Abstract
The archaeal exosome is a prokaryotic protein complex with RNA processing and degrading activities. Recently it was shown that the exosome is localized at the periphery of the cell in the thermoacidophilic archaeon Sulfolobus solfataricus. This localization is most likely mediated by the archaeal DnaG protein and depends on (direct or indirect) hydrophobic interactions with the membrane. A localization of RNA degrading proteins and protein complexes was also demonstrated in several bacteria. In bacteria a subcellular localization was also shown for substrates of these proteins and protein complexes, i.e. chromosomally encoded mRNAs and a small RNA. Thus, despite the missing compartmentalization, a spatial organization of RNA processing and degradation exists in prokaryotic cells. Recent data suggest that the spatial organization contributes to the temporal regulation of these processes.
Introduction
The exosome was first described as an essential protein complex involved in processing and degradation of RNA in Eukarya.Citation1 The core of the eukaryotic exosome shows similarity to the bacterial polynucleotide phosphorylase (PNPase) and to an RNA degrading protein complex in Archaea, named the archaeal nine-subunit exosome.Citation2–Citation7 In Archaea, the exosome strongly interacts with a protein annotated as the bacterial type primase DnaG.Citation4,Citation8 Recently, the DnaG-containing exosome was shown to localize at the periphery of the cells of the termoacidophilic archaeon Sulfolobus solfataricus.Citation9 Localization of RNA processing components is also known from bacteria.Citation10–Citation13 These findings open new perspectives to understand the strategy of prokaryotic cells to achieve spatial and temporal organization of RNA degradation events. Here we summarize the recent data in the field and discuss their implications.
The Archaeal Nine Subunit Exosome and its Interaction with aDnaG
The archaeal exosome is a protein complex with phosphorolytic exoribonuclease activity degrading RNA in 3′ to 5′ direction.Citation5,Citation6,Citation8,Citation14 In a reverse reaction, it synthesizes heteropolymeric RNA tails.Citation15,Citation16 Its nine-subunit core comprises three aRrp41 (archaeal Rrp41) subunits, three aRrp42 subunits and three aRrp4 and/or aCsl4 subunits. The active site is located in aRrp41, which forms together with aRrp42 a catalytically active hexameric ring. The RNA-binding domains containing subunits aRrp4 and/or aCsl4 are located on the top of the hexamer building a trimeric cap. The substrates are recruited by the RNA-binding cap and the 3′-end is threaded into the central channel of the hexamer, where degradation or synthesis takes place.Citation5–Citation7 Both cap proteins influence the substrate specificity of the exosome and its capability to degrade structured RNAs.Citation17
The nine subunit exosome strongly interacts with the archaeal protein DnaG (aDnaG), which is annotated as a bacterial type primase. aDnaG was co-purified with the S. solfataricus exosome and was shown to be in a complex with the exosome in Methanothermobacter thermoautotrophicus, suggesting that it plays a role in RNA metabolism.Citation8,Citation18 The following data support this view and characterize aDnaG as a subunit of the archaeal exosome: (1) aRrp41-depletion is paralleled by aDnaG-depletion in cell-free extracts.Citation8 (2) Essentially identical protein complexes containing the above mentioned subunits of the exosome are purified using antibodies directed against aDnaG or Rrp41.Citation8 (3) aDnaG is strongly associated with the exosome and cannot be eluted separately using MgCl2 or NaCl in concentrations up to 2 M (own unpublished data).
Although not all sequenced Archaea contain an exosome, all of them harbor the gene encoding aDnaG, suggesting an important, exosome-unrelated function for this protein. Recently it was shown that aDnaG of S. solfataricus exhibits primase activity and it was proposed that aDnaG performs, together with the heterodimeric primase of eukaryotic type, replication of DNA in Archaea.Citation19 An essential primase function of aDnaG is a plausible explanation for the high conservation of this protein in Archaea. It is conceivable that aDnaG plays a dual role in exosome-containing Archaea contributing to DNA replication and to (regulation of) RNA processing and degradation.
Subcellular Localization of the Exosome in Archaea
A major part of aRrp41 (representing the nine-subunit exosome) and aDnaG are found in the pellet after ultracentrifugation of S. solfataricus cell-free extract at 100,000 g, indicating that the non-soluble, aDnaG-containing exosome is either bound to the membrane, or to ribosomal subunits, or is part of very large, supramolecular complexes.Citation9 The aDnaG-containing exosome co-sediments with ribosomal subunits in glycerol density gradients and in sucrose density gradients under low salt conditions (150 mM NaCl), but it is not clear whether the exosome specifically interacts with ribosomes. A minor part of the exosome without aDnaG is detectable in fractions of low density representing the soluble, cytoplasmic exosome.Citation4,Citation8,Citation9 A 16 kDa protein of unknown function was coimmunoprecipitated with the low density exosome, suggesting that the soluble and the non-soluble nine-subunit exosome interact with different proteins.Citation8
Under high salt conditions (500 mM ammonium chloride), the aDnaG-containing exosome was enriched in the membrane fraction together with surface layer proteins in sucrose density gradients.Citation9 Western blot analysis was used to detect aDnaG and aRrp41 in the membrane fractions. It should be noted that the composition of the loading buffer and the heat treatment prior to loading on SDS gels may differently influence the resolving and blotting of the highly abundant transmembrane proteins, and of aDnaG and aRrp41, which do not harbor transmembrane domains.Citation20 When a loading buffer with higher SDS and betamercaptoethanol content was applied and samples were heated to 50°C instead of 100°C, gel separation of membrane proteins and western blot detection of aRrp41 were reproducible, but the detection of aDnaG was negatively affected (unpublished results).
In the last years the proteome of S. solfataricus was analyzed by several independent groups and special attempts were made to identify the membrane proteins of this archaeon. The membrane proteins in these studies were either defined as insoluble proteins harboring identifiable transmembrane domains, or as proteins with transmembrane domains, which co-sediment with membranes in sucrose density gradients. Some proteins without transmembrane domains were also found in the insoluble/membrane fractions, and aRrp41 and aDnaG were among them, supporting the view that the exosome is localized at the membrane.Citation21,Citation22 Finally, aDnaG and aRrp41 were detected at the periphery of S. solfataricus cells using immunofluorescence microscopy.Citation9
Taken together, the data strongly suggest that aDnaG is responsible for the localization of the exosome in S. solfataricus and that the aDnaG-containing exosome interacts with the membrane, with (a) transmembrane protein(s) or with a structure beneath the membrane via hydrophobic forces (). The subcellular localization of an RNA degrading protein complex in the third domain of life, Archaea, was not unexpected, since several examples for peripheral localization of RNases and RNase-containing complexes in the other prokaryotic domain of life, Bacteria, were shown recently.Citation10–Citation13 Below we shortly summarize the knowledge on localization of RNases in Bacteria and in Eukarya, which are phylogenetically more closely related to Archaea than to Bacteria.Citation23
Subcellular Localization of RNases in Eukarya and in Bacteria
The eukaryotic cells harbor different compartments delimited by membranes. In this way transcription and maturation of RNA (in the nucleus) are spatially separated from translation (in the cytoplasm). Decay of eukaryotic RNA takes place in both the nucleus and the cytoplasm, since defect RNAs are not transported into the cytoplasm but are degraded in the nucleus, and mRNAs have a limited lifespan in the cytoplasm.Citation24 Usually different RNases are localized in the different compartments. For example, two distinct 5′ to 3′ exoribonucleases operate in the nucleus and in the cytoplasm of eukaryotic cells, respectively.Citation25
The nine-subunit core of the eukaryotic exosome is catalytically inactive but structurally highly similar to the archaeal nine-subunit exosome.Citation2 It is present in the nucleus and in the cytoplasm. The nine-subunit core interacts with at least one ribonucleolytically active subunit, which may be identical in the nucleus and in the cytoplasm, and with additional, compartment-specific proteins.Citation24,Citation26 In the cytoplasm, the exosome was found in specific granules probably involved in AU-rich element mediated mRNA decay.Citation27 These cytoplasmic granules are different from the stress granules, the processing bodies and other particles, in which RNA is translationally arrested and/or degraded, and which contain specific RNA binding proteins and RNases.Citation28,Citation29 These examples illustrate that in eukaryotic cells the spatial organization of RNA processing and degradation is ensured not only via compartmentalization, but also by sub-localization of RNases within the compartments.
In contrast to the eukaryotic cells, the prokaryotic bacterial cells encounter fundamental problems to spatially separate cellular components, since they lack organelles. Recent data on phylogenetically distant bacteria show that RNases and RNase-comprising protein complexes are not evenly distributed in the cytoplasm but are localized. The specific subcellular localization is mediated by different mechanisms which are, in most of the cases, still not elucidated. The emerging principles are direct interaction with the cytoplasmic membrane, formation of or binding to filamentous structures which may interact with the membrane, and localization in the vicinity of the nucleoid by unknown mechanisms.
The gram-negative gamma-proteo-bacterium Escherichia coli, the best studied bacterial model organism, contains an RNA-degrading, membrane-bound protein complex called the degradosome, which is organized by the essential endoribonuclease RNase E.Citation10 RNase E consists of an N-terminal, catalytically active domain and a C-terminal scaffold region, to which the RNA helicase RhlB, enolase and polynucleotide phosphorylase (PNPase) are bound.Citation30 RNase E directly interacts with the membrane via a short amphipathic helix located between the N-terminal and the C-terminal domains (). The membrane localization of RNase E is important for normal growth, but the underlying mechanisms are still unknown.Citation31 The above mentioned degradosomal components can be visualized in the cell as helical, cytoskeleton-like structures. These structures rely on RNase E and RhlB—both proteins are localized in similar helical structures independently of each other.Citation11,Citation32 RhlB itself can assemble in filaments in an ATP-dependent manner, but it is not clear whether it interacts with the membrane or with some structure beneath the membrane.Citation33 The molecular interactions responsible for the appearance of the membrane-bound RNase E in cytoskeleton-like structures are not known.
Both RNase E and RhlB can independently interact with PNPase in RNA-degrading complexes. Therefore, the presence of RNase E or RhlB in the cell is sufficient for localization of PNPase, while enolase is localized solely via its interaction with RNase E ().Citation33,Citation34 PNPase is structurally and functionally similar to the archaeal exosome: both degrade RNA phosphorolytically in 3′-5′ direction and are responsible for the synthesis of heteropolymeric tails, which can be used as loading platforms in the process of RNA degradation.Citation6,Citation15,Citation35 In vitro, changes in the concentration of NDPs and phosphate influence the opposite activities of prokaryotic PNPase and of the archaeal exosome as RNases or RNA tailing enzymes.Citation16,Citation36 It remains an unresolved question how these activities are regulated in the cell and whether the subcellular localization of the enzymes contributes to this regulation.
The gram-negative α-proteobacterium Caulobacter crescentus harbors an RNase E with a different localization in comparison to E. coli. The N-terminal half of C. crescentus RNase E shows strong similarity to the N-terminal, catalytic part of the E. coli protein, while the C-terminal half is not conserved and organizes the C. crescentus degradosome containing PNPase, an RNA helicase and aconitase.Citation37 There is no evidence for the presence of an amphipathic helix in RNase E of C. crescentus, and RNase E is not at the membrane in this organism. However, it is also not randomly distributed in the cytoplasm, but is localized in the vicinity of the nucleoid, where its potential RNA substrates were also found ().Citation13 Interestingly, another ribonuclease, the 3′-5′ exoribonuclease RNase R was detected in helix-like structures in this organism ().Citation12 The mechanisms responsible for the localization of these RNases in C. crescentuis were not explored so far.
Another example for the specific localization of an RNase is found in the gram-positive model organism B. subtilis. It lacks RNase E, but harbors the membrane-bound endoribonuclease RNase Y, which was shown to interact with other proteins, and was proposed to organize a degradosome-like complex at the periphery of the cell ().Citation38–Citation40 As subunits of the B. subtilis degradosome PNPase, the RNA helicase CshA, RNases J1 and J2, enolase and phosphofructokinase were suggested.Citation39,Citation40 However, in another study, the interaction of RNaseJ1 and of the RNase J1-J2 complex with RNase Y was questioned.Citation41
The described parallels and differences to E. coli suggest that subcellular localization of RNases is physiologically important for bacteria, and that based on similar principles, different mechanisms ensuring the correct RNA processing and degradation developed in different organisms.
Consequences of Spatial Organization of RNA Processing and Degradation in Prokaryotes
The finding that RNA-degrading proteins and protein complexes are localized in Bacteria and in Archaea raises questions about the consequences of spatial organization of RNA processing and degradation in prokaryotes. Are the RNases localized to keep them away from the substrates or to keep them in the vicinity of the substrates? If both possibilities are true, then we should expect temporal changes in the localization of RNases and/or RNA substrates. Indeed, an example of such a spatial and temporal regulation of RNA degradation is the cell cycle dependent localization of tmRNA in C. crescentus. As mentioned above, RNase R, which degrades tmRNA, is localized in helix-like structures in this organism. Via its binding to the protein SmpB, tmRNA is also localized in helix-like structures during the G1-phase, but does not colocalize with RNase R and is thus spatially separated (and protected) from RNase R (). The localization of tmRNA is lost after initiation of DNA replication and tmRNA is degraded, although the localization pattern of RNase R does not change.Citation12
Specific localization of RNAs in bacteria seems to be the rule and not the exception. Recently it was shown that in both E. coli and C. crescentus, the mRNAs of chromosomally encoded proteins do not diffuse into the cytoplasm but remain localized in the vicinity of their genetic loci ( and C).Citation13 Different subcellular localization of mRNAs (near the corresponding genetic loci) and RNases (at the membrane, at filaments beneath the membrane or in the cytoplasm) may be important to regulate the controlled RNA degradation. This also implies the existence of mechanisms by which specifically localized RNases or RNAs are released to reach the appropriate interaction partner under certain conditions.
Conclusions
The picture emerges that prokaryotes developed sophisticated mechanisms to organize and regulate spatially and temporally the events of RNA processing and RNA degradation, although they lack organelles and cellular compartmentalization is limited. Attachment to the membrane, formation of cytoskeleton-like structures or interaction with such structures help to arrange the macromolecules dealing with RNA in the proper order, to separate them spatially from each other and from potential substrates, or to enable their interaction with the substrates. The increasing amount of data on the specific localization of different macromolecules and even of individual chromosomal loci in Bacteria, and the detection of many different, on the majority still unexplored, cytoskeletal filaments in Bacteria and in Archaea, point to a highly organized interior of prokaryotic cells.Citation42–Citation50
Figures and Tables
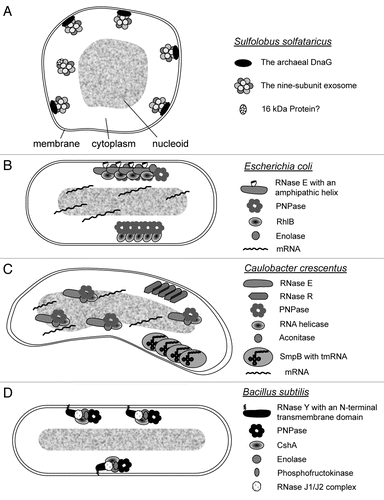
Figure 1 Current models of the subcellular localization of RNAs, RNases and RNase-containing complexes in prokaryotes. (A) Localization of the exosome in S. solfataricus. The nine-subunit exosome and aDnaG localize to the membrane by an unknown mechanism.Citation9 A small fraction of the exosome is soluble and does not contain DnaG, but probably interacts with other proteins like the 16 kDa protein Sso0218.Citation8,Citation9 (B) Localization of the degradosome, the RhIB-PNPase complex and of mRNAs in E. coli. The degradosome is bound to the membrane via an amphipathic helix of RNase E.Citation10,Citation31 The other components of the protein complex, PNPase, RhIB and enolase, bind to the C-terminal part of RNase E.Citation10,Citation30 The degradosome can be visualized in a helical, cytoskeleton-like pattern. The independent RhIB-PNPase complex is also organized in cytoskeleton-like structures, but it is not clear whether it directly interacts with the membrane.Citation11,Citation32 RhIB is capable of forming filaments.Citation33 Chromosomally encoded mRNAs remain in the vicinity of their genetic loci.Citation13 (C) Localization of RNase R, RNase E, tmRNA and mRNAs in C. crescentus. Chromosomally encoded mRNAs remain in the vicinity of their genetic loci. RNase E co-localizes with the nucleoid.Citation13 Thus, the degradosome which is organized by RNase E and contains PNPase, an RNA helicase and aconitase, is probably also localized.Citation37 RNase R was found in a helix-like structure different from the helix-like structure, in which tmRNA bound to SmpB was detected during the G1 phase.Citation12 It is not clear whether RNase R and SmpB interact with the membrane or with some filaments beneath the membrane, or whether these proteins form filaments. (D) Localization of RNase Y in B. subtilis. RNase Y contains an N-terminal transmembrane helix and is localized at the cell periphery.Citation38 It was proposed to organize a subcellularly localized protein complex containing PNPase, the RNA helicase CshA, enolase, phosphofructokinase and the RNases J1 and J2.Citation39,Citation40
Acknowledgements
This work was supported by Deutsche Forschungsgemeinschaft (grant Kl 563/18-3 and IRTG “Enzyme and multienzyme complexes acting on nucleic acids”).
References
- Mitchell P, Petfalski E, Shevchenko A, Mann M, Tollervey D. The exosome: a conserved eukaryotic RNA processing complex containing multiple 3′-5′ exoribonucleases. Cell 1997; 91:457 - 466
- Liu Q, Greimann JC, Lima CD. Reconstitution, activities and structure of the eukaryotic RNA exosome. Cell 2006; 127:1223 - 1237
- Symmons MF, Williams MG, Luisi BF, Jones GH, Carpousis AJ. Running rings around RNA: a superfamily of phosphate-dependent RNases. Trends Biochem Sci 2002; 27:11 - 18
- Evguenieva-Hackenberg E, Walter P, Hochleitner E, Lottspeich F, Klug G. An exosome-like complex in Sulfolobus solfataricus. EMBO Rep 2003; 4:889 - 893
- Büttner K, Wenig K, Hopfner KP. Structural framework for the mechanism of archaeal exosomes in RNA processing. Mol Cell 2005; 20:461 - 471
- Lorentzen E, Walter P, Fribourg S, Evguenieva-Hackenberg E, Klug G, Conti E. The archaeal exosome core is a hexameric ring structure with three catalytic subunits. Nat Struct Mol Biol 2005; 12:575 - 581
- Lorentzen E, Dziembowski A, Lindner D, Seraphin B, Conti E. RNA channelling by the archaeal exosome. EMBO Rep 2007; 8:470 - 476
- Walter P, Klein F, Lorentzen E, Ilchmann A, Klug G, Evguenieva-Hackenberg E. Characterization of native and reconstituted exosome complexes from the hyperthermophilic archaeon Sulfolobus solfataricus. Mol Microbiol 2006; 62:1076 - 1089
- Roppelt V, Hobel CF, Albers SV, Lassek C, Schwarz H, Klug G. The archaeal exosome localizes to the membrane. FEBS Lett 2010; 584:2791 - 2795
- Liou GG, Jane WN, Cohen SN, Lin NS, Lin-Chao S. RNA degradosomes exist in vivo in Escherichia coli as multicomponent complexes associated with the cytoplasmic membrane via the N-terminal region of ribonuclease E. Proc Natl Acad Sci USA 2001; 98:63 - 68
- Taghbalout A, Rothfield L. RNaseE and the other constituents of the RNA degradosome are components of the bacterial cytoskeleton. Proc Natl Acad Sci USA 2007; 104:1667 - 1672
- Russell JH, Keiler KC. Subcellular localization of a bacterial regulatory RNA. Proc Natl Acad Sci USA 2009; 106:16405
- Montero Llopis P, Jackson AF, Sliusarenko O, Surovtsev I, Heinritz J, Emonet T, et al. Spatial organization of the flow of genetic information in bacteria. Nature 2010; 466:77 - 81
- Lorentzen E, Conti E. Structural basis of 3′ end RNA recognition and exoribonucleolytic cleavage by an exosome RNase PH core. Mol Cell 2005; 20:473 - 481
- Portnoy V, Evguenieva-Hackenberg E, Klein F, Walter P, Lorentzen E, Klug G, et al. RNA polyadenylation in Archaea: not observed in Haloferax while the exosome polynucleotidylates RNA in Sulfolobus. EMBO Rep 2005; 6:1188 - 1193
- Evguenieva-Hackenberg E, Roppelt V, Finsterseifer P, Klug G. Rrp4 and Csl4 are needed for efficient degradation but not for polyadenylation of synthetic and natural RNA by the archaeal exosome. Biochemistry 2008; 47:13158 - 13168
- Roppelt V, Klug G, Evguenieva-Hackenberg E. The evolutionarily conserved subunits Rrp4 and Csl4 confer different substrate specificities to the archaeal exosome. FEBS Lett 2010; 584:2931 - 2936
- Farhoud MH, Wessels HJ, Steenbakkers PJ, Mattijssen S, Wevers RA, van Engelen BG, et al. Protein complexes in the archaeon Methanothermobacter thermautotrophicus analyzed by blue native/SDS-PAGE and mass spectrometry. Mol Cell Proteomics 2005; 4:1653 - 1663
- Zuo Z, Rodgers CJ, Mikheikin AL, Trakselis MA. Characterization of a functional DnaG-type primase in archaea: implications for a dual-primase system. J Mol Biol 2010; 397:664 - 676
- von Jagow G, Schägger H. A practical guide to membrane protein purification 1994; Academic Press
- Barry RC, Young MJ, Stedman KM, Dratz EA. Proteomic mapping of the hyperthermophilic and acidophilic archaeon Sulfolobus solfataricus P2. Electrophoresis 2006; 27:2970 - 2983
- Pham TK, Sierocinski P, van der Oost J, Wright PC. Quantitative proteomic analysis of Sulfolobus solfataricus membrane proteins. J Proteome Res 2010; 9:1165 - 1172
- Woese CR, Kandler O, Wheelis ML. Towards a natural system of organisms: proposal for the domains Archaea, Bacteria and Eucarya. Proc Natl Acad Sci USA 1990; 87:4576 - 4579
- Houseley J, LaCava J, Tollervey D. RNA-quality control by the exosome. Nat Rev Mol Cell Biol 2006; 7:529 - 539
- Johnson AW. Rat1p and Xrn1p are functionally interchangeable exoribonucleases that are restricted to and required in the nucleus and cytoplasm, respectively. Mol Cell Biol 1997; 17:6122 - 6130
- Tomecki R, Kristiansen MS, Lykke-Andersen S, Chlebowski A, Larsen KM, Szczesny RJ, et al. The human core exosome interacts with differentially localized processive RNases: hDIS3 and hDIS3L. EMBO J 2010; 29:2342 - 2357
- Lin WJ, Duffy A, Chen CY. Localization of AU-rich element-containing mRNA in cytoplasmic granules containing exosome subunits. J Biol Chem 2007; 282:19958 - 19968
- Anderson P, Kedersha N. RNA granules. J Cell Biol 2006; 172:803 - 808
- Zabolotskaya MV, Grima DP, Lin MD, Chou TB, Newbury SF. The 5′-3′ exoribonuclease Pacman is required for normal male fertility and is dynamically localized in cytoplasmic particles in Drosophila testis cells. Biochem J 2008; 416:327 - 335
- Carpousis AJ. The RNA degradosome of Escherichia coli: an mRNA-degrading machine assembled on RNase E. Annu Rev Microbiol 2007; 61:71 - 87
- Khemici V, Poljak L, Luisi BF, Carpousis AJ. The RNase E of Escherichia coli is a membrane-binding protein. Mol Microbiol 2008; 70:799 - 813
- Taghbalout A, Rothfield L. RNase E and RNA helicase B play central roles in the cytosceletal organization of the RNA degradosome. J Biol Chem 2008; 283:13850 - 13855
- Taghbalout A, Yang Q. Self-assembly of the bacterial cytoskeleton-associated RNA helicase B protein into polymeric filamentous structures. J Bacteriol 2010; 192:3222 - 3226
- Liou GG, Chang HY, Lin CS, Lin-Chao S. DEAD box RhlB RNA helicase physically associates with exoribonuclease PNPase to degrade double-stranded RNA independent of the degradosome-assembling region of RNase E. J Biol Chem 2002; 277:41157 - 41162
- Mohanty BK, Kushner SR. Polynucleotide phosphorylase functions both as a 3′ right-arrow 5′ exonuclease and a poly(A) polymerase in Escherichia coli. Proc Natl. Acad Sci USA 2000; 97:11966 - 11971
- Yehudai-Resheff S, Hirsh M, Schuster G. Polynucleotide phosphorylase functions as both an exonuclease and a poly(A) polymerase in spinach chloroplasts. Mol Cell Biol 2001; 21:5408 - 5416
- Hardwick SW, Chan VS, Broadhurst RW, Luisi BF. An RNA degradosome assembly in Caulobacter crescentus. Nucleic Acids Res 2010; 39:1449 - 1459
- Hunt A, Rawlins JP, Thomaides HB, Errington J. Functional analysis of 11 putative essential genes in Bacillus subtilis. Microbiology 2006; 152:2895 - 2907
- Commichau FM, Rothe FM, Herzberg C, Wagner E, Hellwig D, Lehnik-Habrink M, et al. Novel activities of glycolytic enzymes in Bacillus subtilis: interactions with essential proteins involved in mRNA processing. Mol Cell Proteomics 2009; 8:1350 - 1360
- Lehnik-Habrink M, Pförtner H, Rempeters L, Pietack N, Herzberg C, Stülke J. The RNA degradosome in Bacillus subtilis: identification of CshA as the major RNA helicase in the multiprotein complex. Mol Microbiol 2010; 77:958 - 971
- Mathy N, Hébert A, Mervelet P, Bénard L, Dorléans A, de la Sierra-Gallay IL, et al. Bacillus subtilis ribonucleases J1 and J2 form a complex with altered enzyme behaviour. Mol Microbiol 2010; 75:489 - 498
- Mascarenhas J, Weber MH, Graumann PL. Specific polar localization of ribosomes in Bacillus subtilis depends on active transcription. EMBO Rep 2001; 2:685 - 689
- Weber MH, Volkov AV, Fricke I, Marahiel MA, Graumann PL. Localization of cold shock proteins to cytosolic spaces surrounding nucleoids in Bacillus subtilis depends on active transcription. J Bacteriol 2001; 183:6435 - 6443
- Simmons LA, Grossman AD, Walker GC. Clp and Lon proteases occupy distinct subcellular positions in Bacillus subtilis. J Bacteriol 2008; 190:6758 - 6768
- Viollier PH, Thanbichler M, McGrath PT, West L, Meewan M, McAdams HH, et al. Rapid and sequential movement of individual chromosomal loci to specific subcellular locations during bacterial DNA replication. Proc Natl Acad Sci USA 2004; 101:9257 - 9262
- Briegel A, Dias DP, Li Z, Jensen RB, Frangakis AS, Jensen GJ. Multiple large filament bundles observed in Caulobacter crescentus by electron cryotomography. Mol Microbiol 2006; 62:5 - 14
- Trent JD, Kagawa HK, Yaoi T, Olle E, Zaluzec NJ. Chaperonin filaments: the archaeal cytoskeleton?. Proc Natl Acad Sci USA 1997; 94:5383 - 5388
- Mayer F, Vogt B, Poc C. Immunoelectron microscopic studies indicate the existence of a cell shape preserving cytoskeleton in prokaryotes. Naturwissenschaften 1998; 85:278 - 282
- Hara F, Yamashiro K, Nemoto N, Ohta Y, Yokobori S, Yasunaga T, et al. An actin homolog of the archaeon Thermoplasma acidophilum that retains the ancient characteristics of eukaryotic actin. J Bacteriol 2007; 189:2039 - 2045
- Junglas B, Briegel A, Burghardt T, Walther P, Wirth R, Huber H, et al. Ignicoccus hospitalis and Nanoarchaeum equitans: ultrastructure, cell-cell interaction and 3D reconstruction from serial sections of freeze-substituted cells and by electron cryotomography. Arch Microbiol 2008; 190:395 - 408