Abstract
One of the many important consequences that temperature down-shift has on cells is stabilization of secondary structures of RNAs. This stabilization has wide-spread effects, such as inhibition of expression of several genes due to termination of their transcription and inefficient RNA degradation that adversely affect cell growth at low temperature. Several cold shock proteins are produced to counteract these effects and thus allow cold acclimatization of the cell. The main RNA modulating cold shock proteins of E. coli can be broadly divided into two categories, (1) the CspA family proteins, which mainly affect the transcription and possibly translation at low temperature through their RNA chaperoning function and (2) RNA helicases and exoribonucleases that stimulate RNA degradation at low temperature through their RNA unwinding activity.
Introduction: The Cold Shock Response
Living organisms encounter various stresses, change in temperature being one of the most common. Fluctuations in temperature have widespread effects on the growth and survival of bacteria, which have therefore developed mechanisms that allow them to adapt to these changes. The cold-shock response is one such mechanism; it consists of a number of adaptive changes ranging from alterations in membrane composition to alterations in the global protein profile of the cell. Physiologically, the cold shock response of a bacterium like Escherichia coli manifests itself as an acclimation phase during which cell growth completely (but temporarily) stops immediately after the temperature downshift. During this phase, several cold shock proteins, which help the cells to adopt to low temperature, are produced. After the acclimation phase is over, the synthesis of most of the cold shock proteins decreases to a new basal level and synthesis of non-cold shock proteins resumes, allowing cells to grow at low temperature, albeit at a slower rate (review in ref. Citation1–Citation3). Certain bacteria such as B. subtilis and Lactobacillus lactis do not exhibit a temporary cessation of growth upon cold-shock response but instead immediately proceed to slow-rate low-temperature growth.Citation4,Citation5 These bacteria might be “preconditioned” to low temperature growth even when growing at their physiologically optimal temperature. Cold shock proteins play a critical role in the cold shock response of E. coli. Some of the main cold shock proteins of E. coli are the RNA chaperones CspA,Citation6 CspB,Citation7 CspGCitation8 and CspI,Citation9 RNA helicase CsdA,Citation10 ribosome-binding factor RbfA,Citation11 polynucleotide phosphorylase (PNPase),Citation12–Citation14 transcription factor NusA,Citation15 translation initiation factors IF1,Citation16 and IF2,Citation17 RecA,Citation18 histone-like protein H-NS,Citation19 DNA gyrase,Citation20 pY,Citation21 protein chaperones, Trigger factor,Citation22 Hsc-66,Citation23 HscB,Citation23 ClpB,Citation24 Recombination factor, dihydrolipoamide transferase,Citation25 pyruvate dehydrogenase,Citation25 and trehalose synthesizing proteins.Citation22 The relative amounts of all these proteins (and, doubtless, many others) are increased upon temperature down shift. However, time-resolved analysis of cells undergoing low temperature acclimation reveals a clear sequence of appearance of various cold shock proteins, indicating that the cold shock response is hierarchical, and some proteins are needed at the earliest stages of cold shock response, while others are needed at later stages and may be regulated by proteins of the first kind.
Sensing the Temperature Change at Cellular Level
The cell uses various thermosensors, including membranes, nucleic acids, proteins etc., to register temperature changes (reviewed in ref. Citation26). Some of the major direct consequences of temperature downshift include (i) decrease in membrane fluidity affecting membrane-associated cellular functions such as active transport and protein secretion, (ii) changes in nucleic acid structures, (iii) reduced ribosome function which affects translation, especially of non-cold-shock proteins and (iv) inefficient protein folding. Various mechanisms are adapted by the cell to restore the flexibility of membranes for example, increasing proportion of unsaturated fatty acids (UFAs). The cis-vaccenic acid, with low melting point and high flexibility (homeoviscous adaptation) is one such fatty acid,Citation27 which is formed from palmitoleic acid mediated by the enzyme β-ketoacyl-acyl carrier protein (ACP) synthase II. This enzyme is activated by cold shock.Citation28,Citation29 In the case of B. subtilis and cyanobacteria, cold-inducible desaturases play an important role in alteration of the degree of saturation of fatty acids in membrane phospholipids.Citation30,Citation31 Cold-induced changes in the structure of nucleic acid are of two types, (a) stabilization of the secondary structures of RNA and DNA and (b) increased negative supercoiling of DNA. The increase in negative supercoiling of DNA that occurs upon cold shock affects DNA related functions, such as replication, transcription and recombination.Citation32–Citation34 Stabilization of the secondary structures in RNAs, affects transcription and translation due to hindered movement of RNA polymerase and ribosomes, respectively. RNAs have been proposed to act as cellular thermometers.Citation26,Citation35,Citation36 Certain E. coli proteins such as CspA, the main cold shock protein, and its homologs act as RNA chaperonesCitation37,Citation38 melting the secondary structures in nucleic acids and facilitating transcription and translation at low temperature. This activity of CspA and its homologs is essential for cold acclimation of cells.Citation39,Citation40 Since CspA is among the first (and by far, most abundant) proteins induced upon cold shock, its RNA chaperoning function is considered as one of the main mechanism(s) of cold adaptation. Temperature downshift renders ribosomes nonfunctional in translation of most cellular mRNAs. However, this effect is not as severe for mRNAs of genes encoding cold-shock proteins. This selectivity is due to structural elements present in cold-shock proteins mRNAs that promote translation initiation at low temperature. During the growth lag period after the temperature downshift, cold-shock ribosomal factors such as RbfA and CsdA are produced. These proteins bind ribosomes, converting them to cold-adapted ribosomes that are capable of translating non-cold-shock mRNAs.Citation41
Temperature downshift also leads to changes in certain proteins for example, aspartate chemoreceptor (Tar) of E. coli. These changes include reversible methylation of cytoplasmic signaling/adaptation domain.Citation42 The temperature downshift also causes protein mis-folding, although it is not as severe as that caused by heat shock.Citation43 DNA microrarray analysis of transcripts of E. coli cells subjected to cold shock showed that certain molecular chaperones such as Caseinolytic proteases (Clps), trigger factor and GroEL and GroESCitation39 were induced upon cold shock. Trigger factor (TF) is induced at a modest level 2–3 h after cold shock.Citation22 TF is a peptidyl prolyl isomerase that catalyzes the cis/trans isomerization of peptide bonds which are N-terminal to the proline residue.Citation43 TF can associate with ribosomes and plays an important role in co-translational protein folding.Citation44,Citation45 Cell viability at 4°C showed direct correlation with the intracellular levels of TF, which is presumably due to its ability to help protein synthesis and folding at low temperature.Citation43
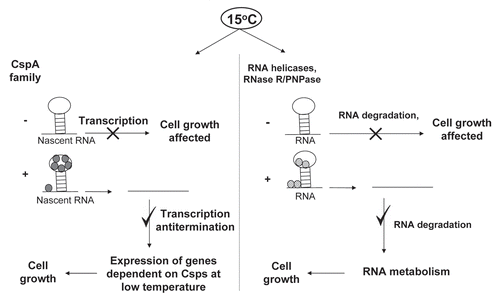
RNA Modulating Cold Shock Proteins
Proteins that restructure RNAs are of different types (reviewed in ref. Citation46): (i) RNA chaperones that melt misfolded RNA molecules and thus promote proper folding, (ii) RNA annealers that accelerate annealing of complementary RNAs, (iii) RNA helicases that resolve the RNA structures using ATP hydrolysis and (iv) specific RNA binding proteins that can also contribute to RNA folding by stabilizing specific RNA structures. Here, we focus only on RNA modulating cold shock proteins of E. coli. The main RNA modulating cold shock proteins of E. coli can be broadly divided into two categories, (i) the CspA family proteins, which mainly affect DNA transcription and possibly RNA translation at low temperature (RNA chaperones), (ii) the helicases and exoribonucleases, which modulate the RNA metabolism at low temperature. The two types of proteins are schematically depicted in .
The E. coli CspA Family
E. coli contains a family of nine homologous proteins (CspA to CspI), out of which only CspA, CspB, CspG and CspI are cold-shock inducible. A number of diverse and seemingly unrelated functions have been attributed to CspA and its homologs in different bacteria, such as, RNA chaperones,Citation38 improved adaptation to cold-shock, stationary phase, nutrient starvation and freezing,Citation47,Citation48 antibiotic biosynthesis,Citation49 UV sensitivity,Citation50 regulation of expression of proteins responding to osmotic stress, oxidative stress and stationary phase (UspA, OsmY, Dps, ProP and KatGCitation51), camphor resistance and chromosome condensation,Citation52,Citation53 downregulation of λ Q-mediated transcription antitermination,Citation54 downregulation of poly(A)-mediated 3′ to 5′ exonucleolytic decay by PNPase,Citation55 inhibition of DNA replication,Citation56 and tolerance to solvents such as toluene.Citation57
The cold-shock induction of CspA and its homologs was studied in detail and was shown to occur at the levels of transcription, mRNA stability and translation.
Regulation at the level of transcription.
The cspA transcript undergoes a 4–5-fold increase upon cold shock as revealed by studies using reporter gene fusions the reporter genes,Citation58,Citation59 primer extension, northern blot analysis and DNA microarray analysis of the global transcript profile of cold-shocked cells.Citation39 No additional factors (other than cold) are necessary for cspA induction. The 15°C induction of cspA is observed even when the gene is fused to non-cognate promoter, however, the level of such heterologous induction is significantly less than that observed with the cognate cspA promoter. Certain elements seem to enhance the level of cspA promoter activity. These include (i) an AT-rich sequence (UP element) immediately upstream of the -35 region;Citation58,Citation59 and (ii) an extended -10 box a TGn motif preceding the -10 box. However, none of these elements seem to specifically contribute to low-temperature activity of the cspA promoter.
The cspA, cspB, cspG and cspI genes possess a long 5′ untranslated region (5′-UTR). This region contains a highly conserved, 11-base sequence termed the ‘cold box’. It has been reported that at high concentrations of CspA, the protein binds the cold-box and thus regulates its own expression. The overproduction of 5′UTR leads to prolonged synthesis of CspA, an effect suppressed by co-overproduction of CspA.Citation60,Citation61
Although CspA is the main cold shock protein, it is also overproduced, albeit at much lower levels, at 37°C during early exponential growth phase. This induction results from the location of the cspA gene near the oriC replication origin, which leads to higher gene dosage and higher stability of cspA mRNA due to lower RNase activity at this stage of growth.Citation62 For similar reasons, 37°C production of CspA is observed upon nutritional upshift.Citation63
Regulation at the level of stabilization of mRNA.
The cspA mRNA is transiently and dramatically stabilized immediately following cold shock. This stabilization is likely the major factor that leads to dramatic induction of CspA at low temperature.Citation64 The 5′-UTR was shown to be responsible for the extreme instability of cspA mRNA at 37°C (a half-life of 12 s) and has a positive effect on its stabilization upon cold shock (a half-life of more than 20 min).Citation59 The cspA promoter is active at 37°C, but the steady-state levels of cspA RNA and CspA synthesis are low at this temperature due to extreme instability of its mRNA. Using enzymatic and chemical probing, it was recently shown that the cspA mRNA undergoes a temperature-dependent structural rearrangement at low temperature, likely resulting from stabilization of an otherwise thermodynamically unstable folding intermediate. The “low temperature” structure is more efficiently translated and somewhat less susceptible to degradation than the 37°C structure.Citation65 Thus, the cspA mRNA acts as a thermometer, sensing temperature downshifts by adopting a functionally distinct structure.Citation65
Regulation at the level of translation.
The mRNAs of cold-inducible CspA homologs and those of ribosome-associated factors like csdA and rbfA, contain an A/T rich sequence located 14-bases downstream of their initiation codons. This element is called Translation-Enhancing Element and is presumed to enhance translation initiation in cold-shock mRNAs.Citation66 As this sequence is complementary to a region in the penultimate stem of 16S rRNA, it was previously thought to help translation initiation by facilitating the formation of translation preinitiation complex through direct interaction with 16S rRNA. However, this view is not universally accepted and the exact mechanism of the stimulatory effect of TEE on translation initiation remains unknown at present.Citation59,Citation67
CspA homologs as transcription antiterminators.
The low-temperature-induced stabilization of the secondary structures of RNA interferes with both transcription and translation elongation. CspA homologs are proposed to act as RNA chaperones by facilitating transcription and translation at low temperature by virtue of their ability to ‘melt’ the secondary structures in nucleic acids. The function of CspA homologs as RNA chaperones promoting transcription antitermination has been studied in considerable detail.Citation37,Citation38,Citation40 Modulation of transcription termination by RNA-binding proteins involves resolving hairpin structures in nascent RNA that can act as transcriptional terminators or pause sites, thus leading to transcript elongation (reviewed in ref. Citation68). The three-dimensional structures of CspA from E. coli and CspB from B. subtilis have been resolved by X-ray crystallography and NMR-analysis and found to be very similar.Citation69–Citation73 The protein consists of five antiparallel β-strands (β1 to β5) that form a β-barrel structure with two β-sheets. Two RNA-binding motifs, RNP1 and RNP2, are located on the β2 and β3 strands, respectively. The proteins have an overall negative surface charge with positively charged amino acids surrounding a surface-exposed aromatic patch (RNP1 W11, F18 and F20 and RNP2 F31, H33 and F34). After the initial approach to an RNA molecule through electrostatic attraction and subsequent binding through stacking of the aromatic RNP side chains with RNA bases, further intramolecular or intermolecular base pairing by a segment of RNA bound to a Csp protein is prevented by charge repulsion.Citation74 CspA and its homologs do not exhibit high degree of specificity for their RNA/DNA substrates.Citation38,Citation75,Citation76 The RNA chaperone activity allows the CspA homologs to act as transcription antiterminators and thus aid in cold acclimation of cells.Citation37,Citation40 CspE, a member of this family, was used as a model to analyze these activities. CspE caused transcription antitermination at every rho-independent terminator tested. Systematic mutations of individual amino acids of CspE showed that only Phe17, Phe30 and His32 are essential for nucleic acid melting activity. The Phe residues initiate the melting process and the His residue completes it. The proteins carrying substitutions of these amino acids with Arg fully retain their nucleic acid binding activity, but lose the ability to cause transcription antitermination and cold acclimation of cells as they lack the melting activity.Citation40,Citation77 DNA microarray analysis of the cold shock response of the wild-type cells, the cells carrying deletion of various csp genes along with the cells overexpressing the wild-type or melting-deficient Csp proteins revealed genes, which require the transcription antitermination activity of Csp proteins for expression at low temperature. The genes which showed strict dependence on Csp proteins for their expression at 15°C, included malE and malK (membrane related functions), mopA and mopB (chaperones), dps, katG, rpoS, uspA (stress response), as well as several others. Several of these genes possess promoter-proximal sequences that contain stem-loop structures, which could stabilize at low temperature and block transcript elongation. These sequences, termed Csp-responsive transcription terminators, are proposed to be physiological targets of Csp proteins. Csp-dependent melting of these structures is proposed to allow transcription of downstream genes to continue at low temperature.Citation78
Helicases and Exoribonucleases
SrmB and CsdA, the DEAD-box helicases involved in cold shock acclimation.
The DEAD-box RNA helicase family proteins play important roles in many cellular processes such as processing, transport or degradation of RNA or ribosome biogenesis (reviewed in ref. Citation79). The E. coli encodes five DEAD-box RNA helicase family proteins, CsdA, DbpA, RhlB, RhlE and SrmB.Citation80 SrmB and CsdA seem to play some role in the cold acclimation of cells and are briefly described below.
SrmB.
SrmB is involved in ribosome biogenesis. In fact, the srmB gene was originally isolated as a multicopy suppressor of mutations in the ribosomal protein L24 gene rplX.Citation81 Deletion of srmB results in (i) slow-growth phenotype at low temperature, (ii) deficit in free 50S ribosomal subunits and (iii) accumulation of a new ribosomal particle sedimenting around 40S. Thus, it was suggested that there is a step of 50S assembly, which involves a structural rearrangement that, at least at low temperature, requires SrmB.Citation82
CsdA.
The requirement of CsdA at low temperature is more pronounced than that of SrmB and the deletion of csdA gene severely impairs growth at low temperature.Citation83,Citation84 CsdA has been assigned multiple cellular functions including ribosome biogenesis, translation initiation and degradation of mRNAs.
The possibility of CsdA being involved in the biogenesis of ribosomal subunits was first suggested by an observation that its gene, deaD/csdA, can act as a multicopy suppressor of a missense mutation in the rpsB gene that encodes the ribosomal protein S2.Citation10 Further, overexpression of CsdA restores the incorporation of mutant S2 (as well as the S1 protein) into 30S ribosomal subunits.Citation85 Recently, it was shown that CsdA is also involved in the biogenesis of the 50S ribosomal subunitsCitation79 and associates with 50S precursors at low temperature.Citation83
CsdA is homologous to eukaryotic translation initiation factor eIF4A. EIF4A catalyzes ATP-dependent unwinding of RNA duplexes and stimulates translational initiation. It was thus suggested that CsdA too may be involved in assisting the translation by promoting translation initiation of structured mRNAs.Citation86
The role of CsdA in mRNA degradation was suggested by the observations that it (i) is found in degradosomes in cold-adapted cultures,Citation87–Citation89 and (ii) is involved in efficient and selective degradation of Csp mRNAs by unwinding the mRNA secondary structure that impedes the processive activity of PNPase.Citation87 Although CsdA was shown to be involved in important physiological processes, it was not clear which of its partial activities (or their combination) play a role in its essential cold-shock function. Our data suggest that the main way through which CsdA contributes to cold acclimation is through its involvement in mRNA decay. The helicase activity of CsdA is pivotal for promoting the degradation of mRNAs stabilized at low temperature: helicase-deficient CsdA mutants do not complement cold sensitivity of the csdA deletion cells. The correlation between the helicase activity of CsdA and stability of mRNAs of cold-inducible genes was further shown using the cspA mRNA, which was significantly stabilized in the ΔcsdA cells. Again, this effect was counteracted by overexpression of the wild-type CsdA, but not helicase-deficient CsdA mutants.Citation90 We used csdA deletion cells and screened an E. coli genomic library for protein(s) that can complement the essential CsdA function during cold acclimation. We observed that another DEAD-box RNA helicase, RhlE, complemented the csdA deletion.Citation90 Other groups reported similar findings.Citation79,Citation91
CsdA and SrmB share several properties: they (i) unwind nucleic acid duplexes with 3′ or 5′ extensions, (ii) stabilize certain mRNAs,Citation92 (iii) bind to RNase E88, (iv) participate in 50S assembly, probably by modulating RNA structures through their unwinding activity and (v) act as RNA chaperones that prevent and/or resolve misfolding. Despite these similarities, overexpression of SrmB does not suppress the cold sensitivity of the csdA deletion mutant and vice versa.Citation83 The reasons for this specificity remain presently unknown.
Cold-inducible Exoribonucleases
RNase R.
PNPase, RNase R and RNase II are the three major 3′-to-5′ processing exoribonucleases in E. coli. These enzymes are primarily involved in RNA metabolism. Both PNPase,Citation93,Citation94 and RNase R95 are induced by cold shock and are suggested to be the universal degraders of structured RNA in the cell.Citation96,Citation97 RNase II is not cold shock inducible. Interestingly, RNase II, but not RNase R can complement the cold shock function of PNPase.Citation98 RNase II and RNase R belong to the RNR family. Based on sequence analysis and comparison with the RNase II structure,Citation99–Citation101 RNase R consists of a central nuclease domain, two cold-shock (CSD) domains near the N-terminal region of the protein, an S1 domain and a highly basic C-terminal region.Citation102
While not detected in our complementation screen of csdA deletion strain (above) overproduction of RNase R or CspA complemented the cold-sensitive phenotype of the deletion strain.Citation90 The fact that CspA is an RNA chaperone and RNase R is an exoribonuclease further supported the hypothesis that the primary role of CsdA in cold acclimation is in mRNA decay and its helicase activity is pivotal for promoting degradation of mRNAs stabilized at low temperature.Citation90 Interestingly, in Bacillus subtilis it was shown that cold-induced helicases and Csps work in together to rescue misfolded mRNA molecules and maintain proper initiation of translation at low temperatures.Citation103
PNPase and RNase II overproduction did not complement CsdA helicase mutants.Citation90 This observation led us to hypothesize that complementation of the cold-shock function of CsdA by RNase R is not merely due to its ability to degrade secondary structures in RNAs, but instead may be due to a helicase activity. To test this prediction, mutant proteins bearing point mutations within the domain responsible for the ribonucleic activity were created. We observed that RNase R mutants devoid of ribonuclease activity retained their ability to complement the cold shock function of CsdA. Purified ribonuclease-negative mutant proteins also exhibited helicase activity in vitro. RNase R has three cold shock domains that are known to possess unwinding activity in other proteins (most notably, CspA). Analysis of RNase R mutants revealed that only one cold shock domain of RNase R, the CSD2 domain, is important for its helicase activity. In vivo studies showed that this domain is not essential for the ribonuclease activity of RNase R.
PNPase.
Regulation of expression. PNPase is encoded by the pnp gene.Citation13,Citation14 Its expression is post-transcriptionally autoregulated at the level of both translation and mRNA stability.Citation104 It has been shown that PNPase binds to the 5′ end of RNase III-processed pnp transcript, leading to the inhibition of translation and channeling of pnp mRNA into degradation pathway.Citation105–Citation107 During the cold acclimation phase this regulation is temporarily relieved leading to stabilization of the pnp mRNA, making it extremely abundant. CspA-dependent transcription antitermination at intercistronic intrinsic terminators also plays a role in transient induction of promoter-distal pnp gene. It has also been shown that suppression of Rho-dependent transcription termination within the pnp gene and its restoration by PNPase constitutes an autogenous regulatory circuit that modulates pnp expression during cold acclimation phase. The 5′-untranslated region of pnp mRNA plays a role in this phenomenon.Citation108
Structure-function. PNPase is a homotrimer. Each 711 amino acid monomer consists of two RNA binding domains, KH and S1, located at the C-terminal portion of the polypeptide. The duplicated core of the protein is responsible for the catalytic function and homotrimerization.Citation109–Citation111 Both first and second core domains mediate the catalysis of the phosphorolytic reaction. Both the phosphorolytic and RNA binding activities are required for autogenous regulation of PNPase.Citation106 It has also been shown that the KH and S1 domains of PNPase are important for its proper degradosome activity at low temperature. Note that at low temperatures, the stem-loop structures present in the target mRNAs are more stable.Citation112
PNPase is one of the main exoribonucleases in the cell;Citation113 it promotes processive degradation of RNA. PNPase is essential at low temperature for cell growth, although its exact role is not fully known.Citation114,Citation115 Recent data suggest that the ribonuclease activity of PNPase is critical for its cold shock function.Citation98 By selectively degrading csp RNAs PNPase decreases the production of CspA and its cold-induced homologs at the end of the acclimation phase. In a pnp mutant, expression of CspA homologs was significantly prolonged upon cold shock.Citation87,Citation116 PNPase has been shown to be associated with endonuclease RNase E and other proteins in the RNA degradosome.Citation117–Citation119 In vitro, PNPase catalyzes phosphorolytic degradation of RNA, releasing nucleoside 5′-diphosphate from the 3′ end of the substrate and also carries out the reverse reaction of nucleoside 5′-diphosphate polymerization with the release of phosphate;Citation120 both the phosphorolytic and polymerization activities are allosterically inhibited by ATP.Citation121 The polymerization activity of PNPase is responsible for residual RNA tailing observed in E. coli mutants devoid of the main polyadenylating enzyme PAP I.Citation122,Citation123 PNPase-dependent RNA tailing and degradation occur mainly at low ATP concentrations, whereas other enzymes may play a more significant role at high energy charge.
RNA Metabolism at Low Temperature
The RNA metabolism at low temperature may be different than that at 37°C and may require (i) the assistance of proteins that destabilize RNA secondary structures that are stabilized upon temperature downshift, making them accessible to ribonucleases and (ii) cold-inducible ribonucleases that can carry out RNA degradation efficiently and selectively to allow cell growth at low temperature. Note that both CsdA and PNPase are essential only at low temperature. Interestingly, these two proteins seem to function independently of each other as they cannot complement each other's functions. RNase R, in spite of being an 3′–5′ exoribonuclease like PNPase, cannot complement its cold shock function, but can complement that of CsdA by virtue of its unwinding activity. This implies that these two groups of proteins have distinct RNA targets. A detailed, comparative analysis of the RNA targets of these proteins should elaborate on how these proteins affect the RNA metabolism at low temperature.
Concluding Remarks
Stabilization of the secondary structures in RNA affects transcription of certain genes and also renders some RNAs inaccessible to degradation by ribonucleases. This results in inhibition or extensive slowing down of cell growth at low temperature. Cells produce two main groups of cold shock proteins, which modulate the RNA secondary structures and thus play important role(s) in the cold-shock adaptation of cells. It is interesting why cells need so many different proteins to facilitate RNA degradation at low temperature. While at least some of the proteins discussed here can partially complement for each others absence at cold shock, the complementation is never complete (and sometimes is totally absent). The result suggest multiple and only partly overlapping targets (presumably, nascent or already transcribed RNAs) that cold shock proteins act upon. Elucidation of these targets and determining the modes of action of various cold shock proteins on each target will be necessary for complete understanding of cellular adaptation to low temperatures.
Figures and Tables
Acknowledgements
This work was supported by NIH RO3 Grant 76900 to S.P. and G.M. “RO1 64530, Russian Academy of Sciences Presidium Molecular and Cell Biology Program grant and Federal Program “Scientific and pedagogical cadre of innovative Russia” 2009–2013,” State contract 02.740.11.0771 to K.S.
References
- Phadtare S. Recent developments in bacterial coldshock response. Curr Issues Mol Biol 2004; 6:125 - 136
- Weber MH, Marahiel MA. Bacterial cold shock responses. Sci Prog 2003; 86:9 - 75
- Ermolenko DN, Makhatadze GI. Bacterial cold-shock proteins. Cell Mol Life Sci 2002; 59:1902 - 1913
- Willimsky G, Bang H, Fischer G, Marahiel MA. Characterization of cspB, a Bacillus subtilis inducible cold shock gene affecting cell viability at low temperatures. J Bacteriol 1992; 174:6326 - 6335
- Chapot-Chartier MP, Schouler C, Lepeuple AS, Gripon JC, Chopin MC. Characterization of cspB, a cold-shock-inducible gene from Lactococcus lactis and evidence for a family of genes homologous to the Escherichia coli cspA major cold shock gene. J Bacteriol 1997; 179:5589 - 5593
- Goldstein J, Pollitt NS, Inouye M. Major cold shock protein of Escherichia coli. Proc Nat Acad Sci USA 1990; 87:283 - 287
- Lee SJ, Xie A, Jiang W, Etchegaray JP, Jones PG, Inouye M. Family of the major cold-shock protein, CspA (CS7.4), of Escherichia coli, whose members show a high sequence similarity with the eukaryotic Y-box binding proteins. Mol Microbiol 1994; 11:833 - 839
- Nakashima K, Kanamaru K, Mizuno T, Horikoshi K. A novel member of the cspA family of genes that is induced by cold shock in Escherichia coli. J Bacteriol 1996; 178:2994 - 2997
- Wang N, Yamanaka K, Inouye M. CspI, the ninth member of the CspA family of Escherichia coli, is induced upon cold shock. J Bacteriol 1999; 181:1603 - 1609
- Toone WM, Rudd KE, Friesen JD. deaD, a new Escherichia coli gene encoding a presumed ATP-dependent RNA helicase, can suppress a mutation in rpsB, the gene encoding ribosomal protein S2. J Bacteriol 1991; 173:3291 - 3302
- Dammel CS, Noller HF. Suppression of a cold-sensitive mutation in 16S rRNA by overexpression of a novel ribosome-binding factor, RbfA. Genes Develop 1995; 9:626 - 637
- Donovan WP, Kushner SR. Polynucleotide phosphorylase and ribonuclease II are required for cell viability and mRNA turnover in Escherichia coli K-12. Proc Nat Acad Sci USA 1986; 83:120 - 124
- Reiner AM. Characterization of polynucleotide phosphorylase mutants of Escherichia coli. J Bacteriol 1969; 97:1437 - 1443
- Reiner AM. Isolation and mapping of polynucleotide phosphorylase mutants of Escherichia coli. J Bacteriol 1969; 97:1431 - 1436
- Friedman DI, Olson ER, Georgopoulos C, Tilly K, Herskowitz I, Banuett F. Interactions of bacteriophage and host macromolecules in the growth of bacteriophage lambda. Microbiol Rev 1984; 48:299 - 325
- Cummings HS, Hershey JW. Translation initiation factor IF1 is essential for cell viability in Escherichia coli. J Bacteriol 1994; 176:198 - 205
- Gualerzi CO, Pon CL. Initiation of mRNA translation in prokaryotes. Biochem 1990; 29:5881 - 5889
- Walker GC. Mutagenesis and inducible responses to deoxyribonucleic acid damage in Escherichia coli. Microbiol Rev 1984; 48:60 - 93
- Dersch P, Kneip S, Bremer E. The nucleoid-associated DNA-binding protein H-NS is required for the efficient adaptation of Escherichia coli K-12 to a cold environment. Mol Gen Genet 1994; 245:255 - 259
- Jones PG, Krah R, Tafuri SR, Wolffe AP. DNA gyrase, CS7.4 and the cold shock response in Escherichia coli. J Bacteriol 1992; 174:5798 - 5802
- Agafonov DE, Kolb VA, Spirin AS. Ribosome-associated protein that inhibits translation at the aminoacyl-tRNA binding stage. EMBO Rep 2001; 2:399 - 402
- Kandror O, DeLeon A, Goldberg AL. Trehalose synthesis is induced upon exposure of Escherichia coli to cold and is essential for viability at low temperatures. Proc Nat Acad Sci USA 2002; 99:9727 - 9732
- Lelivelt MJ, Kawula TH. Hsc66, an Hsp70 homolog in Escherichia coli, is induced by cold shock but not by heat shock. J Bacteriol 1995; 177:4900 - 4907
- Porankiewicz J, Clarke AK. Induction of the heat shock protein ClpB affects cold acclimation in the cyanobacterium Synechococcus sp. strain PCC 7942. J Bacteriol 1997; 179:5111 - 5117
- Jones PG, Inouye M. The cold-shock responseóa hot topic. Mol Microbiol 1994; 11:811 - 818
- Klinkert B, Narberhaus F. Microbial thermosensors. Cell Mol Life Sci 2009; 66:2661 - 2676
- Sinensky M. Homeoviscous adaptationóa homeostatic process that regulates the viscosity of membrane lipids in Escherichia coli. Proc Nat Acad Sci USA 1974; 71:522 - 525
- Garwin JL, Cronan JE Jr. Thermal modulation of fatty acid synthesis in Escherichia coli does not involve de novo enzyme synthesis. J Bacteriol 1980; 141:1457 - 1459
- Garwin JL, Klages AL, Cronan JE Jr. Beta-ketoacyl-acyl carrier protein synthase II of Escherichia coli. Evidence for function in the thermal regulation of fatty acid synthesis. J Biol Chem 1980; 255:3263 - 3265
- Aguilar PS, Cronan JE Jr, de Mendoza D. A Bacillus subtilis gene induced by cold shock encodes a membrane phospholipid desaturase. J Bacteriol 1998; 180:2194 - 2200
- Murata N, Wada H. Acyl-lipid desaturases and their importance in the tolerance and acclimatization to cold of cyanobacteria. Biochem J 1995; 308:1 - 8
- Wang JY, Syvanen M. DNA twist as a transcriptional sensor for environmental changes. Mol Microbiol 1992; 6:1861 - 1866
- Mizushima T, Kataoka K, Ogata Y, Inoue R, Sekimizu K. Increase in negative supercoiling of plasmid DNA in Escherichia coli exposed to cold shock. Mol Microbiol 1997; 23:381 - 386
- Krispin O, Allmansberger R. Changes in DNA supertwist as a response of Bacillus subtilis towards different kinds of stress. FEMS Microbiol Lett 1995; 134:129 - 135
- Narberhaus F, Waldminghaus T, Chowdhury S. RNA thermometers. FEMS Microbiol Rev 2006; 30:3 - 16
- Storz G. An RNA thermometer. Genes Develop 1999; 13:633 - 636
- Bae W, Xia B, Inouye M, Severinov K. Escherichia coli CspA-family RNA chaperones are transcription antiterminators. Proc Nat Acad Sci USA 2000; 97:7784 - 7789
- Jiang W, Hou Y, Inouye M. CspA, the major cold-shock protein of Escherichia coli, is an RNA chaperone. J Biol Chem 1997; 272:196 - 202
- Phadtare S, Inouye M. Genome-wide transcriptional analysis of the cold shock response in wild-type and cold-sensitive, quadruple-csp-deletion strains of Escherichia coli. J Bacteriol 2004; 186:7007 - 7014
- Phadtare S, Inouye M, Severinov K. The nucleic acid melting activity of Escherichia coli CspE is critical for transcription antitermination and cold acclimation of cells. J Biol Chem 2002; 277:7239 - 7245
- Jones PG, Inouye M. RbfA, a 30S ribosomal binding factor, is a cold-shock protein whose absence triggers the cold-shock response. Mol Microbiol 1996; 21:1207 - 1218
- Nishiyama SI, Umemura T, Nara T, Homma M, Kawagishi I. Conversion of a bacterial warm sensor to a cold sensor by methylation of a single residue in the presence of an attractant. Mol Microbiol 1999; 32:357 - 365
- Kandror O, Goldberg AL. Trigger factor is induced upon cold shock and enhances viability of Escherichia coli at low temperatures. Proc Nat Acad Sci USA 1997; 94:4978 - 4981
- Maier R, Eckert B, Scholz C, Lilie H, Schmid FX. Interaction of trigger factor with the ribosome. J Mol Biol 2003; 326:585 - 592
- Blaha G, Wilson DN, Stoller G, Fischer G, Willumeit R, Nierhaus KH. Localization of the trigger factor binding site on the ribosomal 50S subunit. J Mol Biol 2003; 326:887 - 897
- Rajkowitsch L, Chen D, Stampfl S, Semrad K, Waldsich C, Mayer O, et al. RNA chaperones, RNA annealers and RNA helicases. RNA Biol 2007; 4:118 - 130
- Derzelle S, Hallet B, Ferain T, Delcour J, Hols P. Improved adaptation to cold-shock, stationary-phase and freezing stresses in Lactobacillus plantarum overproducing cold-shock proteins. Appl Environ Microbiol 2003; 69:4285 - 4290
- Yamanaka K, Inouye M. Growth-phase-dependent expression of cspD, encoding a member of the CspA family in Escherichia coli. J Bacteriol 1997; 179:5126 - 5130
- Martinez-Costa OH, Zalacain M, Holmes DJ, Malpartida F. The promoter of a cold-shock-like gene has pleiotropic effects on Streptomyces antibiotic biosynthesis. FEMS Microbiol Lett 2003; 220:215 - 221
- Mangoli S, Sanzgiri VR, Mahajan SK. A common regulator of cold and radiation response in Escherichia coli. J Environ Pathol Toxicol Oncol 2001; 20:23 - 26
- Phadtare S, Inouye M. Role of CspC and CspE in regulation of expression of RpoS and UspA, the stress response proteins in Escherichia coli. J Bacteriol 2001; 183:1205 - 1214
- Hu KH, Liu E, Dean K, Gingras M, DeGraff W, Trun NJ. Overproduction of three genes leads to camphor resistance and chromosome condensation in Escherichia coli. Genetics 1996; 143:1521 - 1532
- Sand O, Gingras M, Beck N, Hall C, Trun N. Phenotypic characterization of overexpression or deletion of the Escherichia coli crcA, cspE and crcB genes. Microbiology 2003; 149:2107 - 2117
- Hanna MM, Liu K. Nascent RNA in transcription complexes interacts with CspE, a small protein in E. coli implicated in chromatin condensation. J Mol Biol 1998; 282:227 - 239
- Feng Y, Huang H, Liao J, Cohen SN. Escherichia coli poly(A)-binding proteins that interact with components of degradosomes or impede RNA decay mediated by polynucleotide phosphorylase and RNase E. J Biol Chem 2001; 276:31651 - 31656
- Yamanaka K, Zheng W, Crooke E, Wang YH, Inouye M. CspD, a novel DNA replication inhibitor induced during the stationary phase in Escherichia coli. Mol Microbiol 2001; 39:1572 - 1584
- Segura A, Godoy P, van Dillewijn P, Hurtado A, Arroyo N, Santacruz S, et al. Proteomic analysis reveals the participation of energy- and stress-related proteins in the response of Pseudomonas putida DOT-T1E to toluene. J Bacteriol 2005; 187:5937 - 5945
- Goldenberg D, Azar I, Oppenheim AB, Brandi A, Pon CL, Gualerzi CO. Role of Escherichia coli cspA promoter sequences and adaptation of translational apparatus in the cold shock response. Mol Gen Genet 1997; 256:282 - 290
- Mitta M, Fang L, Inouye M. Deletion analysis of cspA of Escherichia coli: requirement of the AT-rich UP element for cspA transcription and the downstream box in the coding region for its cold shock induction. Mol Microbiol 1997; 26:321 - 335
- Jiang W, Fang L, Inouye M. The role of the 5′-end untranslated region of the mRNA for CspA, the major cold-shock protein of Escherichia coli, in cold-shock adaptation. J Bacteriol 1996; 178:4919 - 4925
- Fang L, Hou Y, Inouye M. Role of the cold-box region in the 5′ untranslated region of the cspA mRNA in its transient expression at low temperature in Escherichia coli. J Bacteriol 1998; 180:90 - 95
- Brandi A, Spurio R, Gualerzi CO, Pon CL. Massive presence of the Escherichia coli ëmajor cold-shock proteiní CspA under non-stress conditions. EMBO J 1999; 18:1653 - 1659
- Yamanaka K, Inouye M. Induction of CspA, an E. coli major cold-shock protein, upon nutritional upshift at 37 degrees C. Genes Cells 2001; 6:279 - 290
- Phadtare S, Severinov K. Extended -10 motif is critical for activity of the cspA promoter but does not contribute to low-temperature transcription. J Bacteriol 2005; 187:6584 - 6589
- Giuliodori AM, Di Pietro F, Marzi S, Masquida B, Wagner R, Romby P, et al. The cspA mRNA is a thermosensor that modulates translation of the cold-shock protein CspA. Mol Cell 2010; 37:21 - 33
- Qing G, Xia B, Inouye M. Enhancement of Translation Initiation by A/T-Rich Sequences Downstream of the Initiation Codon in Escherichia coli. J Mol Microbiol Biotechnol 2004; 6:133 - 144
- Moll I, Huber M, Grill S, Sairafi P, Mueller F, Brimacombe R, et al. Evidence against an Interaction between the mRNA downstream box and 16S rRNA in translation initiation. J Bacteriol 2001; 183:3499 - 3505
- Stulke J. Control of transcription termination in bacteria by RNA-binding proteins that modulate RNA structures. Arch Microbiol 2002; 177:433 - 440
- Feng W, Tejero R, Zimmerman DE, Inouye M, Montelione GT. Solution NMR structure and backbone dynamics of the major cold-shock protein (CspA) from Escherichia coli: evidence for conformational dynamics in the single-stranded RNA-binding site. Biochemistry 1998; 37:10881 - 10896
- Newkirk K, Feng W, Jiang W, Tejero R, Emerson SD, Inouye M, et al. Solution NMR structure of the major cold shock protein (CspA) from Escherichia coli: identification of a binding epitope for DNA. Proc Nat Acad Sci USA 1994; 91:5114 - 5118
- Schindelin H, Jiang W, Inouye M, Heinemann U. Crystal structure of CspA, the major cold shock protein of Escherichia coli. Proc Nat Acad Sci USA 1994; 91:5119 - 5123
- Schindelin H, Marahiel MA, Heinemann U. Universal nucleic acid-binding domain revealed by crystal structure of the B. subtilis major cold-shock protein. Nature 1993; 364:164 - 168
- Schnuchel A, Wiltscheck R, Czisch M, Herrler M, Willimsky G, Graumann P, et al. Structure in solution of the major cold-shock protein from Bacillus subtilis. Nature 1993; 364:169 - 171
- Graumann PL, Marahiel MA. A superfamily of proteins that contain the cold-shock domain. Trends Biochem Sci 1998; 23:286 - 290
- Phadtare S, Inouye M. Sequence-selective interactions with RNA by CspB, CspC and CspE, members of the CspA family of Escherichia coli. Mol Microbiol 1999; 33:1004 - 1014
- Lopez MM, Yutani K, Makhatadze GI. Interactions of the cold shock protein CspB from Bacillus subtilis with single-stranded DNA. Importance of the T base content and position within the template. J Biol Chem 2001; 276:15511 - 15518
- Phadtare S, Tyagi S, Inouye M, Severinov K. Three amino acids in Escherichia coli CspE surface-exposed aromatic patch are critical for nucleic acid melting activity leading to transcription antitermination and cold acclimation of cells. J Biol Chem 2002; 277:46706 - 46711
- Phadtare S, Tadigotla V, Shin WH, Sengupta A, Severinov K. Analysis of Escherichia coli global gene expression profiles in response to overexpression and deletion of CspC and CspE. J Bacteriol 2006; 188:2521 - 2527
- Iost I, Dreyfus M. DEAD-box RNA helicases in Escherichia coli. Nucleic Acids Res 2006; 34:4189 - 4197
- Linder P, Lasko PF, Ashburner M, Leroy P, Nielsen PJ, Nishi K, et al. Birth of the D-E-A-D box. Nature 1989; 337:121 - 122
- Nishi K, Morel-Deville F, Hershey JW, Leighton T, Schnier J. An eIF-4A-like protein is a suppressor of an Escherichia coli mutant defective in 50S ribosomal subunit assembly. Nature 1988; 336:496 - 498
- Charollais J, Pflieger D, Vinh J, Dreyfus M, Iost I. The DEAD-box RNA helicase SrmB is involved in the assembly of 50S ribosomal subunits in Escherichia coli. Mol Microbiol 2003; 48:1253 - 1265
- Charollais J, Dreyfus M, Iost I. CsdA, a cold-shock RNA helicase from Escherichia coli, is involved in the biogenesis of 50S ribosomal subunit. Nucleic Acids Research 2004; 32:2751 - 2759
- Jones PG, Mitta M, Kim Y, Jiang W, Inouye M. Cold shock induces a major ribosomal-associated protein that unwinds double-stranded RNA in Escherichia coli. Proc Nat Acad Sci USA 1996; 93:76 - 80
- Moll I, Grill S, Grundling A, Blasi U. Effects of ribosomal proteins S1, S2 and the DeaD/CsdA DEAD-box helicase on translation of leaderless and canonical mRNAs in Escherichia coli. Mol Microbiol 2002; 44:1387 - 1396
- Lu J, Aoki H, Ganoza MC. Molecular characterization of a prokaryotic translation factor homologous to the eukaryotic initiation factor eIF4A. Int J Biochem Cell Biol 1999; 31:215 - 229
- Yamanaka K, Inouye M. Selective mRNA degradation by polynucleotide phosphorylase in cold shock adaptation in Escherichia coli. J Bacteriol 2001; 183:2808 - 2816
- Khemici V, Toesca I, Poljak L, Vanzo NF, Carpousis AJ. The RNase E of Escherichia coli has at least two binding sites for DEAD-box RNA helicases: functional replacement of RhlB by RhlE. Mol Microbiol 2004; 54:1422 - 1430
- Prudíhomme-Genereux A, Beran RK, Iost I, Ramey CS, Mackie GA, Simons RW. Physical and functional interactions among RNase E, polynucleotide phosphorylase and the cold-shock protein, CsdA: evidence for a ëcold shock degradosomeí. Mol Microbiol 2004; 54:1409 - 1421
- Awano N, Xu C, Ke H, Inoue K, Inouye M, Phadtare S. Complementation Analysis of the Cold-Sensitive Phenotype of the Escherichia coli csdA Deletion Strain. J Bacteriol 2007; 189:5808 - 5815
- Jain C. The E. coli RhlE RNA helicase regulates the function of related RNA helicases during ribosome assembly. RNA 2008; 14:381 - 389
- Iost I, Dreyfus M. mRNAs can be stabilized by DEAD-box proteins. Nature 1994; 372:193 - 196
- Jones PG, VanBogelen RA, Neidhardt FC. Induction of proteins in response to low temperature in Escherichia coli. J Bacteriol 1987; 169:2092 - 2095
- Zangrossi S, Briani F, Ghisotti D, Regonesi ME, Tortora P, Deho G. Transcriptional and post-transcriptional control of polynucleotide phosphorylase during cold acclimation in Escherichia coli. Mol Microbiol 2000; 36:1470 - 1480
- Cairrao F, Cruz A, Mori H, Arraiano CM. Cold shock induction of RNase R and its role in the maturation of the quality control mediator SsrA/tmRNA. Mol Microbiol 2003; 50:1349 - 1360
- Cheng ZF, Deutscher MP. Quality control of ribosomal RNA mediated by polynucleotide phosphorylase and RNase R. Proc Nat Acad Sci USA 2003; 100:6388 - 6393
- Li Z, Reimers S, Pandit S, Deutscher MP. RNA quality control: degradation of defective transfer RNA. EMBO J 2002; 21:1132 - 1138
- Awano N, Inouye M, Phadtare S. RNase activity of polynucleotide phosphorylase is critical at low temperature in Escherichia coli and is complemented by RNase II. J Bacteriol 2008; 190:5924 - 5933
- Zuo Y, Vincent HA, Zhang J, Wang Y, Deutscher MP, Malhotra A. Structural basis for processivity and single-strand specificity of RNase II. Mol Cell 2006; 24:149 - 156
- Frazao C, McVey CE, Amblar M, Barbas A, Vonrhein C, Arraiano CM, et al. Unravelling the dynamics of RNA degradation by ribonuclease II and its RNA-bound complex. Nature 2006; 443:110 - 114
- McVey CE, Amblar M, Barbas A, Cairrao F, Coelho R, Romao C, et al. Expression, purification, crystallization and preliminary diffraction data characterization of Escherichia coli ribonuclease II (RNase II). Acta crystallographica 2006; 62:684 - 687
- Vincent HA, Deutscher MP. The Roles of Individual Domains of RNase R in Substrate Binding and Exoribonuclease Activity: THE NUCLEASE DOMAIN IS SUFFICIENT FOR DIGESTION OF STRUCTURED RNA. J Biol Chem 2009; 284:486 - 494
- Hunger K, Beckering CL, Wiegeshoff F, Graumann PL, Marahiel MA. Cold-induced putative DEAD box RNA helicases CshA and CshB are essential for cold adaptation and interact with cold shock protein B in Bacillus subtilis. J Bacteriol 2006; 188:240 - 248
- Jarrige AC, Mathy N, Portier C. PNPase autocontrols its expression by degrading a double-stranded structure in the pnp mRNA leader. EMBO J 2001; 20:6845 - 6855
- Portier C, Dondon L, Grunberg-Manago M, Regnier P. The first step in the functional inactivation of the Escherichia coli polynucleotide phosphorylase messenger is a ribonuclease III processing at the 5′ end. EMBO J 1987; 6:2165 - 2170
- Briani F, Del Favero M, Capizzuto R, Consonni C, Zangrossi S, Greco C, et al. Genetic analysis of polynucleotide phosphorylase structure and functions. Biochimie 2007; 89:145 - 157
- Garcia-Mena J, Das A, Sanchez-Trujillo A, Portier C, Montanez C. A novel mutation in the KH domain of polynucleotide phosphorylase affects autoregulation and mRNA decay in Escherichia coli. Mol Microbiol 1999; 33:235 - 248
- Marchi P, Longhi V, Zangrossi S, Gaetani E, Briani F, Deho G. Autogenous regulation of Escherichia coli polynucleotide phosphorylase during cold acclimation by transcription termination and antitermination. Mol Genet Genomics 2007; 278:75 - 84
- Gibson TJ, Thompson JD, Heringa J. The KH domain occurs in a diverse set of RNA-binding proteins that include the antiterminator NusA and is probably involved in binding to nucleic acid. FEBS Lett 1993; 324:361 - 366
- Symmons MF, Jones GH, Luisi BF. A duplicated fold is the structural basis for polynucleotide phosphorylase catalytic activity, processivity and regulation. Structure 2000; 8:1215 - 1216
- Regnier P, Grunberg-Manago M, Portier C. Nucleotide sequence of the pnp gene of Escherichia coli encoding polynucleotide phosphorylase. Homology of the primary structure of the protein with the RNA-binding domain of ribosomal protein S1. J Biol Chem 1987; 262:63 - 68
- Matus-Ortega ME, Regonesi ME, Pina-Escobedo A, Tortora P, Deho G, Garcia-Mena J. The KH and S1 domains of Escherichia coli polynucleotide phosphorylase are necessary for autoregulation and growth at low temperature. Biochim Biophys Acta 2007; 1769:194 - 203
- Mohanty BK, Kushner SR. Genomic analysis in Escherichia coli demonstrates differential roles for polynucleotide phosphorylase and RNase II in mRNA abundance and decay. Mol Microbiol 2003; 50:645 - 658
- Piazza F, Zappone M, Sana M, Briani F, Deho G. Polynucleotide phosphorylase of Escherichia coli is required for the establishment of bacteriophage P4 immunity. J Bacteriol 1996; 178:5513 - 5521
- Luttinger A, Hahn J, Dubnau D. Polynucleotide phosphorylase is necessary for competence development in Bacillus subtilis. Mol Microbiol 1996; 19:343 - 356
- Polissi A, De Laurentis W, Zangrossi S, Briani F, Longhi V, Pesole G, et al. Changes in Escherichia coli transcriptome during acclimatization at low temperature. Res Microbiol 2003; 154:573 - 580
- Nurmohamed S, Vaidialingam B, Callaghan AJ, Luisi BF. Crystal structure of Escherichia coli polynucleotide phosphorylase core bound to RNase E, RNA and manganese: implications for catalytic mechanism and RNA degradosome assembly. J Mol Biol 2009; 389:17 - 33
- Carpousis AJ. The RNA degradosome of Escherichia coli: an mRNA-degrading machine assembled on RNase E. Ann Rev Microbiol 2007; 61:71 - 87
- Liou GG, Jane WN, Cohen SN, Lin NS, Lin-Chao S. RNA degradosomes exist in vivo in Escherichia coli as multicomponent complexes associated with the cytoplasmic membrane via the N-terminal region of ribonuclease E. Proc Nat Acad Sci USA 2001; 98:63 - 68
- Littauer UZ. From polynucleotide phosphorylase to neurobiology. J Biol Chem 2005; 280:38889 - 38897
- Del Favero M, Mazzantini E, Briani F, Zangrossi S, Tortora P, Deho G. Regulation of Escherichia coli polynucleotide phosphorylase by ATP. J Biol Chem 2008; 283:27355 - 27359
- Mohanty BK, Kushner SR. Polynucleotide phosphorylase, RNase II and RNase E play different roles in the in vivo modulation of polyadenylation in Escherichia coli. Mol Microbiol 2000; 36:982 - 994
- Mohanty BK, Kushner SR. Polynucleotide phosphorylase functions both as a 3′ right-arrow 5′ exonuclease and a poly(A) polymerase in Escherichia coli. Proc Nat Acad Sci USA 2000; 97:11966 - 11971