although the relevance of this widespread association of mRNAs with actin motors remains to be demonstrated, a functionally related subset of mRNAs coding for cytoskeletal regulators was identified that specifically binds to Myo3p. This set includes mRNAs encoding Las17p (an actin nucleation promoting factor [NPF]), Vpr1p (can stimulate the NPF activity of Myo5p), and Bbc1p (an interactor of Las17p and Myo3/5p), but also ASH1 mRNA. Myo3p, together with Myo5p, belongs to the type I myosin motors. They localize to cortical actin patches at the plasma membrane, where they play a direct role in clathrin-mediated endocytosis.Citation80 Due to the strong bias for actin patch nucleator mRNAs to associate with Myo3p, Casolari et al. investigated the dynamics of ASH1, VPR1, LAS17 and BBC1 mRNAs in further detail using live cell imaging and 3D-superlocalizationCitation79 and they provided evidence for Myo3p-dependent localization of these mRNAs at cortical actin patches. It will be interesting to see in the future how Myo3p can facilitate this process, which partner proteins are involved, and to what extent mRNA localization contributes to endocytosis and actin dynamics.
A unique pattern of mRNA localization was found for ABP140 mRNA (). Rather than being bud-localized, this transcript accumulates at the distal pole of the mother cell. ABP140 encodes an actin- and tRNA binding protein whose function remains largely unclear.Citation81,82 It has been used to decorate actin cables, which are bundles of actin filaments that cross the bud neck (between mother cell and bud). While Abp140p is associated with actin cables throughout the cell via its N-terminal actin-binding domain (ABD), ABP140 mRNA mainly accumulates close to the distal pole. Its trafficking is dependent on actin retrograde flow but independent of the SHE machinery.Citation83 Proper localization of ABP140 mRNA requires its ABD (present within the first 17 amino acids) and the immediately following amino acid stretch (in total the first 67 amino acids). Kilchert and Spang demonstrated that asymmetric ABP140 mRNA localization requires the association of the transcript with ribosomes and active translation of the N-terminus containing the ABD (). The model derived from these data suggests a co-translational transport consisting of a ternary complex of ABP140 transcript, translating ribosome, and the emerging nascent polypeptide chain that can tether to actin cables and thereby follow actin movement to the distal pole by retrograde flow. This co-translational mRNA targeting mechanism is not exceptional but adds to other previously reported examples including mammalian Dia1 mRNA, signal recognition particle-dependent nascent chain targeting to the ER, and co-translational targeting to mitochondria (see above).Citation84
Outlook
During the past few years hundreds of localized mRNAs were discovered in various subcellular regions of budding yeast. Ranging from the incipient bud site, the bud tip, the shmoo tip, the distal pole of the mother cell, cortical actin patches below the plasma membrane, as well as the surface of various organelles like the ER, mitochondria, and peroxisomes, localized mRNAs have been discovered in every “corner” of the cell. Thus, even in a unicellular organism the concept of mRNA localization appears to be widespread. This is, at least to some extent, in agreement with the presence of a large repertoire of RNA binding proteins, of which only few have been assigned a function. Extensive studies on localized ASH1 mRNAs have taught us that its fate is already determined co-transcriptionally by binding of a specific RBP (She2p), which serves also to recruit other RBPs into the complex. Given the wealth of known She2p targets that are localized e.g., at different stages of the cell cycle or via ER-linked or -independent ways, one of the outstanding questions is to what extent does the protein composition differ for those mRNPs and how will it change during the lifetime of an individual mRNA; which accessory/specific factors determine its subsequent association with unique motor complexes and target destinations like the ER membrane? As RBPs can bind RNA with low affinity, it is conceivable that posttranslational modification of RBPs or the interaction with other proteins can modify target affinity and specificity and allow for mRNP remodeling. Thus, one of the urgent tasks in the field is to determine the exact composition of mRNPs as well as the potential posttranslational modifications of the proteins within. It has also become obvious that different modes of mRNA targeting like RBP-mediated and co-translational localization, cooperate on certain mRNAs to achieve their targeting. However, it is still open to what extent these pathways participate in the localization of individual mRNAs and what their contribution to processes like polarization, secretion, and organelle function really is. Addressing these questions needs identification and dissection of the molecular interactions of the numerous mRNAs and RBPs by multidisciplinary or even systemic approaches. If we follow this road, budding yeast might pave the way to a first global understanding of the contribution of mRNA localization in the overall physiology of a cell.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
We would like to thank D. Niessing and A. Niedner for comments on the manuscript.
References
- Martin KC, Ephrussi A. mRNA localization: gene expression in the spatial dimension. Cell 2009; 136:719-30; PMID:19239891; http://dx.doi.org/10.1016/j.cell.2009.01.044
- St Johnston D, Ahringer J. Cell polarity in eggs and epithelia: parallels and diversity. Cell 2010; 141:757-74; PMID:20510924; http://dx.doi.org/10.1016/j.cell.2010.05.011
- Condeelis J, Singer RH. How and why does beta-actin mRNA target? Biol Cell 2005; 97:97-110; PMID:15601261; http://dx.doi.org/10.1042/BC20040063
- Doyle M, Kiebler MA. Mechanisms of dendritic mRNA transport and its role in synaptic tagging. EMBO J 2011; 30:3540-52; PMID:21878995; http://dx.doi.org/10.1038/emboj.2011.278
- Jambhekar A, Derisi JL. Cis-acting determinants of asymmetric, cytoplasmic RNA transport. RNA 2007; 13:625-42; PMID:17449729; http://dx.doi.org/10.1261/rna.262607
- Gagnon JA, Mowry KL. Molecular motors: directing traffic during RNA localization. Crit Rev Biochem Mol Biol 2011; 46:229-39; PMID:21476929; http://dx.doi.org/10.3109/10409238.2011.572861
- Long RM, Singer RH, Meng X, Gonzalez I, Nasmyth K, Jansen RP. Mating type switching in yeast controlled by asymmetric localization of ASH1 mRNA. Science 1997; 277:383-7; PMID:9219698; http://dx.doi.org/10.1126/science.277.5324.383
- Bertrand E, Chartrand P, Schaefer M, Shenoy SM, Singer RH, Long RM. Localization of ASH1 mRNA particles in living yeast. Mol Cell 1998; 2:437-45; PMID:9809065; http://dx.doi.org/10.1016/S1097-2765(00)80143-4
- Böhl F, Kruse C, Frank A, Ferring D, Jansen RP. She2p, a novel RNA-binding protein tethers ASH1 mRNA to the Myo4p myosin motor via She3p. EMBO J 2000; 19:5514-24; PMID:11032818; http://dx.doi.org/10.1093/emboj/19.20.5514
- Shen Z, St-Denis A, Chartrand P. Cotranscriptional recruitment of She2p by RNA pol II elongation factor Spt4-Spt5/DSIF promotes mRNA localization to the yeast bud. Genes Dev 2010; 24:1914-26; PMID:20713510; http://dx.doi.org/10.1101/gad.1937510
- Heym RG, Zimmermann D, Edelmann FT, Israel L, Ökten Z, Kovar DR, Niessing D. In vitro reconstitution of an mRNA-transport complex reveals mechanisms of assembly and motor activation. J Cell Biol 2013; 203:971-84; PMID:24368805; http://dx.doi.org/10.1083/jcb.201302095
- Weis BL, Schleiff E, Zerges W. Protein targeting to subcellular organelles via MRNA localization. Biochim Biophys Acta 2013; 1833:260-73; PMID:23457718; http://dx.doi.org/10.1016/j.bbamcr.2012.04.004
- Cosma MP. Daughter-specific repression of Saccharomyces cerevisiae HO: Ash1 is the commander. EMBO Rep 2004; 5:953-7; PMID:15459746; http://dx.doi.org/10.1038/sj.embor.7400251
- Jansen RP, Dowzer C, Michaelis C, Galova M, Nasmyth K. Mother cell-specific HO expression in budding yeast depends on the unconventional myosin myo4p and other cytoplasmic proteins. Cell 1996; 84:687-97; PMID:8625407; http://dx.doi.org/10.1016/S0092-8674(00)81047-8
- Bobola N, Jansen RP, Shin TH, Nasmyth K. Asymmetric accumulation of Ash1p in postanaphase nuclei depends on a myosin and restricts yeast mating-type switching to mother cells. Cell 1996; 84:699-709; PMID:8625408; http://dx.doi.org/10.1016/S0092-8674(00)81048-X
- Goode BL, Eck MJ. Mechanism and function of formins in the control of actin assembly. Annu Rev Biochem 2007; 76:593-627; PMID:17373907; http://dx.doi.org/10.1146/annurev.biochem.75.103004.142647
- Wesche S, Arnold M, Jansen R-P. The UCS domain protein She4p binds to myosin motor domains and is essential for class I and class V myosin function. Curr Biol 2003; 13:715-24; PMID:12725728; http://dx.doi.org/10.1016/S0960-9822(03)00264-1
- Paquin N, Chartrand P. Local regulation of mRNA translation: new insights from the bud. Trends Cell Biol 2008; 18:105-11; PMID:18262421; http://dx.doi.org/10.1016/j.tcb.2007.12.004
- Chartrand P, Meng XH, Singer RH, Long RM. Structural elements required for the localization of ASH1 mRNA and of a green fluorescent protein reporter particle in vivo. Curr Biol 1999; 9:333-6; PMID:10209102; http://dx.doi.org/10.1016/S0960-9822(99)80144-4
- Sladewski TE, Bookwalter CS, Hong M-S, Trybus KM. Single-molecule reconstitution of mRNA transport by a class V myosin. Nat Struct Mol Biol 2013; 20:952-7; PMID:23812374; http://dx.doi.org/10.1038/nsmb.2614
- Olivier C, Poirier G, Gendron P, Boisgontier A, Major F, Chartrand P. Identification of a conserved RNA motif essential for She2p recognition and mRNA localization to the yeast bud. Mol Cell Biol 2005; 25:4752-66; PMID:15899876; http://dx.doi.org/10.1128/MCB.25.11.4752-4766.2005
- Jambhekar A, McDermott K, Sorber K, Shepard KA, Vale RD, Takizawa PA, DeRisi JL. Unbiased selection of localization elements reveals cis-acting determinants of mRNA bud localization in Saccharomyces cerevisiae. Proc Natl Acad Sci U S A 2005; 102:18005-10; PMID:16326802; http://dx.doi.org/10.1073/pnas.0509229102
- Müller M, Richter K, Heuck A, Kremmer E, Buchner J, Jansen R-P, Niessing D. Formation of She2p tetramers is required for mRNA binding, mRNP assembly, and localization. RNA 2009; 15:2002-12; PMID:19710186; http://dx.doi.org/10.1261/rna.1753309
- Shen Z, Paquin N, Forget A, Chartrand P. Nuclear shuttling of She2p couples ASH1 mRNA localization to its translational repression by recruiting Loc1p and Puf6p. Mol Biol Cell 2009; 20:2265-75; PMID:19244342; http://dx.doi.org/10.1091/mbc.E08-11-1151
- Du T-G, Jellbauer S, Müller M, Schmid M, Niessing D, Jansen R-P. Nuclear transit of the RNA-binding protein She2 is required for translational control of localized ASH1 mRNA. EMBO Rep 2008; 9:781-7; PMID:18566598; http://dx.doi.org/10.1038/embor.2008.112
- Niedner A, Müller M, Moorthy BT, Jansen R-P, Niessing D. Role of Loc1p in assembly and reorganization of nuclear ASH1 messenger ribonucleoprotein particles in yeast. Proc Natl Acad Sci U S A 2013; 110:E5049-58; PMID:24324176; http://dx.doi.org/10.1073/pnas.1315289111
- Heuck A, Du T-G, Jellbauer S, Richter K, Kruse C, Jaklin S, Müller M, Buchner J, Jansen R-P, Niessing D. Monomeric myosin V uses two binding regions for the assembly of stable translocation complexes. Proc Natl Acad Sci U S A 2007; 104:19778-83; PMID:18056806; http://dx.doi.org/10.1073/pnas.0706780104
- Dunn BD, Sakamoto T, Hong M-SS, Sellers JR, Takizawa PA. Myo4p is a monomeric myosin with motility uniquely adapted to transport mRNA. J Cell Biol 2007; 178:1193-206; PMID:17893244; http://dx.doi.org/10.1083/jcb.200707080
- Takizawa PA, Sil A, Swedlow JR, Herskowitz I, Vale RD. Actin-dependent localization of an RNA encoding a cell-fate determinant in yeast. Nature 1997; 389:90-3; PMID:9288973; http://dx.doi.org/10.1038/38015
- Toi H, Fujimura-Kamada K, Irie K, Takai Y, Todo S, Tanaka K. She4p/Dim1p interacts with the motor domain of unconventional myosins in the budding yeast, Saccharomyces cerevisiae. Mol Biol Cell 2003; 14:2237-49; PMID:12808026; http://dx.doi.org/10.1091/mbc.E02-09-0616
- Beach DL, Salmon ED, Bloom K. Localization and anchoring of mRNA in budding yeast. Curr Biol 1999; 9:569-78; PMID:10359695; http://dx.doi.org/10.1016/S0960-9822(99)80260-7
- Amberg DC, Zahner JE, Mulholland JW, Pringle JR, Botstein D. Aip3p/Bud6p, a yeast actin-interacting protein that is involved in morphogenesis and the selection of bipolar budding sites. Mol Biol Cell 1997; 8:729-53; PMID:9247651; http://dx.doi.org/10.1091/mbc.8.4.729
- Gonsalvez GB, Little JL, Long RM. ASH1 mRNA anchoring requires reorganization of the Myo4p-She3p-She2p transport complex. J Biol Chem 2004; 279:46286-94; PMID:15328357; http://dx.doi.org/10.1074/jbc.M406086200
- Gonzalez I, Buonomo SB, Nasmyth K, von Ahsen U. ASH1 mRNA localization in yeast involves multiple secondary structural elements and Ash1 protein translation. Curr Biol 1999; 9:337-40; PMID:10209099; http://dx.doi.org/10.1016/S0960-9822(99)80145-6
- Park H-O, Bi E. Central roles of small GTPases in the development of cell polarity in yeast and beyond. Microbiol Mol Biol Rev 2007; 71:48-96; PMID:17347519; http://dx.doi.org/10.1128/MMBR.00028-06
- Aronov S, Gerst JE. Involvement of the late secretory pathway in actin regulation and mRNA transport in yeast. J Biol Chem 2004; 279:36962-71; PMID:15192110; http://dx.doi.org/10.1074/jbc.M402068200
- Aronov S, Gelin-Licht R, Zipor G, Haim L, Safran E, Gerst JE. mRNAs encoding polarity and exocytosis factors are cotransported with the cortical endoplasmic reticulum to the incipient bud in Saccharomyces cerevisiae. Mol Cell Biol 2007; 27:3441-55; PMID:17339339; http://dx.doi.org/10.1128/MCB.01643-06
- Slessareva JE, Dohlman HG. G protein signaling in yeast: new components, new connections, new compartments. Science 2006; 314:1412-3; PMID:17138892; http://dx.doi.org/10.1126/science.1134041
- Merlini L, Dudin O, Martin SG. Mate and fuse: how yeast cells do it. Open Biol 2013; 3:130008-8; PMID:23466674; http://dx.doi.org/10.1098/rsob.130008
- Guo M, Aston C, Burchett SA, Dyke C, Fields S, Rajarao SJ, Uetz P, Wang Y, Young K, Dohlman HG. The yeast G protein alpha subunit Gpa1 transmits a signal through an RNA binding effector protein Scp160. Mol Cell 2003; 12:517-24; PMID:14536090; http://dx.doi.org/10.1016/S1097-2765(03)00307-1
- Gelin-Licht R, Paliwal S, Conlon P, Levchenko A, Gerst JE. Scp160-dependent mRNA trafficking mediates pheromone gradient sensing and chemotropism in yeast. Cell Rep 2012; 1:483-94; PMID:22832273; http://dx.doi.org/10.1016/j.celrep.2012.03.004
- Frey S, Pool M, Seedorf M. Scp160p, an RNA-binding, polysome-associated protein, localizes to the endoplasmic reticulum of Saccharomyces cerevisiae in a microtubule-dependent manner. J Biol Chem 2001; 276:15905-12; PMID:11278502; http://dx.doi.org/10.1074/jbc.M009430200
- West M, Zurek N, Hoenger A, Voeltz GK. A 3D analysis of yeast ER structure reveals how ER domains are organized by membrane curvature. J Cell Biol 2011; 193:333-46; PMID:21502358; http://dx.doi.org/10.1083/jcb.201011039
- Tavassoli S, Chao JT, Young BP, Cox RC, Prinz WA, de Kroon AIPM, Loewen CJR. Plasma membrane–endoplasmic reticulum contact sites regulate phosphatidylcholine synthesis. EMBO Rep 2013; 14:434-40; PMID:23519169; http://dx.doi.org/10.1038/embor.2013.36
- Du Y, Ferro-Novick S, Novick P. Dynamics and inheritance of the endoplasmic reticulum. J Cell Sci 2004; 117:2871-8; PMID:15197242; http://dx.doi.org/10.1242/jcs.01286
- Estrada P, Kim J, Coleman J, Walker L, Dunn B, Takizawa P, Novick P, Ferro-Novick S. Myo4p and She3p are required for cortical ER inheritance in Saccharomyces cerevisiae. J Cell Biol 2003; 163:1255-66; PMID:14691136; http://dx.doi.org/10.1083/jcb.200304030
- Schmid M, Jaedicke A, Du T-G, Jansen R-P. Coordination of endoplasmic reticulum and mRNA localization to the yeast bud. Curr Biol 2006; 16:1538-43; PMID:16890529; http://dx.doi.org/10.1016/j.cub.2006.06.025
- Fundakowski J, Hermesh O, Jansen R-P. Localization of a subset of yeast mRNAs depends on inheritance of endoplasmic reticulum. Traffic 2012; 13:1642-52; PMID:22994588; http://dx.doi.org/10.1111/tra.12011
- Kraut-Cohen J, Afanasieva E, Haim-Vilmovsky L, Slobodin B, Yosef I, Bibi E, Gerst JE. Translation- and SRP-independent mRNA targeting to the endoplasmic reticulum in the yeast Saccharomyces cerevisiae. Mol Biol Cell 2013; 24:3069-84; PMID:23904265; http://dx.doi.org/10.1091/mbc.E13-01-0038
- Shepard KA, Gerber AP, Jambhekar A, Takizawa PA, Brown PO, Herschlag D, DeRisi JL, Vale RD. Widespread cytoplasmic mRNA transport in yeast: identification of 22 bud-localized transcripts using DNA microarray analysis. Proc Natl Acad Sci U S A 2003; 100:11429-34; PMID:13679573; http://dx.doi.org/10.1073/pnas.2033246100
- Oeffinger M, Wei KE, Rogers R, DeGrasse JA, Chait BT, Aitchison JD, Rout MP. Comprehensive analysis of diverse ribonucleoprotein complexes. Nat Methods 2007; 4:951-6; PMID:17922018; http://dx.doi.org/10.1038/nmeth1101
- Genz C, Fundakowski J, Hermesh O, Schmid M, Jansen R-P. Association of the yeast RNA-binding protein She2p with the tubular endoplasmic reticulum depends on membrane curvature. J Biol Chem 2013; 288:32384-93; PMID:24056370; http://dx.doi.org/10.1074/jbc.M113.486431
- Schwartz TU. Origins and evolution of cotranslational transport to the ER. Adv Exp Med Biol 2007; 607:52-60; PMID:17977458; http://dx.doi.org/10.1007/978-0-387-74021-8_4
- Kraut-Cohen J, Gerst JE. Addressing mRNAs to the ER: cis sequences act up! Trends Biochem Sci 2010; 35:459-69; PMID:20346679; http://dx.doi.org/10.1016/j.tibs.2010.02.006
- Hermesh O, Jansen R-P. Take the (RN)A-train: localization of mRNA to the endoplasmic reticulum. Biochim Biophys Acta 2013; 1833:2519-25; PMID:23353632; http://dx.doi.org/10.1016/j.bbamcr.2013.01.013
- Ast T, Cohen G, Schuldiner M. A network of cytosolic factors targets SRP-independent proteins to the endoplasmic reticulum. Cell 2013; 152:1134-45; PMID:23452858; http://dx.doi.org/10.1016/j.cell.2013.02.003
- Palazzo AF, Springer M, Shibata Y, Lee C-S, Dias AP, Rapoport TA. The signal sequence coding region promotes nuclear export of mRNA. PLoS Biol 2007; 5:e322; PMID:18052610; http://dx.doi.org/10.1371/journal.pbio.0050322
- Prilusky J, Bibi E. Studying membrane proteins through the eyes of the genetic code revealed a strong uracil bias in their coding mRNAs. Proc Natl Acad Sci U S A 2009; 106:6662-6; PMID:19366666; http://dx.doi.org/10.1073/pnas.0902029106
- Neupert W, Herrmann JM. Translocation of proteins into mitochondria. Annu Rev Biochem 2007; 76:723-49; PMID:17263664; http://dx.doi.org/10.1146/annurev.biochem.76.052705.163409
- Kellems RE, Butow RA. Cytoplasmic-type 80 S ribosomes associated with yeast mitochondria. I. Evidence for ribosome binding sites on yeast mitochondria. J Biol Chem 1972; 247:8043-50; PMID:4629740
- Kellems RE, Allison VF, Butow RA. Cytoplasmic type 80 S ribosomes associated with yeast mitochondria. II. Evidence for the association of cytoplasmic ribosomes with the outer mitochondrial membrane in situ. J Biol Chem 1974; 249:3297-303; PMID:4598123
- Garcia M, Darzacq X, Delaveau T, Jourdren L, Singer RH, Jacq C. Mitochondria-associated yeast mRNAs and the biogenesis of molecular complexes. Mol Biol Cell 2007; 18:362-8; PMID:17108321; http://dx.doi.org/10.1091/mbc.E06-09-0827
- Marc P, Margeot A, Devaux F, Blugeon C, Corral-Debrinski M, Jacq C. Genome-wide analysis of mRNAs targeted to yeast mitochondria. EMBO Rep 2002; 3:159-64; PMID:11818335; http://dx.doi.org/10.1093/embo-reports/kvf025
- Corral-Debrinski M, Blugeon C, Jacq C. In yeast, the 3′ untranslated region or the presequence of ATM1 is required for the exclusive localization of its mRNA to the vicinity of mitochondria. Mol Cell Biol 2000; 20:7881-92; PMID:11027259; http://dx.doi.org/10.1128/MCB.20.21.7881-7892.2000
- Margeot A, Blugeon C, Sylvestre J, Vialette S, Jacq C, Corral-Debrinski M. In Saccharomyces cerevisiae, ATP2 mRNA sorting to the vicinity of mitochondria is essential for respiratory function. EMBO J 2002; 21:6893-904; PMID:12486010; http://dx.doi.org/10.1093/emboj/cdf690
- Sylvestre J, Margeot A, Jacq C, Dujardin G, Corral-Debrinski M. The role of the 3′ untranslated region in mRNA sorting to the vicinity of mitochondria is conserved from yeast to human cells. Mol Biol Cell 2003; 14:3848-56; PMID:12972568; http://dx.doi.org/10.1091/mbc.E03-02-0074
- Garcia M, Delaveau T, Goussard S, Jacq C. Mitochondrial presequence and open reading frame mediate asymmetric localization of messenger RNA. EMBO Rep 2010; 11:285-91; PMID:20224577; http://dx.doi.org/10.1038/embor.2010.17
- Gadir N, Haim-Vilmovsky L, Kraut-Cohen J, Gerst JE. Localization of mRNAs coding for mitochondrial proteins in the yeast Saccharomyces cerevisiae. RNA 2011; 17:1551-65; PMID:21705432; http://dx.doi.org/10.1261/rna.2621111
- Quenault T, Lithgow T, Traven A. PUF proteins: repression, activation and mRNA localization. Trends Cell Biol 2011; 21:104-12; PMID:21115348; http://dx.doi.org/10.1016/j.tcb.2010.09.013
- García-Rodríguez LJ, Gay AC, Pon LA. Puf3p, a Pumilio family RNA binding protein, localizes to mitochondria and regulates mitochondrial biogenesis and motility in budding yeast. J Cell Biol 2007; 176:197-207; PMID:17210948; http://dx.doi.org/10.1083/jcb.200606054
- Gerber AP, Herschlag D, Brown PO. Extensive association of functionally and cytotopically related mRNAs with Puf family RNA-binding proteins in yeast. PLoS Biol 2004; 2:E79; PMID:15024427; http://dx.doi.org/10.1371/journal.pbio.0020079
- Saint-Georges Y, Garcia M, Delaveau T, Jourdren L, Le Crom S, Lemoine S, Tanty V, Devaux F, Jacq C. Yeast mitochondrial biogenesis: a role for the PUF RNA-binding protein Puf3p in mRNA localization. PLoS One 2008; 3:e2293; PMID:18523582; http://dx.doi.org/10.1371/journal.pone.0002293
- Eliyahu E, Pnueli L, Melamed D, Scherrer T, Gerber AP, Pines O, Rapaport D, Arava Y. Tom20 mediates localization of mRNAs to mitochondria in a translation-dependent manner. Mol Cell Biol 2010; 30:284-94; PMID:19858288; http://dx.doi.org/10.1128/MCB.00651-09
- Olivas W, Parker R. The Puf3 protein is a transcript-specific regulator of mRNA degradation in yeast. EMBO J 2000; 19:6602-11; PMID:11101532; http://dx.doi.org/10.1093/emboj/19.23.6602
- Ma C, Agrawal G, Subramani S. Peroxisome assembly: matrix and membrane protein biogenesis. J Cell Biol 2011; 193:7-16; PMID:21464226; http://dx.doi.org/10.1083/jcb.201010022
- Zipor G, Haim-Vilmovsky L, Gelin-Licht R, Gadir N, Brocard C, Gerst JE. Localization of mRNAs coding for peroxisomal proteins in the yeast, Saccharomyces cerevisiae. Proc Natl Acad Sci U S A 2009; 106:19848-53; PMID:19903887; http://dx.doi.org/10.1073/pnas.0910754106
- Katz ZB, Wells AL, Park HY, Wu B, Shenoy SM, Singer RH. β-Actin mRNA compartmentalization enhances focal adhesion stability and directs cell migration. Genes Dev 2012; 26:1885-90; PMID:22948660; http://dx.doi.org/10.1101/gad.190413.112
- Mingle LA, Okuhama NN, Shi J, Singer RH, Condeelis J, Liu G. Localization of all seven messenger RNAs for the actin-polymerization nucleator Arp2/3 complex in the protrusions of fibroblasts. J Cell Sci 2005; 118:2425-33; PMID:15923655; http://dx.doi.org/10.1242/jcs.02371
- Casolari JM, Thompson MA, Salzman J, Champion LM, Moerner WE, Brown PO. Widespread mRNA association with cytoskeletal motor proteins and identification and dynamics of myosin-associated mRNAs in S. cerevisiae. PLoS One 2012; 7:e31912; PMID:22359641; http://dx.doi.org/10.1371/journal.pone.0031912
- Weinberg J, Drubin DG. Clathrin-mediated endocytosis in budding yeast. Trends Cell Biol 2012; 22:1-13; PMID:22018597; http://dx.doi.org/10.1016/j.tcb.2011.09.001
- Riedl J, Crevenna AH, Kessenbrock K, Yu JH, Neukirchen D, Bista M, Bradke F, Jenne D, Holak TA, Werb Z, et al. Lifeact: a versatile marker to visualize F-actin. Nat Methods 2008; 5:605-7; PMID:18536722; http://dx.doi.org/10.1038/nmeth.1220
- D’Silva S, Haider SJ, Phizicky EM. A domain of the actin binding protein Abp140 is the yeast methyltransferase responsible for 3-methylcytidine modification in the tRNA anti-codon loop. RNA 2011; 17:1100-10; PMID:21518804; http://dx.doi.org/10.1261/rna.2652611
- Kilchert C, Spang A. Cotranslational transport of ABP140 mRNA to the distal pole of S. cerevisiae. EMBO J 2011; 30:3567-80; PMID:21792172; http://dx.doi.org/10.1038/emboj.2011.247
- Liao G, Ma X, Liu G. An RNA-zipcode-independent mechanism that localizes Dia1 mRNA to the perinuclear ER through interactions between Dia1 nascent peptide and Rho-GTP. J Cell Sci 2011; 124:589-99; PMID:21266463; http://dx.doi.org/10.1242/jcs.072421
