Abstract
Messenger RNA export from the nucleus to the cytoplasm plays an essential role in linking transcription to translation and consequently regulation of protein expression. mRNA export requires a series of events: pre-mRNA processing, ribonucleoprotein targeting to the NPC (nuclear pore complexes), and translocation through nuclear pores to the cytoplasm. Interestingly, the conventional nuclear export machinery, exportins and the Ran GTPase, is not required for mRNA export. Instead, a protein complex consisting of a number of RNA binding proteins is essential for this event including the Aly/REF protein. Phosphoinositide signaling regulates a variety of cellular functions including pre-mRNA splicing and mRNA export. In fact, a phospholipase C-dependent inositol polyphosphate kinase pathway is required for efficient mRNA export. Recently, we showed that Aly is a physiological target of nuclear phosphoinositide-3-kinase (PI3K) signaling, which regulates Aly localization as well as Aly function in cell proliferation and mRNA export through nuclear Akt-mediated phosphorylation and phosphoinositide association. Hence, water-soluble inositol polyphosphates and phosphatidylinositol lipids play pivotal roles in modulating mRNA export.
Nuclear mRNA Export Machinery
The nucleus of the eukaryotic cell is surrounded by a double nuclear membrane that compartmentalizes the genetic material from the protein translation machinery in the cytoplasm. To allow exchange of macromolecules between the nucleus and the cytoplasm, the nuclear envelope contains a large number of nuclear pore complexes (NPCs) that serve as aqueous channels for high molecular weight molecules.Citation1 Small molecules of a size less than ~40–60 kDa can pass freely through NPCs. However, large molecules or cargoes require a “ticket” for targeting to and transport through NPCs. For most cargoes that enter and/or exit the nucleus, this “ticket” is a signal recognized by a large family of soluble nuclear transport receptors termed the importin/exportin/karyopherin family.Citation2
Many classes of RNAs are exported from the nucleus in complex with these conventional transport receptors.Citation3 However, different classes of RNA are exported by distinct mechanisms. Each class of RNA forms complexes with different receptors prior to export to the cytoplasm. For instance, tRNA is exported via directly binding to its receptor, exportin-t.Citation4 However, rRNA, which interacts with exportin-1, is exported in pre-ribosomal particles containing ribosomal proteins, several rRNA species, and non-ribosomal proteins.Citation5 Moreover, snRNAs are transiently transported to the cytoplasm for assembly into snRNP particles and also need exportin-1 for export.Citation6 Interestingly, importins/exportins do not directly mediate mRNA export. In the case of mRNA export, Mex67/TAP (S. cerevisiae/mammalian), which is not related to the importin/exportin family of proteins, is the primary mRNA nuclear export receptor.Citation7 Mex67 interacts with both poly(A)+ RNA and nuclear pore complexes and cells mutant for Mex67 show rapid accumulation of poly(A)+ RNA in the nucleus.Citation8 Furthermore, Mex67 forms a heterodimer with a small protein designated Mtr2,Citation9 and this Mex67-Mtr2 heterodimer acts as a nuclear transport receptor like importin β that is essential for mRNA export.Citation10 Another key player in mRNA export is the conserved nuclear protein known as Yra1 in yeast and Aly/REF in metazoans.Citation11,Citation12 Aly interacts directly with Tap, suggesting a conserved role for Yra1/Aly in mRNA export.Citation12 The direct functional evidence in support of Aly as a key mRNA export factor comes from the observation that recombinant Aly promotes export of mRNA in Xenopus oocytes.Citation13 Microinjection of antibodies to Aly blocks mRNA export without affecting other transport pathways. Although Aly is primarily nuclear, it shuttles between the nucleus and the cytoplasm, consistent with a nuclear export function.Citation14
The mRNA export machinery is intimately coupled to the pre-mRNA processing/splicing machinery. Aly is recruited to mRNAs during splicing.Citation14 Furthermore, studies in mammalian cells reveal that Aly colocalizes with pre-mRNA splicing factors in nuclear “speckles,” which are storage sites for components of the pre-mRNA processing machinery.Citation13 Aly/Yra1 also forms a stoichiometric complex with a conserved RNA helicase termed Sub2 in yeastCitation15 and UAP56 in mammals.Citation16 The spliced mature mRNA has exon-exon junctions and several proteins that comprise the exon-exon junction complex (EJC) accumulate in this region.Citation17 The EJC is assembled concomitantly with splicing and binds tightly to a newly formed mRNA at a position 24 nucleotides upstream of these splice junctions in mammalian cells.Citation18 Thus, EJC formation serves as one of the markers of the maturation of mRNA. Interestingly, the Aly/REF protein is one of the EJC components. Thus, ALY/REF selectively binds to both mature mRNA and the mRNA export receptor.Citation14
Nonetheless, Aly proteins are dispensable for mRNA export in Drosophila and C. elegans, suggesting functional redundancy or complexity of this adaptor protein in these organisms; by contrast, Yra1 is essential for mRNA export and cell growth in budding yeast.Citation19-Citation21
In the case of multiple intron-containing transcripts, EJC component proteins are gathered at accumulating exon-exon junctions even though pre-mRNA splicing is not completely finished. Nevertheless, immature mRNA is sequestered in the nucleus through a mechanism of intron binding to nuclear proteins. Once splicing and additional maturation steps are completed, the mature mRNA-EJC complex (mRNP) is exported to the cytoplasm where mRNA is translated. This course of events suggests that the EJC can ensure not only proper mRNA maturation but also direct appropriate subcellular localization of the processed transcript. On the other hand, the molecular mechanisms regulating nuclear export of intronless transcripts (such as histone H2A, β-globin or transfected cDNA) as well as how export factors are recruited onto intronless mRNAs are unknown. Some mRNAs like histone H2A contain specific sequences that act as sites for specific export factors independent of splicing.Citation22 SRp20 and 9G8, which belong to a serine-arginine rich (SR) family of RNA binding proteins, specifically associate with a sequence in specific intronless transcripts to facilitate nuclear export by directly recruiting the TAP mRNA export receptor.Citation23 Nevertheless, some other intronless transcripts lack the above-mentioned specific sequences. Recent studies show that addition of a poly (A) tail onto the 3'-end of mRNA and the 5'-end cap structure are also important for nuclear export of intronless transcripts.Citation24,Citation25 Aly can bind to both mature and immature mRNA transcripts, and it plays a role in the export of intronless mRNA in the absence of specific export sequences. For instance, the RNA helicase UAP56 recruits intronless mRNA to Aly in an ATP-dependent manner.Citation26 Moreover, Aly can also bind to the 5' cap and this interaction is required to promote export of intronless mRNA. Likewise, SR proteins play a general role in export of both intronless and spliced mRNAs. Experiments carried out in Xenopus oocytes show that nuclear export of spliced mRNA (ftz) and intronless mRNA (β-globin) is inhibited after microinjection of antibody against SR proteins.Citation27 Taken together, all of this evidence strongly suggests a general role for Aly/REF in modulating export of both intronless and spliced mRNA transcripts from the nucleus.
Nuclear Phosphoinositide and Related Protein Signaling
Phosphoinositide and its downstream signaling effectors regulate a variety of cellular activities. In classical phosphoinositide signaling, extracellular stimuli trigger phospholipid metabolism by different classes of kinases, phosphatases and phospholipases. Following metabolism of phosphoinositides, second messengers such as inositol phosphates are produced and subsequently provoke numerous cellular events. A large body of evidence demonstrates that almost all of the phosphoinositides and the enzymes responsible for the metabolism of inositol lipids can be found in the nucleus.Citation28,Citation29 Accumulating evidence suggests that nuclear phosphatidylinositol lipids modulate a broad range of nuclear processes including chromatin structure, pre-mRNA splicing, cell cycle progression and nuclear response to DNA damage.Citation30-Citation33 However, the nuclear phosphoinositide cycle is independent of the counterpart in the cytoplasm. For instance, the levels of nuclear phosphoinositol lipids decrease more than 50% during S phase, whereas the levels of their cytoplasmic counterparts remain constant throughout S phase, indicating that specific nuclear turnover of these lipids is activated during DNA synthesis.Citation34 When mouse erythroleukemia (MEL) cells are induced to differentiate, the levels of nuclear PI(4,5)P2 (phosphatidylinositol 4,5-diphosphate) increase.Citation35 PI(4,5)P2 can be found in Triton X-100 insoluble nuclear fractions, consistent with an association with the nuclear matrix region and/or nuclear speckles, which house many pre-mRNA processing proteins.Citation36 Not only PI(4,5)P2 but also phosphatidylinositol monophosphate kinase (PIPK)Iα, which uses PI4P as a substrate to generate PI(4,5)P2, localizes to nuclear speckles, suggesting a role for nuclear phosphoinositides in pre-mRNA splicing. Most recently, Anderson and his colleagues show that PIPKIα co-localizes at nuclear speckles and interacts with a newly identified non-canonical poly(A) polymerase, which we have termed Star-PAP (nuclear speckle targeted PIPKIα regulated-poly(A) polymerase) and that the activity of Star-PAP can be specifically regulated by PtdIns4,5P2. Star-PAP and PIPKIα function together in a complex to control the expression of select mRNAs.Citation37 PI(3,4,5)P3 is produced by class IA phosphatidylinositol 3-kinase (PI3K) from PI(4,5)P2. Class IA PI3K and its activity have been found in the nucleus of a variety of cell types and organs. Previously, we reported that in nerve growth factor (NGF) treated PC12 cells, the level of nuclear PI(3,4,5)P3 is increased by nuclear PI3K.Citation38,Citation39 In our effort to search for nuclear receptors for PI(3,4,5)P3, we identified nucleophasmin/B23 as one of the binding targets.Citation40 Aly was also one of the binding proteins identified. In our recent study, we provided further evidence demonstrating that Aly directly binds phosphoinositides.Citation41 Mapping experiments suggest that the N terminus of Aly is the binding domain for phosphoinositides. Protein sequence analysis reveals that this region contains numerous basic residues. Mutation of R27/29/31 or R79/K81 into alanine abolishes Aly binding to PI(3,4,5)P3, suggesting that these residues play an essential role in binding to the heavily negatively charged phosphoinositide head groups. Presumably, the clusters of positively charged arginine residues in the N terminus and arginine-79/lysine-81 may form a pocket that binds to the phosphorylated inositol head group of phosphoinositol lipids. Thus, alteration of one cluster of positively charged residues results in deformation of the delicate three-dimensional conformation, leading to loss of binding affinity by Aly to phosphoinositides. Phosphoinositides binding by Aly regulates its subcellular residency, mRNA export and cell proliferation activities.Citation41
As a key downstream effector of PI3K signaling cascade, the phosphorylated and activated Akt kinase also translocates into the nucleus.Citation42 Indeed, some Akt substrates, such as the FoxO family transcription factorsCitation43 and the transcriptional co-activator p300,Citation44 are resident in the nucleus. To search for nuclear Akt substrates contributing to blocking apoptosis, we recently identified acinus, a nuclear factor required for apoptotic chromatin condensation. Acinus is an SR protein and essential for RNA splicing.Citation45,Citation46 Acinus is predominantly located in the nucleus where it induces apoptotic chromatin condensation following cleavage by caspases.Citation47 We demonstrated that acinus is a direct target of nuclear Akt.Citation48 Akt phosphorylation of acinus on serine 422 and 573 confers resistance to caspase cleavage in the nucleus and the inhibition of subsequent acinus-dependent chromatin condensation (). Moreover, we identified a nucleolar protein Ebp1 (ErbB3 binding protein), which also inhibits the DNA fragmentation activity of Caspase-activated DNase during apoptosis.Citation49 Ebp1 exerts its pro-survival action through forming a complex with nuclear Akt, a process that is regulated by PKC-mediated phosphorylation. Therefore, our finding provides further evidence that nuclear Akt inhibits apoptosis not only through phosphorylating apoptotic effectors but also through binding to survival partners in the nucleus.
Figure 1. Nuclear Akt phosphorylates SR proteins. Acinus and Aly both contain RRM (RNA recognition domain) and reside in the nuclear speckles. These two proteins are also implicated in pre-mRNA splicing. Akt phosphorylates both of them, regulating their subnuclear residency and biological functions. Arrows indicate Akt phosphorylation residues.
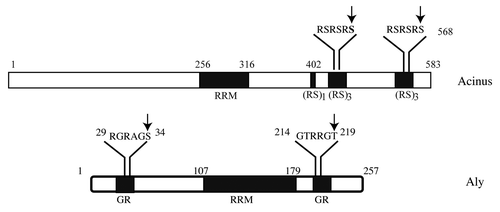
Recently we reported that nuclear Akt phosphorylates Aly and regulates its cellular activities.Citation41 Aly contains an RNA recognition domain (RRM) and GR (Glycine-Arginine)-rich domain in both the amino and carboxy-termini (). In exploring the sequence of Aly, we noticed that amino acids 29–34, RGRAGS and 214–219, GTRRGT, correspond to a motif that is identified as a consensus Akt phosphorylation element present in numerous Akt substrates. Employing an in vitro kinase assay, site-directed mutagenesis and metabolic labeling, we found that Aly acts as a physiological substrate of nuclear Akt and showed that nuclear Akt phosphorylates Aly on both S34 and T219. However, the T219 residue in Aly is a major Akt phosphorylation site. Interestingly, the T219A but not S34A mutation abolishes Akt association with Aly, fitting with the observation that T219 and not S34 is the predominant phosphorylation site.Citation41 Interestingly, Aly is also a caspase-3 substrate. Unlike acinus, Akt phosphorylation does not protect Aly from proteolytic degradation. However, the physiological significance of this finding remains elusive. Conceivably, Aly is degraded by caspases during programmed cell death, but it is not implicated in promoting cell survival.
The Effect of Phosphoinositides on Aly mRNA Export Activity
The processing and transport of mRNA are tightly linked, such that splicing, polyadenylation and capping all precede and affect the export process.Citation3 Alternative splicing of pre-mRNAs can be regulated by extracellular signals such as growth factors, cytokines, hormones and stress stimuli.Citation50 For instance, insulin-activated PI3K signaling, which has been implicated in mammary epithelial-mesenchymal interaction, regulates fibronectin alternative splicing.Citation51 Consistent with this report, we found that nuclear PI3K signaling regulates mRNA export through downstream nuclear PI(3,4,5)P3 and nuclear Akt.Citation41 We show that Aly directly interacts with nuclear PI(4,5)P2 and PI(3,4,5)P3, which is essential for proper localization of Aly to nuclear speckles. Knock down of Aly significantly decreases mRNA export. Moreover, depletion of Aly selectively decreases expression of cyclin A and cyclin B1 but not other G1 cyclins. In addition, BrdU incorporation is significantly decreased in HeLa cells when Aly is inactivated. These results support the idea that Aly is required for both mRNA export and cell cycle progression in HeLa cells. These findings are consistent with a recent report that Yra1, the budding yeast homolog of Aly, is required for S phase entry and proper Dia2 binding to replication origins.Citation52 Thus, Aly modulates cell cycle progression and plays a role in DNA replication, in line with a previous report that PI3K is important for both transition from G1 to S phase and S-phase progression.Citation53,Citation54 While wild-type Aly is exclusively localized to nuclear speckles, Aly mutants R27/29/31A and R79A/K81A, which fail to associate with PI(3,4,5)P3, only partially colocalize with the nuclear speckle marker, SC-35.Citation41 Interestingly, these mutants aggregate in the nucleoplasm outside nuclear speckles, indicating that PI(3,4,5)P3 binding somehow regulates nuclear speckle localization of Aly. Both Aly mutants R27/29/31A and R79A/K81A also displayed a decreased activity in mRNA export compared to wild-type Aly and control, suggesting that these basic residues are essential for the role of Aly in mRNA export.Citation41 This observation fits with a previous report that nuclear phosphoinositides influence pre-mRNA splicing and chromatin structure.Citation55
Aly T219 phosphorylation by Akt does not promote cell survival; instead, it strongly enhances cell proliferation and a block to T219 phosphorylation profoundly blocks BrdU incorporation and cell proliferation.Citation41 Interestingly, the cell growth activity of Aly is also regulated by phosphoinositides. Overexpression of Aly mutants deficient for binding to nuclear PI(3,4,5)P3 substantially suppresses cell proliferation. As mentioned above, these mutants also aggregate in the nucleoplasm outside nuclear speckles. Conceivably, proper localization of Aly to nuclear speckles may be necessary for the cell growth activity we have linked to Aly function. To assess the effect of Akt phosphorylation on Aly-mediated mRNA export, we transfected HeLa cells with various GFP-Aly constructs. Noticeably, the unphosphorylatable T219A mutant of Aly markedly decreased mRNA export, suggesting that Akt phosphorylation is required for proper function of Aly in mRNA export.Citation41
Mounting evidence supports the idea that cell cycle progression and nuclear phosphoinositide metabolism are linked. Although the levels of total cellular phosphoinositides remain constant throughout the cell cycle, nuclear phosphoinositides fluctuate significantly in a cell cycle-dependent manner.Citation34,Citation56 Nuclear speckles are highly dynamic, and their morphology is tightly linked to the state of mRNA transcription. Inhibition of mRNA transcription induces these structures to become larger and fewer in number and phosphatidylinositol phosphate kinases and PI(4,5)P2 reorganize in a similar manner.Citation57 The presence of PI(4,5)P2 in these speckles within the nucleus has been linked to its involvement in pre-mRNA splicing.Citation58 In fact, when PI(4,5)P2 was immunoprecipitated from HeLa cell nuclear extracts, some proteins co-purified, resulting in a specific inhibition of pre-mRNA splicing in these depleted extracts.Citation58 Now, we show that PI(4,5)P2 and PI(3,4,5)P3 robustly bind to Aly in nuclear speckles and thus the involvement of nuclear phosphoinositides in pre-mRNA splicing and/or mRNA export might be direct through modulation of Aly function. Nevertheless, the possibility that PI(4,5)P2 or PI(3,4,5)P3 binds to nuclear matrix proteins and serves as a structural interface between the enzymatic core of the spliceosome and the matrix itself also cannot be excluded. Identification of Aly, a nuclear speckle protein implicated in mRNA export, as one of the nuclear phosphoinositol lipid binding targets is certainly of great help in understanding the exact and multifaceted functions of these nuclear lipids. The mTOR signaling plays a major role in cell growth along the Akt signaling. Indeed, mTOR regulates several cellular mechanisms such as transcription initiation via the S6 kinase (S6K). In 2004, a novel S6K1 substrate, named SKAR (S6K1 Aly/REF-like target) was isolated. SKAR also localizes in the nuclear speckles and is deposited at the EJC during splicing.Citation59,Citation60 Thus nuclear PI3K/Akt downstream pathways play an essential role in regulating mRNA export.
Mature mRNA export involves three key steps: the initial step of mature mRNA export is generation of cargo-carrier complex, such as the EJC and TAP complex. The next step is the passage through NPC channels. The last step is to release mRNA from the NPC and associated carrier proteins. The detailed mechanism of how cargo-receptor complexes translocate through the nuclear pore is still a topic of much debate; however, there is consensus that weak interactions between the mRNA-carrier protein complex and phenylalanine glycine (FG) repeat-containing nucleoporins in the NPC channel facilitate this movement.Citation61 Indeed, the first time that nuclear phosphoinositide metabolites were linked to nuclear export was in the final dissociation step of the mRNA export process.Citation62 In a yeast genetic screen with a nuclear pore mutant, Phospholipase C (PLC1) and inositol phosphate kinases genes were identified and subsequently shown to be necessary for proper mRNA export in budding yeast. Thus, previous studies demonstrate that inositol polyphosphate kinases (IPK) and inositol pyrophosphate synthases (IPS) are necessary for mRNA export.Citation63 Phospholipase C and two inositol polyphosphate (IP) kinases constitute a signaling pathway that regulates nuclear messenger RNA export through production of inositol hexakisphosphate (IP6).Citation64 Recent mechanistic studies reveal that the DEAD-box helicase, Dbp5, promotes dissociation of Mex67 (TAP in mammals) and other mRNA binding proteins from transcripts following passage through the NPC.Citation65,Citation66 Importantly, Dbp5 is activated by Gle1 and IP6.Citation67 Gle1 is an essential for mRNA nuclear export in yeast that is localized to the cytoplasmic face of the nuclear pore. This localization suggests that Dbp5-mediated remodeling at Gle1 may act as a terminal step in the export of mature RNA messages to the cytoplasm.Citation68 Thus, metabolites from nuclear PI(4,5)P2, especially highly phosphorylated molecules, including polyphosphate inositols and PI(3,4,5)P3, regulate mRNA export. The discovery that nuclear Akt phosphorylates Aly and modulates its mRNA export activity provides novel insight into the molecular mechanism of how PI3K signaling regulates cell cycle progression, pre-mRNA splicing, mRNA export and cell proliferation.
Acknowledgements
This work is supported by grant from National Institute of Health (RO1, CA127119) to K. Ye. We are thankful for Dr. Anita H. Corbett at Department of Biochemistry, Emory University for her critical reading and editing of the manuscript.
References
- D’Angelo MA, Hetzer MW. Structure, dynamics and function of nuclear pore complexes. Trends Cell Biol 2008; 18:456 - 66
- Pemberton LF, Paschal BM. Mechanisms of receptor-mediated nuclear import and nuclear export. Traffic 2005; 6:187 - 98
- Kohler A, Hurt E. Exporting RNA from the nucleus to the cytoplasm. Nat Rev Mol Cell Biol 2007; 8:761 - 73
- Gorlich D, Kutay U. Transport between the cell nucleus and the cytoplasm. Annu Rev Cell Dev Biol 1999; 15:607 - 60
- Warner JR. Nascent ribosomes. Cell 2001; 107:133 - 6
- Ohno M, Segref A, Bachi A, Wilm M, Mattaj IW. PHAX, a mediator of U snRNA nuclear export whose activity is regulated by phosphorylation. Cell 2000; 101:187 - 98
- Reed R, Magni K. A new view of mRNA export: separating the wheat from the chaff. Nat Cell Biol 2001; 3:201 - 4
- Segref A, Sharma K, Doye V, Hellwig A, Huber J, Luhrmann R, et al. Mex67p, a novel factor for nuclear mRNA export, binds to both poly(A)+ RNA and nuclear pores. EMBO J 1997; 16:3256 - 71
- Kadowaki T, Hitomi M, Chen S, Tartakoff AM. Nuclear mRNA accumulation causes nucleolar fragmentation in yeast mtr2 mutant. Mol Biol Cell 1994; 5:1253 - 63
- Santos-Rosa H, Moreno H, Simos G, Segref A, Fahrenkrog B, Pante N, et al. Nuclear mRNA export requires complex formation between Mex67p and Mtr2p at the nuclear pores. Mol Cell Biol 1998; 18:6826 - 38
- Strasser K, Hurt E. Yra1p, a conserved nuclear RNA-binding protein, interacts directly with Mex67p and is required for mRNA export. EMBO J 2000; 19:410 - 20
- Stutz F, Bachi A, Doerks T, Braun IC, Seraphin B, Wilm M, et al. REF, an evolutionary conserved family of hnRNP-like proteins, interacts with TAP/Mex67p and participates in mRNA nuclear export. RNA 2000; 6:638 - 50
- Rodrigues JP, Rode M, Gatfield D, Blencowe BJ, Carmo-Fonseca M, Izaurralde E. REF proteins mediate the export of spliced and unspliced mRNAs from the nucleus. Proc Natl Acad Sci USA 2001; 98:1030 - 5
- Zhou Z, Luo MJ, Straesser K, Katahira J, Hurt E, Reed R. The protein Aly links pre-messenger-RNA splicing to nuclear export in metazoans. Nature 2000; 407:401 - 5
- Strasser K, Hurt E. Splicing factor Sub2p is required for nuclear mRNA export through its interaction with Yra1p. Nature 2001; 413:648 - 52
- Luo ML, Zhou Z, Magni K, Christoforides C, Rappsilber J, Mann M, et al. Pre-mRNA splicing and mRNA export linked by direct interactions between UAP56 and Aly. Nature 2001; 413:644 - 7
- Le Hir H, Izaurralde E, Maquat LE, Moore MJ. The spliceosome deposits multiple proteins 20–24 nucleotides upstream of mRNA exon-exon junctions. EMBO J 2000; 19:6860 - 9
- Tange TO, Nott A, Moore MJ. The ever-increasing complexities of the exon junction complex. Curr Opin Cell Biol 2004; 16:279 - 84
- Gatfield D, Izaurralde E. REF1/Aly and the additional exon junction complex proteins are dispensable for nuclear mRNA export. J Cell Biol 2002; 159:579 - 88
- Longman D, Johnstone IL, Caceres JF. The Ref/Aly proteins are dispensable for mRNA export and development in Caenorhabditis elegans.. RNA 2003; 9:881 - 91
- Preker PJ, Kim KS, Guthrie C. Expression of the essential mRNA export factor Yra1p is autoregulated by a splicing-dependent mechanism. RNA 2002; 8:969 - 80
- Huang Y, Wimler KM, Carmichael GG. Intronless mRNA transport elements may affect multiple steps of pre-mRNA processing. EMBO J 1999; 18:1642 - 52
- Huang Y, Steitz JA. Splicing factors SRp20 and 9G8 promote the nucleocytoplasmic export of mRNA. Mol Cell 2001; 7:899 - 905
- Nojima T, Hirose T, Kimura H, Hagiwara M. The interaction between cap-binding complex and RNA export factor is required for intronless mRNA export. J Biol Chem 2007; 282:15645 - 51
- Fuke H, Ohno M. Role of poly (A) tail as an identity element for mRNA nuclear export. Nucleic Acids Res 2008; 36:1037 - 49
- Taniguchi I, Ohno M. ATP-dependent recruitment of export factor Aly/REF onto intronless mRNAs by RNA helicase UAP56. Mol Cell Biol 2008; 28:601 - 8
- Masuyama K, Taniguchi I, Kataoka N, Ohno M. SR proteins preferentially associate with mRNAs in the nucleus and facilitate their export to the cytoplasm. Genes Cells 2004; 9:959 - 65
- D’Santos CS, Clarke JH, Divecha N. Phospholipid signalling in the nucleus. Een DAG uit het leven van de inositide signalering in de nucleus. Biochim Biophys Acta 1998; 1436:201 - 32
- Bacqueville D, Deleris P, Mendre C, Pieraggi MT, Chap H, Guillon G, et al. Characterization of a G protein-activated phosphoinositide 3-kinase in vascular smooth muscle cell nuclei. J Biol Chem 2001; 276:22170 - 6
- Gillooly DJ, Morrow IC, Lindsay M, Gould R, Bryant NJ, Gaullier JM, et al. Localization of phosphatidylinositol 3-phosphate in yeast and mammalian cells. EMBO J 2000; 19:4577 - 88
- Yokogawa T, Nagata S, Nishio Y, Tsutsumi T, Ihara S, Shirai R, et al. Evidence that 3'-phosphorylated polyphosphoinositides are generated at the nuclear surface: use of immunostaining technique with monoclonal antibodies specific for PI 3,4-P(2). FEBS Lett 2000; 473:222 - 6
- Gozani O, Karuman P, Jones DR, Ivanov D, Cha J, Lugovskoy AA, et al. The PHD finger of the chromatin-associated protein ING2 functions as a nuclear phosphoinositide receptor. Cell 2003; 114:99 - 111
- Rana RA, Cataldi A, Di Pietro R, Mazzotti G, Centurione L, Robuffo I, et al. Evidence for an early and transient involvement of nuclear inositol lipids in subcellular signalling events related to DNA repair processes. Cell Signal 1994; 6:475 - 80
- York JD, Majerus PW. Nuclear phosphatidylinositols decrease during S-phase of the cell cycle in HeLa cells. J Biol Chem 1994; 269:7847 - 50
- Cocco L, Gilmour RS, Ognibene A, Letcher AJ, Manzoli FA, Irvine RF. Synthesis of polyphosphoinositides in nuclei of Friend cells. Evidence for polyphosphoinositide metabolism inside the nucleus which changes with cell differentiation. Biochem J 1987; 248:765 - 70
- Boronenkov IV, Loijens JC, Umeda M, Anderson RA. Phosphoinositide signaling pathways in nuclei are associated with nuclear speckles containing pre-mRNA processing factors. Mol Biol Cell 1998; 9:3547 - 60
- Mellman DL, Gonzales ML, Song C, Barlow CA, Wang P, Kendziorski C, et al. PtdIns4,5P2-regulated nuclear poly(A) polymerase controls expression of select mRNAs. Nature 2008; 451:1013 - 7
- Ye K, Hurt KJ, Wu FY, Fang M, Luo HR, Hong JJ, et al. Pike. A nuclear gtpase that enhances PI3kinase activity and is regulated by protein 4.1N. Cell 2000; 103:919 - 30
- Ye K, Aghdasi B, Luo HR, Moriarity JL, Wu FY, Hong JJ, et al. Phospholipase C gamma 1 is a physiological guanine nucleotide exchange factor for the nuclear GTPase PIKE. Nature 2002; 415:541 - 4
- Ahn JY, Liu X, Cheng D, Peng J, Chan PK, Wade PA, et al. Nucleophosmin/B23, a nuclear PI(3,4,5)P(3) receptor, mediates the antiapoptotic actions of NGF by inhibiting CAD. Mol Cell 2005; 18:435 - 45
- Okada M, Jang SW, Ye K. Akt phosphorylation and nuclear phosphoinositide association mediate mRNA export and cell proliferation activities by ALY. Proc Natl Acad Sci USA 2008; 105:8649 - 54
- Xuan Nguyen TL, Choi JW, Lee SB, Ye K, Woo SD, Lee KH, et al. Akt phosphorylation is essential for nuclear translocation and retention in NGF-stimulated PC12 cells. Biochem Biophys Res Commun 2006; 349:789 - 98
- Arden KC, Biggs WH 3rd. Regulation of the FoxO family of transcription factors by phosphatidylinositol-3 kinase-activated signaling. Arch Biochem Biophys 2002; 403:292 - 8
- Huang WC, Chen CC. Akt phosphorylation of p300 at Ser-1834 is essential for its histone acetyltransferase and transcriptional activity. Mol Cell Biol 2005; 25:6592 - 602
- Tange TO, Shibuya T, Jurica MS, Moore MJ. Biochemical analysis of the EJC reveals two new factors and a stable tetrameric protein core. RNA 2005; 11:1869 - 83
- Jang SW, Yang SJ, Ehlen A, Dong S, Khoury H, Chen J, et al. Serine/arginine protein-specific kinase 2 promotes leukemia cell proliferation by phosphorylating acinus and regulating cyclin A1. Cancer Res 2008; 68:4559 - 70
- Sahara S, Aoto M, Eguchi Y, Imamoto N, Yoneda Y, Tsujimoto Y. Acinus is a caspase-3-activated protein required for apoptotic chromatin condensation. Nature 1999; 401:168 - 73
- Hu Y, Yao J, Liu Z, Liu X, Fu H, Ye K. Akt phosphorylates acinus and inhibits its proteolytic cleavage, preventing chromatin condensation. EMBO J 2005; 24:3543 - 54
- Ahn JY, Liu X, Liu Z, Pereira L, Cheng D, Peng J, et al. Nuclear Akt associates with PKC-phosphorylated Ebp1, preventing DNA fragmentation by inhibition of caspase-activated DNase. EMBO J 2006; 25:2083 - 95
- Stamm S. Signals and their transduction pathways regulating alternative splicing: a new dimension of the human genome. Hum Mol Genet 2002; 11:2409 - 16
- Blaustein M, Pelisch F, Coso OA, Bissell MJ, Kornblihtt AR, Srebrow A. Mammary epithelial-mesenchymal interaction regulates fibronectin alternative splicing via phosphatidylinositol 3-kinase. J Biol Chem 2004; 279:21029 - 37
- Swaminathan S, Kile AC, MacDonald EM, Koepp DM. Yra1 is required for S phase entry and affects Dia2 binding to replication origins. Mol Cell Biol 2007; 27:4674 - 84
- Jones SM, Klinghoffer R, Prestwich GD, Toker A, Kazlauskas A. PDGF induces an early and a late wave of PI3-kinase activity, and only the late wave is required for progression through G1. Curr Biol 1999; 9:512 - 21
- Dangi S, Cha H, Shapiro P. Requirement for phosphatidylinositol-3 kinase activity during progression through S-phase and entry into mitosis. Cell Signal 2003; 15:667 - 75
- Martelli AM, Manzoli L, Cocco L. Nuclear inositides: facts and perspectives. Pharmacol Ther 2004; 101:47 - 64
- Clarke JH, Letcher AJ, D’Santos CS, Halstead JR, Irvine RF, Divecha N. Inositol lipids are regulated during cell cycle progression in the nuclei of murine erythroleukaemia cells. Biochem J 2001; 357:905 - 10
- Anderson RA, Boronenkov IV, Doughman SD, Kunz J, Loijens JC. Phosphatidylinositol phosphate kinases, a multifaceted family of signaling enzymes. J Biol Chem 1999; 274:9907 - 10
- Osborne SL, Thomas CL, Gschmeissner S, Schiavo G. Nuclear PtdIns(4,5)P2 assembles in a mitotically regulated particle involved in pre-mRNA splicing. J Cell Sci 2001; 114:2501 - 11
- Richardson CJ, Broenstrup M, Fingar DC, Julich K, Ballif BA, Gygi S, et al. SKAR is a specific target of S6 kinase 1 in cell growth control. Curr Biol 2004; 14:1540 - 9
- Ma XM, Yoon SO, Richardson CJ, Julich K, Blenis J. SKAR links pre-mRNA splicing to mTOR/S6K1-mediated enhanced translation efficiency of spliced mRNAs. Cell 2008; 133:303 - 13
- Rodriguez MS, Dargemont C, Stutz F. Nuclear export of RNA. Biol Cell 2004; 96:639 - 55
- York JD, Odom AR, Murphy R, Ives EB, Wente SR. A phospholipase C-dependent inositol polyphosphate kinase pathway required for efficient messenger RNA export. Science 1999; 285:96 - 100
- York JD. Regulation of nuclear processes by inositol polyphosphates. Biochim Biophys Acta 2006; 1761:552 - 9
- Odom AR, Stahlberg A, Wente SR, York JD. A role for nuclear inositol 1,4,5-trisphosphate kinase in transcriptional control. Science 2000; 287:2026 - 9
- Lund MK, Guthrie C. The DEAD-box protein Dbp5p is required to dissociate Mex67p from exported mRNPs at the nuclear rim. Mol Cell 2005; 20:645 - 51
- Tran EJ, Zhou Y, Corbett AH, Wente SR. The DEAD-box protein Dbp5 controls mRNA export by triggering specific RNA:protein remodeling events. Mol Cell 2007; 28:850 - 9
- Alcazar-Roman AR, Tran EJ, Guo S, Wente SR. Inositol hexakisphosphate and Gle1 activate the DEAD-box protein Dbp5 for nuclear mRNA export. Nat Cell Biol 2006; 8:711 - 6
- Watkins JL, Murphy R, Emtage JL, Wente SR. The human homologue of Saccharomyces cerevisiae Gle1p is required for poly(A)+ RNA export. Proc Natl Acad Sci USA 1998; 95:6779 - 84