Abstract
Previous work led to the hypothesis that SRrp86, a related member of the SR protein superfamily, can interact with and modulate the activity of other SR proteins. Here, we sought to test this hypothesis by examining the effect of changing SRrp86 concentrations on overall alternative splicing patterns. SpliceArrays were used to examine 9,854 splicing events in wild-type cells, cells overexpressing SRrp86, and cells treated with siRNAs to knockdown SRrp86. From among the 500 splicing events exhibiting altered splicing under these conditions, the splicing of c-Jun and IκBβ were validated as being regulated by SRrp86 resulting in altered regulation of their downstream targets. In both cases, functionally distinct isoforms were generated that demonstrate the role SRrp86 plays in controlling alternative splicing.
Introduction
Eukaryotic pre-mRNA transcripts contain coding and noncoding regions called exons and introns, respectively.Citation1,Citation2 To encode functional proteins, the noncoding regions must be removed and the coding regions spliced together. Genes that contain multiple exons can be spliced to form many different mature transcripts via alternative splicing producing tissue- and developmental stage-specific transcripts.Citation3,Citation4 Transcriptome-wide analyses have concluded that about 95% of human genes undergo alternative splicing.Citation5,Citation6
The process of spliceosome formation and the identification of splice sites is directed by cis-acting elements, including the 5′ splice site, 3′ splice site, polypyrimidine tract and branch point sequence. Because splice sites are short and degenerate, the spliceosome is faced with the challenge of efficiently and precisely identifying correct splice sites which is all the more remarkable because most transcripts contain a large number of potential splice sites, some of which are actually better matches to canonical splice sites than the correct ones.Citation1,Citation7–Citation9 In addition, introns can be 20 times longer than their neighboring exons, especially in higher eukaryotes.Citation10–Citation12 Thus, while canonical splice sites are necessary to identify correct exon/intron boundaries, they are not sufficient to prevent the use of incorrect sites. To overcome this, additional factors are needed to direct the spliceosome to bona fide splice sites. These factors bind to a variety of mostly short and highly degenerate RNA sequences. The combination of multiple splicing components binding to weak RNA sequences underscores the challenge faced by the spliceosome to maintain fidelity and prevent aberrant splicing yet, at the same time, enable differential splice site choice and alternative splicing.
One of the best characterized RNA elements that help to define splice sites are referred to as splicing enhancers.Citation13 Splicing enhancers are purine-rich sequences found in both exons and introns and are recognized by trans-acting splicing factors, mostly SR proteins. SR proteins contain one or two N-terminal RNA recognition motifs (RRM) and a C-terminal domain rich in serine and arginine dipeptides (RS domain).Citation14 The RRM imparts substrate specificity through sequence-specific RNA binding ability. The RS domain participates in protein-protein interactions that guide spliceosomal components to splice sites and helps define exon boundaries in a phosphorylation-dependent manner. SR proteins are required for the identification of constitutive splice sites and also for regulated alternative splicing decisions.Citation15 RS domain-splicing factor interactions promote U2 binding to the branch point sequence and help recruit the U4/U6-U5 tri-snRNP to the spliceosome.Citation16 Likewise, the SR proteins ASF/SF2 and SC35 interact with the U1 snRNP protein component, U1-70K, and with U2AFCitation35 thus physically bridging 3′ and 5′ splice sites.Citation17–Citation22 The formation of such bridge complexes across both exons and introns is crucial to identify splice sites. Initial work studying splicing with simple two exon, one intron substrates suggested that splice site identification occurs across introns.Citation23–Citation26 However, exon definition best explains the finding that mutation of an internal 5′ splice site can inhibit splicing of the upstream intron resulting in exon skipping rather than intron retention.Citation27 Additionally, 3′ splice sites of internal exons are not efficiently recognized if there is no 5′ splice site at the other end of the exon.Citation28 Together, it appears that the formation of bridge complexes, across both exons and introns, is key to accurate identification of splice sites.
Previously, we identified an SR-related member of the SR protein superfamily, SRrp86, and showed that it can alter the activity of core SR proteins through direct protein-protein interaction.Citation29–Citation32 SRrp86 has an overall structure similar to SR proteins with one N-terminal RRM and two C-terminal RS domains but it differs from core SR proteins by activity and by the presence of an EK-rich domain separating the two RS domains. The EK domain appears to mimic a phosphorylated RS domain, consistent with experiments showing that EK domain peptides inhibit spliceosome formation and splicing in vitro and that fusion of the EK domain to SRp75 inhibits its activity.Citation31 SRrp86 appears to modulate the function of other SR proteins as both a mediator and repressor of bridge complex formation through its RS domains. If this hypothesis is correct, then the concentration of SRrp86 should significantly affect global splicing patterns. Here, we sought to broadly test the effect of changing the concentration of SRrp86 on alternative splicing.
Results
Overexpression and knockdown of SRrp86.
To determine the effect of altered levels of SRrp86 on alternative splicing, we chose to isolate RNA from two different cell lines (HeLa and H1299) following either overexpression or knockdown of SRrp86 (). For overexpression, a rat SRrp86 expression vector was transfected into cells and proteins were isolated after 48 hours. For knockdown, siRNAs targeting SRrp86 were transfected into cells after which proteins were isolated. Neither treatment caused any noticeable phenotype. Protein samples from overexpression, knockdown and control cells were analyzed by western blot using a rabbit polyclonal antibody directed towards SRrp86. As shown, the knockdown in both cell lines consistently resulted in loss of at least 90% of the endogenous protein. Overexpression of SRrp86 resulted in an approximate 5–20-fold increase in SRrp86 levels.
SpliceArray analysis of global splicing regulated by SRrp86.
To analyze the global effects of changing concentrations of SRrp86 on alternative splicing, we used microarrays designed by ExonHit with 50,909 probes that recognize 9,854 alternative splicing events in 1,160 genes. We chose probes specifc for genes involved in calcium-mediated signaling, cancer, the MAPKKK pathway, and receptor protein tyrosine kinase signaling, as well as genes in the ribonucleoprotein family.
For each event, probes detect novel and constitutive exon sequences, exon-exon junctions, exon-intron junctions, and invariant exonic sequences (). The invariant probes control for expression level changes. If signals obtained using spliced isoform-specific probes change to the same magnitude that an invariant probe signal changes, the event is likely the result of altered mRNA expression levels rather than an alternative splicing event. This occurred in approximately 50% of the genes with significant probes. We ignored such events and focused on splicing events that appeared to be unrelated to expression levels.
On average, splicing changes were detected in about 10% of the 1,160 genes on the array for approximately 500 alternative splicing events. Examination of the differentially spliced genes affected by SRrp86 showed a wide spectrum of gene function including cell cycle regulation, RNA binding and processing, transcription regulation, cell signaling and apoptosis. After applying the criteria above, we narrowed the splicing changes to 32 events which changed under the different conditions. Based on the overall agreement of the probe set for each event, this meant a change in an isoform-specific probe and no change in a non-specific probe ( and , all significant probes are available in Suppl. Tables 1–4). Interestingly, most of the events we detected were cell-type specific; only four events were detected in both cell lines (CDKN1A, COX6C, MDM2, SHC1). This likely reflects the fact that both cell lines express a distinct set of splicing factors and other RNA binding proteins that can modulate the effects of changing levels of SRrp86. Nevertheless, based on these events, changing concentrations of SRrp86 result in the activation some splicing events while repressing others. This agrees with the a role for SRrp86 in activating certain SR proteins and inhibiting others.
SRrp86 regulates NFκB signaling by altering IκBβ splicing.
To validate the array results, we directly tested the splicing of two genes in H1299 cells, IκBβ (gene name NFKBIB) and c-Jun (gene name JUN). We would have preferred to analyze events that occurred in both cell types but unfortunately, none were previously characterized or shown to functionally alter alternatively spliced isoforms. Thus, we first chose to examine the splicing behavior of IκBβ since the SpliceArray showed a reciprocal relationship between splicing and either overexpression or knockdown of SRrp86 in H1299 cells and because we could analyse the functional consequences of the two splice choices. IκBβ isoforms play a key role in regulating NFκB signaling. The NFκB family of transcription factors are sequestered in the cytoplasm by the inhibitor of κB proteins, IκBα and IκBβ.Citation33,Citation34 In response to activating stimuli, the IκB proteins are phosphorylated by the Iκ kinases and degraded by the proteasome.Citation35 This frees NFκB to enter the nucleus and activate transcription of target genes. In human cells, the IκBβ gene produces two proteins by alternative splicing ().Citation36 The two proteins differ in their C-termini, with the smaller protein, IκBβ2, being produced by an intron retention event. The SpliceArray indicated that overexpression of SRrp86 stimulated removal of this intron and therefore production of the larger protein, IκBβ1 (). In contrast, knock down of SRrp86 had the opposite effect with more retention of the intron and consequent increased relative production of the IκBβ2 isoform. These two isoforms differ in their stringency of NFκB inhibition and in their ability to be degraded in response to NFκB-activating stimuli.Citation36 Thus, SRrp86 levels should have profound effects on NFκB activity.
To direcly test the effect of changing SRrp86 levels on NFκB, we utilized a luciferase reporter system and TNFα treatment to analyze stimulated and unstimulated NFκB activity. Upon stimulation, IκBβ1 is rapidly degraded whereas IκBβ2 is only weakly degraded which serves to limit the activity of NFκB.Citation36 Thus, a balance between these two isoforms, in addition to the function of other IκB proteins, enables precise regulation of NFκB activity.
From the SpliceArray data, overexpression of SRrp86 resulted in loss of IκBβ2 expression, which should increase basal NFκB activity. Knockdown of SRrp86 resulted in the loss of IκBβ1 which should decrease basal NFκB activity. To directly test these predictions, cells were transfected with the luciferase reporter in the presence or absence of either SRrp86 for overexpression or siRNAs to knockdown SRrp86. After transfection, cells were stimulated by treatment with TNFα and analyzed for luciferase activity (). As shown, overexpression of SRrp86 increased basal NFκB activity relative to control levels (). This result is consistent with the SpliceArray and western data showing a loss of IκBβ2 expression upon overexpression of SRrp86. After treatment with TNFα increased NFκB activity was detected upon overexpression of SRrp86. Here, the decrease in the relative levels of IκBβ2 isoform after SRrp86 overexpression serves to increase activation upon stimulation. On the other hand, knockdown of SRrp86 had the opposite effect with decreased basal NFκB activity relative to control, consistent with the proposed role for IκBβ1. Knockdown of SRrp86 also caused decreased stimulated NFκB activity relative to control levels due to increased expression of IκBβ2, which is only weakly degraded upon stimulation and therefore sequesters NFκB in the cytoplasm. Interestingly, the fold change upon induction with TNFα remained similar regardless of SRrp86 levels. Also, it is of note that the level of NFκB activity in stimulated control cells could be achieved simply by overexpressing SRrp86 without TNFα treatment. Thus, altered SRrp86 levels can have dramatic effects on NFκB signaling irrespective of other signaling cascades.
SRrp86 regulates c-Jun splicing and function.
Given the important role that NFκB plays in regulating signaling and cell growth, the second splicing event we chose to explore further was a unique splicing event detected in cells overexpressing SRrp86 that creates a functionally distinct isoform of c-Jun. In this case, we did not observe a reciprocal splicing effect dependent on SRrp86 levels but we did observe a novel splicing event due to SRrp86 that would be predicted to alter the activity of this oncogene. c-Jun is the central component of the Activator Protein 1 (AP-1) family and is required for normal development.Citation37–Citation39 As an oncogene, it has increased activity in tumors.Citation40,Citation41 c-Jun, a one exon gene, is normally not spliced. However, the SpliceArray detected an unusual novel intron splicing event upon overexpression of SRrp86. This event results in loss of half of the 5′ UTR and 75% of the open reading frame which prevents production of c-Jun protein (). To confirm the loss of c-Jun protein levels, western blots were performed on proteins isolated from H1299 cells transfected with vectors driving expression of SRrp86. As shown, overexpression of SRrp86 resulted in the loss of c-Jun protein expression, consistent with the SpliceArray results (). The lack of any effect of SRrp86 knockdown on c-Jun protein levels indicates that there is a threshold of SRrp86 required to affect cJun splicing and that the endogenous level in these cells is below that threshold.
Since c-Jun is the main component of AP-1 transcription factor complexes, loss of c-Jun expression should result in decreased AP-1 activity. We directly tested this using a reporter construct in which luciferase expression was placed under the control of an AP-1-regulated promoter. Upon overexpression of SRrp86, loss of c-Jun activity should reduce luciferase levels. Thus, we transfected cells with the AP-1-regulated luciferase reporter alone or in conjunction with the SRrp86 expression vector and then analyzed the cellular lysates for luciferase activity (). As shown, overexpression of SRrp86 resulted in a 50% decrease in AP-1 activity as compared to AP-1 activity in the presence of endogenous levels of SRrp86 (). This is consistent with both the SpliceArray data and the western blots shown above and indicates that cells which exhibit increased expression of SRrp86 should show decreased expression of c-Jun, and more specifically, downstream AP-1 target genes.
Discussion
SRrp86 as a global regulator of splicing.
The SpliceArray technology provides a powerful means to systematically detect splice variants and perform transcriptome profiling. The combination of exon-exon junction, exon body and exon-intron junction probes allows detection of multiple alternative splicing events across many genes. This allows expression differences to be monitored under any combination of conditions to gain a deeper understanding of gene expression. Here, we were interested in analyzing how SRrp86 concentrations affect alternative splicing. Upon overexpression and knockdown of SRrp86 in two human cell lines, we detected numerous splicing changes. Because this particular array examines only a subset of genes, it is likely that other splicing events regulated by SRrp86 will be discovered in the future. Despite this limitation, we discovered many altered splicing events dependent on the concentration of SRrp86. These results are consistent with a genome-wide RNAi screen in C. elegans that showed that knock down of the SRrp86 homolog, rsp-7, results in embryonic lethality and a slow growth phenotype.Citation42 In addition, these results are also consistent with previous data indicating that SRrp86 regulates the function of core SR proteins, both positively and negatively.
The most common types of alternative splicing events detected upon changes in SRrp86 levels were novel exons, exon skipping and novel introns. The least common type of event detected was intron retention. Whether SRrp86 is directly or indirectly regulating all of these splicing events is unknown. For example, changing levels of SRrp86 led to altered splicing of transcripts that encode proteins that are themselves regulators of splicing (hnRNP C and hnRNP L). Additionally, western blots performed using an SR protein family specific antibody revealed that the relative ratios of different SR proteins were not affected by more than 10% by SRrp86 expression level. However, there was an overall decrease in SR protein expression upon knockdown of SRrp86 (data not shown). Thus, some of the splicing events detected by the array could be a result of altered hnRNP C or hnRNP L function or secondary effects of altered SR protein expression and not directly related to the change in SRrp86 expression level. Nevertheless, the results clearly illustrate how changing the concentration of one splicing regulator can affect numerous splicing decisions and have dramatic functional consequences.
SRrp86 and c-Jun function.
c-Jun is the central component of the AP-1 transcription factor family, whose members are basic leucine-zipper (bZIP) proteins, and is involved in transformation, tumor aggressiveness, cell cycle progression, differentiation and apoptosis.Citation43 AP-1 is activated and c-Jun induced, both at the transcriptional and posttranslational levels, in response to growth factors, chemokines, extracellular matrix, UV light, proinflammatory cytokines, neurotransmitters and other oncogene-mediated signal transduction networks.Citation43
SRrp86 is expressed differentially across various cell types. In rat tissue, SRrp86 is highly expressed in the brain and testis but has low expression in the liver and undetecteable levels in the kidney.Citation29 Thus, SRrp86 may play a role in regulating tissue specific c-Jun activity. Interestingly, c-Jun plays a proapoptotic role in the brain where SRrp86 is highly expressed.Citation29 SRrp86 could be involved in regulating c-Jun levels in neurons to prevent inappropriate induction of apoptosis.
As further study investigates the role of c-Jun in development and disease, the role of SRrp86 in regulating c-Jun expression will become clear as will the significance of c-Jun alternative splicing. Additionally, the possibility remains that SRrp86 could itself be a target gene of c-Jun. If that is in fact the case, then SRrp86 and c-Jun could form a negative feedback loop with each protein regulating the other with the ultimate goal of preventing overexpression of c-Jun.
SRrp86 in NFκB signaling.
The NFκB family of transcription factors respond to cytokines, growth factors and stress stimuli while the majority of NFκB target genes are involved in the immune response, inflammation, cell adhesion, proliferation and survival.Citation44 The inhibitor of κB (IκB) proteins contain ankyrin-like repeats that allow them to bind NFκB proteins and retain them in the cytoplasm by blocking their nuclear localization signals.Citation34 In response to stimuli, the IκB proteins are phosphorylated, ubiquitinated and degraded by the proteasome.Citation45–Citation48 Destruction of IκB allows NFκB to enter the nucleus and regulate gene expression. While IκBα and IκBβ have similar biochemical functions, they also have many differences. IκBα has a nuclear export sequence and only blocks the nuclear localization sequence on one of the proteins in the dimer.Citation49,Citation50 This property allows it to shuttle between the nucleus and cytoplasm but maintain a predominantly cytoplasmic localization.Citation51 IκBβ lacks a nuclear export sequence and blocks the nuclear localization sequences on both dimeric NFκB proteins.Citation51–Citation54 IκBα can dislodge NFκB from DNA but IκBβ cannot.Citation52,Citation55 However, IκBβ can bind NFκB-DNA complexes and prevent IκBα from dislodging NFκB.
The role of each of the two IκBβ isoforms is supported by the change in NFκB activity observed upon overexpression and knock down of SRrp86. Overexpression of SRrp86 resulted in expression of IκBβ1 leading to increased NFκB activity. Knock down of SRrp86 caused increased relative IκBβ2 expression leading to both decreased NFκB constitutive and stimulated activity. Dissecting the role that regulation of this splicing event might play in development and disease is not entirely clear. Understanding the regulation of SRrp86 itself in different cell types and environments and understanding its role in regulating NFκB in different scenarios is imperative to a full understanding of the consequences of differential splicing of IκBβ.
Materials and Methods
Microarrays.
For overexpression analysis, Hela and H1299 cells were transfected with a flag-tagged SRrp86 (rat) expression construct using TransIT LT1 (Mirus). For knockdown, the same cell lines were transfected with siRNAs (20 nM) targeting human SRrp86 (5′-GGC AGG GGG UCC UCG TCU UUA-3′) for four consecutive days using TransIT TKO (Mirus). Total RNA was harvested using TRI reagent (Molecular Research Center) according to the manufacturer's protocol and stored in ethanol at −20°C.
ExonHit Therapeutics (Gaithersburg, MD, USA) designed custom Agilent 244K arrays to contain the following gene lists: calcium mediated signaling, cancer genes, MAPKKK pathway, miscellaneous signaling pathways, receptor protein tyrosine kinase signaling, and ribonucleoproteins. The array contains probes to allow detection of a total of 9,854 splicing events in 1,160 genes. To account for potential dye bias, duplicate hybridizations with dye swaps were performed.
The arrays were performed using RNA isolated from HeLa and H1299 cell lines under normal culture conditions. Overexpression and knockdown of SRrp86 was performed identically as for protein isolation (), except total RNA was harvested. The RNA (5 µg total RNA) was then reverse transcribed into first strand cDNA using random primers and indirectly labeled with either Cy3 or Cy5. Each sample was labeled using a dye swap with duplicate hybridizations to control for potential dye bias. The cDNA product was purified and equal amounts of labeled material were used for hybridization to the array following Agilent's Two-Color Microarray-Based Gene Expression Analysis manual (document # G4140-90050). After hybridization and extensive washing, the arrays were scanned and the signals were background subtracted and dye normalized. Processed signals were quantile normalized across arrays for comparison. ANOVA analyses (p < 0.0001) were performed to compile statistically significant changes. Finally, each splicing event was analyzed to rule out changes detected due to transcript expression level differences rather than splicing changes.
Arrays were scanned using Agilent's Microarray Scanner. Images were analyzed with the Feature Extraction software, version 9.1.3 (FE9.1.3), using all default settings. Data files were analyzed at ExonHit using Partek® Genomics Suite. The processed signals (background subtracted, dye normalized, floored values) were imported into Partek from the Agilent Feature Extraction data file. Data were quantile normalized across arrays. An ANOVA analysis was performed using log-transformed data to select significant probes (p < 0.0001), and two-way comparisons between control and treated groups were performed.
Western analysis.
Proteins were separated by SDS-PAGE and transferred to PVDF membranes. For detection of SRrp86, membranes were blocked for 30 min in wash buffer (10 mM Tris (pH 8), 0.9% NaCl and 0.1% Tween-20) containing 5% nonfat dry milk. Membranes were then incubated for 1 h with rabbit polyclonal anti-SRrp86 antibodyCitation30 diluted 1:10,000 in blocking buffer, washed with blocking buffer, incubated for 1 h in a 1:5,000 dilution of HRP-conjugated anti-rabbit antibody (Amersham), washed again with blocking buffer, and visualized using ECL reagents (Amersham). IκBβ isoforms were detected using a 1:200 dilution of a rabbit polyclonal anti-IκBβ antibody (N-20, Santa Cruz) in blocking buffer. c-Jun was detected using a 1:200 dilution of a rabbit monoclonal antibody (9165, Cell Signaling Technology) in wash buffer with 5% BSA and incubated overnight at 4°C. α-Tubulin was detected using a 1:10,000 dilution of a rabbit polyclonal antibody (ab15246, abcam) in blocking buffer.
Luciferase assays.
H1299 cells were transfected with 2,000 ng of either c-Jun promoter- or NFκB promoter-firefly luciferase plasmid and 500 ng of TK-renilla luciferase plasmid. Cells were treated with TNFα 18 h after transfection, harvested 6 h later, and the lysates either analyzed immediately or frozen at −80°C until analysis. Luciferase expression was analyzed using a dual-luciferase reporter assay system (Promega) and a Fusion plate reader (Perkin Elmer). SRrp86 overexpression experiments were performed with transfection of the SRrp86 expression vector occurring 24 h before transfection of the luciferase plasmids. SRrp86 knockdown experiments were performed with transfection of the siRNA for four consecutive days and the fourth day coinciding with the transfection of the luciferase plasmids. Data were statistically analyzed using paired, 2-tailed t-tests.
Abbreviations
| AP-1 | = | activator protein 1 |
| bZIP | = | basic leucine-zipper |
| cDNA | = | complementary DNA |
| Cy | = | cyanine |
| ECL | = | enhanced chemiluminescence |
| hnRNP | = | heterogeneous nuclear ribonucleoprotein |
| HRP | = | horseradish peroxidase |
| IκB | = | inhibitor κB |
| mM | = | millimolar |
| mRNA | = | messenger RNA |
| NFκB | = | nuclear factor κB |
| PAGE | = | polyacrylamide gel electrophoresis |
| PVDF | = | polyvinylidene fluoride |
| RNAi | = | RNA interference |
| RRM | = | RNA recognition motif |
| SDS | = | sodium dodecyl sulfate |
| siRNA | = | small interfereing RNA |
| SR protein | = | serine/arginine-rich protein |
| TNF | = | tumor necrosis factor |
| UTR | = | untranslated region |
Figures and Tables
Figure 1 Altered Expression of SRrp86. Knockdown of SRrp86 was achieved by transfecting siRNAs using TransIT TKO (Mirus) for four consecutive days. Overexpression was achieved by transfecting a rat SRrp86 expression vector using TransIT LT1 (Mirus). Protein samples were harvested and separated on 8% SDS-PAGE gels, transferred and probed with either a rabbit polyclonal antibody to SRrp86 or an antibody to α-tubulin. (A) SRrp86 knockdown in HeLa cells. (B) SRrp86 knockdown in H1299 cells. (C) SRrp86 overexpression in HeLa cells. (D) SRrp86 overexpression in H1299 cells.
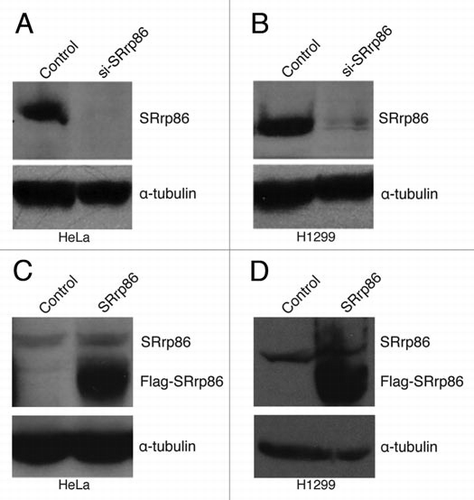
Figure 2 Probe design for the SpliceArray. Six probes were used to detect specific splicing events. Two of the probes, T and F, are designed to detect invariant mRNA regions to control for changes in expression levels. If mRNA detection with these probes changes across conditions, the changes are likely to reflect transcriptional effects and not altered splicing. Probes B, C, D and E span exon-exon junctions or reside entirely within variable exon sequences. Changes in mRNA levels detected with these probes relative to the invariant T and F probes indicate a change in splicing pattern.
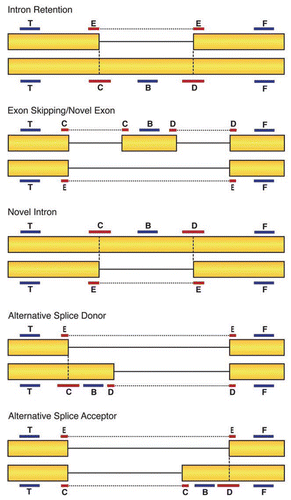
Figure 3 SRrp86 regulates an alternative splicing event in IκBβ and affects NFκB signaling. (A) The SpliceArray detected an alternative intron inclusion event between exons 5 and 6 of the IκBβ gene (NFKBIB). Under normal conditions both isoforms are expressed, but overexpression of SRrp86 promotes splicing of the intron and production of isoform 1. (B) Western blots were performed to detect changes in isoform expression in H1299 cells. Knock down of SRrp86 resulted in increased isoform 2 expression relative to isoform 1. Overexpression of SRrp86 resulted in increased expression of isoform 1 relative to isoform 2. (C) Flow chart outlining the effect SRrp86 expression on luciferase expression. SRrp86 enhances isoform 1 expression which allows for increased levels of basal and TNFá-stimulated NFκB activity. Loss of SRrp86 expression results in decreased levels of isoform 1 while maintaining the repressive activity of isoform 2 with the overall result being decreased NFκB activity. (D) Luciferase assays were performed to determine the effect of changing SRrp86 levels on NFκB signaling. Changes in the ratio of the two IκBβ isoforms due to SRrp86 were monitored using a construct containing the firefly luciferase gene under the control of NFκB. Basal (−) and stimulated (+) levels of NFκB activity were determined after treatment of cells with TNFα for 6 hr. Error bars represent SEM; *represents p < 0.05 compared to unstimulated control and **represents p < 0.05 compared to stimulated control by paired, 2-tailed t-test; n = 4.
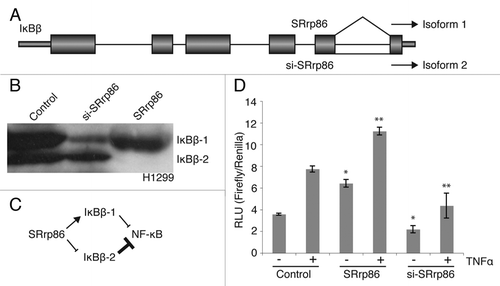
Figure 4 SRrp86 regulates a novel intron event in c-Jun. (A) The SpliceArray detected an aberrant splicing event in c-Jun upon overexpression of SRrp86. Normally, c-Jun produces a single exon mRNA but overexpression of SRrp86 caused removal of part of the 5′ UTR and 75% of the open reading frame. mRNAs resulting from this splicing event are incapable of expressing c-Jun protein. (B) Western blots were performed to analyze the effect of SRrp86 overexpression on c-Jun levels in H1299 cells. Overexpression of SRrp86 resulted in loss of c-Jun expression. Knockdown of SRrp86 had no effect. α-Tubulin was used as a loading control. (C) Flow chart outlining the effect of SRrp86 on luciferase expression using a reporter construct. Briefly, SRrp86 blocks c-Jun function and thus its binding to the AP-1-regulated promoter that drives luciferase expression. (D) Luciferase reporter assays were used to analyze the effect of the loss of c-Jun protein due to SRrp86 overexpression on AP-1 transcription factor complex activity. The AP-1 luciferase construct contains two copies of the AP-1 consensus DNA binding sequence in the pGL-3 promoter vector (Promega). Error bars represent SEM; *represents p < 0.01 by paired, 2-tailed t-test; n = 3.
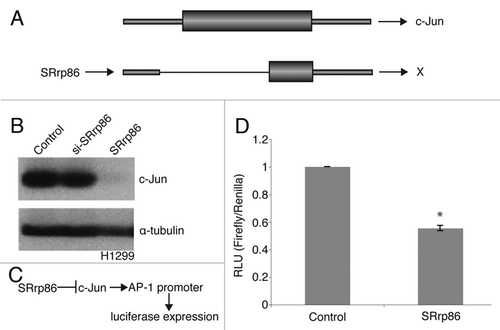
Table 1 SRrp86-regulated alternative splicing events in h1299 cells
Table 2 SRrp86-regulated alternative splicing events in heLa cells
Additional material
Download Zip (213.6 KB)Acknowledgements
This study was supported by an NIH grant to J.G.P. (GM 62487). We would like to thank Ruth Ann Veach (Vanderbilt) for the NFκB luciferase reporter construct and Ann Richmond (Vanderbilt) for the c-Jun luciferase reporter construct.
References
- Burge CB, Tuschl T, Sharp PA. Gesteland RF, Cech TR, Atkins JF. Splicing of precursors to mRNAs by spliceosomes. The RNA World 1999; Cold Spring Harbor, NY Cold Spring Harbor Laboratory Press 525 - 560
- Rio DC. Splicing of pre-mRNA: mechanisms, regulation and role in development. Curr Opin Genet Dev 1993; 3:574 - 584
- Black DL. Mechanisms of alternative pre-messenger RNA splicing. Annu Rev Biochem 2003; 72:291 - 336
- Modrek B, Lee C. A genomic view of alternative splicing. Nat Genet 2002; 30:13 - 19
- Pan Q, Shai O, Lee LJ, Frey BJ, Blencowe BJ. Deep surveying of alternative splicing complexity in the human transcriptome by high-throughput sequencing. Nat Genet 2008; 40:1413 - 1415
- Wang ET, Sandberg R, Luo S, Khrebtukova I, Zhang L, Mayr C, et al. Alternative isoform regulation in human tissue transcriptomes. Nature 2008; 456:470 - 476
- Cartegni L, Chew S, Krainer A. Listening to silence and understanding nonsense: exonic mutations that affect splicing. Nat Rev Genet 2002; 3:285 - 298
- Roca X, Sachidanandam R, Krainer AR. Intrinsic differences between authentic and cryptic 5′ splice sites. Nucleic Acids Res 2003; 31:6321 - 6333
- Sun H, Chasin LA. Multiple splicing defects in an intronic false exon. Mol Cell Biol 2000; 20:6414 - 6425
- Consortium IHGS. Initial sequencing and analysis of the human genome. Nature 2001; 409:860 - 921
- Hawkins JD. A survey on intron and exon lengths. Nuc Acids Res 1988; 16:9893 - 9908
- Venter J, et al. The sequence of the human genome. Science 2001; 291:1304 - 1351
- Blencowe BJ. Exonic splicing enhancers: mechanism of action, diversity and role in human genetic disease. Trends Biochem Sci 2000; 25:106 - 110
- Birney E, Kumar S, Krainer AR. Analysis of the RNA recognition motif and RS and RGG domains: conservation in metazoan pre-mRNA splicing factors. Nuc Acids Res 1993; 21:5803 - 5816
- Schaal TD, Maniatis T. Multiple distinct enhancers inthe protein-coding sequences of a constitutively spliced pre-mRNA. Mol Cell Biol 1999; 19:261 - 273
- Roscigno RF, Garcia-Blanco MA. SR proteins escort the U4/U6 tri-snRNP to the spliceosome. RNA 1995; 1:692 - 706
- Amrein H, Hedley ML, Maniatis T. The role of specific protein-RNA and protein-protein interactions in positive and negative control of pre-mRNA splicing by transformer-2. Cell 1994; 76:735 - 746
- Jamison SF, Pasman Z, Wang J, Will C, Lührmann R, Manley JL, et al. U1 snRNP-ASF/SF2 interaction and 5′ splice site recognition: characterization of required elements. Nuc Acids Res 1995; 23:3260 - 3267
- Kohtz JD, Jamison SF, Will CL, Zuo P, Lührmann R, Garcia-Blanco MA, et al. Protein-protein interactions and 5′ splice site recognition in mammalian mRNA precursors. Nature 1994; 368:119 - 124
- Reed R. Initial splice-site recognition and pairing during pre-mRNA splicing. Curr Opin Gen Dev 1996; 6:215 - 220
- Staknis D, Reed R. SR proteins promote the first specific recognition of pre-mRNA and are present together with the U1 small nuclear ribonucleoprotein particle in a general splicing enhancer complex. Mol Cell Biol 1994; 14:7670 - 7682
- Wang Z, Hoffmann HM, Grabowski PJ. Intrinsic U2AF binding is modulated by exon enhancer signals in parallel with changes in splicing activity. RNA 1995; 1:21 - 35
- Lamond AI, Konarska MM, Sharp PA. A mutational analysis of spliceosome assembly: evidence for splice site collaboration during spliceosome formation. Genes Dev 1987; 1:532 - 143
- Michaud S, Reed R. A functional association between the 5′ and 3′ splice site is established in the earliest prespliceosome complex (E) in mammals. Genes Dev 1993; 7:1008 - 1020
- Ruby SW, Abelson J. Pre-mRNA splicing in yeast. TIGS 1991; 7:79 - 85
- Wu JY, Maniatis T. Specific interactions between proteins implicated in splice site selection and regulated alternative splicing. Cell 1993; 75:1061 - 1070
- Talerico M, Berget SM. Effect of 5′ splice site mutations on splicing of the preceding intron. Mol Cell Biol 1990; 10:6299 - 6305
- Robberson BL, Cote G, Berget SM. Exon definition may facilitate splice site selection in RNAs with multiple exons. Mol Cell Biol 1990; 10:84094
- Barnard D, Li J, Peng R, Patton JG. Regulation of alternative splicing by SRrp86 through co-activation and repression of specific SR proteins. RNA 2002; 8:526 - 533
- Barnard DC, Patton JG. Identification and characterization of a novel serine-arginine rich splicing regulatory protein. Mol Cell Biol 2000; 20:3049 - 3057
- Li J, Barnard DC, Patton JG. A unique glutamic acidlysine (EK) domain acts as a splicing inhibitor. J Biol Chem 2002; 277:39485 - 39492
- Li J, Hawkins IC, Harvey CD, Jenning JL, Link AJ, Patton JG. Regulation of alternative splicing by SRrp86 and its interacting proteins. Mol Cell Biol 2003; 23:7437 - 7447
- Baeuerle PA, Baltimore D. IkappaB: a specific inhibitor of the NFkappaB transcription factor. Science 1988; 242:540 - 546
- Beg AA, Baldwin AS Jr. The IkappaB proteins: multifunctional regulators of Rel/NFkappaB transcription factors. Genes Dev 1993; 7:2064 - 2070
- Scheidereit C. IkappaB kinase complexes: gateways to NFkappaB activation and transcription. Oncogene 2006; 25:6685 - 6705
- Hirano F, Chung M, Tanaka H, Maruyama N, Makino I, Moore DD, et al. Alternative splicing variants of IkappaB beta establish differential NFkappaB signal responsiveness in human cells. Mol Cell Biol 1998; 18:2596 - 2607
- Hilberg F, Aguzzi A, Howells N, Wagner EF. c-jun is essential for normal mouse development and hepatogenesis. Nature 1993; 365:179 - 181
- Johnson RS, van Lingen B, Papaioannou VE, Spiegelman BM. A null mutation at the c-jun locus causes embryonic lethality and retarded cell growth in culture. Genes Dev 1993; 7:1309 - 1317
- Karin M. The regulation of AP-1 activity by mitogen-activated protein kinases. J Biol Chem 1995; 270:16483 - 16486
- Ritke MK, Bergoltz VV, Allan WP, Yalowich JC. Increased c-jun/AP-1 levels in etoposide-resistant human leukemia K562 cells. Biochem Pharmacol 1994; 48:525 - 533
- Szabo E, Riffe ME, Steinberg SM, Birrer MJ, Linnoila RI. Altered cJUN expression: an early event in human lung carcinogenesis. Cancer Res 1996; 56:305 - 315
- Simmer F, Moorman C, van der Linden AM, Kuijk E, van den Berghe PV, Kamath RS, et al. Genome-wide RNAi of C. elegans using the hypersensitive rrf-3 strain reveals novel gene functions. PLoS Biol 2003; 1:12
- Eferl R, Wagner EF. AP-1: a double-edged sword in tumorigenesis. Nat Rev Cancer 2003; 3:859 - 868
- Hayden MS, Ghosh S. Signaling to NFkappaB. Genes Dev 2004; 18:2195 - 2224
- DiDonato JA, Mercurio F, Karin M. Phosphorylation of IkappaBalpha precedes but is not sufficient for its dissociation from NFkappaB. Mol Cell Biol 1995; 15:1302 - 1311
- DiDonato J, Mercurio F, Rosette C, Wu-Li J, Suyang H, Ghosh S, et al. Mapping of the inducible IkappaB phosphorylation sites that signal its ubiquitination and degradation. Mol Cell Biol 1996; 16:1295 - 1304
- Chen Z, Hagler J, Palombella VJ, Melandri F, Scherer D, Ballard D, et al. Signal-induced site-specific phosphorylation targets IkappaBalpha to the ubiquitin-proteasome pathway. Genes Dev 1995; 9:1586 - 1597
- Scherer DC, Brockman JA, Chen Z, Maniatis T, Ballard DW. Signal-induced degradation of IkappaBalpha requires site-specific ubiquitination. Proc Natl Acad Sci USA 1995; 92:11259 - 11263
- Huxford T, Huang DB, Malek S, Ghosh G. The crystal structure of the IkappaBalpha/NFkappaB complex reveals mechanisms of NFkappaB inactivation. Cell 1998; 95:759 - 770
- Jacobs MD, Harrison SC. Structure of an IkappaBalpha/NFkappaB complex. Cell 1998; 95:749 - 758
- Malek S, Chen Y, Huxford T, Ghosh G. IkappaBbeta, but not IkappaBalpha, functions as a classical cytoplasmic inhibitor of NFkappaB dimers by masking both NFkappaB nuclear localization sequences in resting cells. J Biol Chem 2001; 276:45225 - 45235
- Suyang H, Phillips R, Douglas I, Ghosh S. Role of unphosphorylated, newly synthesized IkappaBbeta in persistent activation of NFkappaB. Mol Cell Biol 1996; 16:5444 - 5449
- Chen Y, Vallee S, Wu J, Vu D, Sondek J, Ghosh G. Inhibition of NFkappaB activity by IkappaBbeta in association with kappaB-Ras. Mol Cell Biol 2004; 24:3048 - 3056
- Chen Y, Wu J, Ghosh G. KappaB-Ras binds to the unique insert within the ankyrin repeat domain of IkappaBbeta and regulates cytoplasmic retention of IkappaBbeta x NFkappaB complexes. J Biol Chem 2003; 278:23101 - 23106
- DeLuca C, Petropoulos L, Zmeureanu D, Hiscott J. Nuclear IkappaBbeta maintains persistent NFkappaB activation in HIV-1-infected myeloid cells. J Biol Chem 1999; 274:13010 - 13016