Abstract
The biosynthesis of steroids and the production of spermatozoa are two major functions of the mammalian testis, which are tightly controlled by gonadotropins and numerous locally produced factors. Among these are the estrogens that are produced within the seminiferous epithelium via the irreversible transformation of androgens (C19) into estrogens (C18) by aromatase. We have recently reported that male germ cells are the new source of estrogens in the testis. For instance, estrogen receptors (ER) are found mainly in spermatids that give rise spermatozoa. Moreover, it is noteworthy that GPR30 (a transmembrane ER) induces rapid responses after estradiol binding, which, in turn, modulates cyclins and proapoptotic factors (e.g., BAX) to affect germ cell cycle progression and apoptosis. In summary, at least in the animal species that were studied thus far, germ cells are the major source and the target of estrogens, affecting normal male gonadal development and spermatogenesis, in particular spermiogenesis. These findings have also shed new light on the possible adverse effects of endocrine disruptors having estrogenic activities that can cause abnormal development of the male genital tract.
Male Germ Cells as a New Source of Estrogens in the Testis More than 80 years have past since the pioneering work of ZondekCitation1 who demonstrated that the gonad in stallions produces estrogens. This was followed by a study by Dorrington et al. reporting that the rat testis is able to synthesize estrogens from androgens. In the testis, as in other tissues, cytochrome P450 aromatase is the enzyme that irreversibly transforms androgens into estrogens. In fetal and immature animals, Sertoli cells are the main source of estrogens,Citation5–Citation7 and the expression of aromatase in Leydig cells is very low.Citation3,Citation4 Regardless, the cellular sites of aromatisation change during development, and in the adult mammalian testis, aromatase is localized mostly in Leydig cells.Citation4 During the last decade, there has been growing evidence that within the seminiferous tubules, germ cells, at least in the species studied so far, also participate in the production of testicular estrogens.Citation8 For example, Nitta et al.Citation9 have shown that adult mouse germ cells express a functional aromatase and that estradiol output is equivalent to that of Leydig cells. Similar to Leydig cells, aromatase expression is relatively low in adult rat Sertoli cells,Citation10 and Sertoli cell aromatase is negatively controlled by germ cells.Citation11 In addition, we have demonstrated that adult rat germ cells are an important source of estrogens and that the level of aromatase contributed by germ cells represents ∼60% of that of the whole testis.Citation8 Moreover, numerous aromatase transcripts were found to be unable to encode a functional protein.Citation12 Interestingly, aromatase activity is much higher in late haploid germ cells than in less differentiated germ cells,Citation8 illustrating these quiescent cells have the ability to “regulate” their fate and development during spermatogenesis. Very recently, we have also reported the presence of aromatase mRNA in gonocytes and preleptotene spermatocytes (Bois et al. submitted data), illustrating that all testicular cells express aromatase except peritubular myoid cells.Citation13 Additionally, studies using genetic models have demonstrated the significance of germ cell derived estrogens on male reproductive function. For instance, male mice deficient in aromatase (ArKO) develop normally and are able to breed and to produce litters; however starting at the age of 5 months and onwards, ArKO males present with a failure of spermatogenesis and by the age of one year, they are all infertile. Specifically, spermatogenesis is abnormal in these ArKO mice with a blockage of germ cell maturation at the spermatid stage, leading to a 50% decrease in the number of round and elongated spermatids and their apoptosis.Citation22
Male Germ Cells: A Target for Estrogens
In order to exert a biological effect, estrogens must bind to estrogen receptors (ER), which in turn modulates the transcription of estrogen-responsive target genes (genomic effect). Alternatively, estrogens can activate different signaling pathways by interacting with membrane-bound binding proteins (non-genomic effect). Two types of nuclear ERs are known to exist: ERα (ESR1) and ERβ (ESR2). The distribution of these ERs has been described in the male rat gonad, but there is still debate about their localization.Citation8 Likewise, we have recently reported the presence of transcripts for both ERs in the adult rat testisCitation14 and showed the presence of ERs proteins in purified germ cells.Citation15,Citation16 It has been speculated that round spermatids may be one of the target cell types of estrogens since high levels of ERβ have been demonstrated in these cells.Citation17
Additional evidence came from observations of patients with either congenital estrogen deficiency or estrogen resistance, as well as from animal models such as mice deficient in aromatase (ArKO) or in ERs (ERKO). It should be noted that impaired sperm motility and germ cells arrest (at the spermatid stage) were observed in both aromatase deficient men and mice.Citation18,Citation19 Thus, these observations illustrate a putative link between the lack of estrogen activity and infertility in men, illustrating the important role played by estrogens on male reproductive function. Conversely, exposure of adult male rats to a high phytoestrogen diet disrupts spermatogenesis via an increase in germ cell apoptosis,Citation20 and overexpression of aromatase in mice leads to male infertility in all or in 50% of animals when it takes place either in fetal life or at puberty, respectively.Citation21 In short, there is a delicate balance between androgens and estrogens to maintain normal testicular physiology and reproductive function with aromatase being an important molecule in these cellular events ().
In several mammalian species the synthesis and the role of estrogens have been recently explored. In the black bear (Ursus americanus), the presence of aromatase has been reported at the beginning of testicular recrudescence in Sertoli cells and then in round and elongating spermatids during the mating season.Citation23 In the Siberian hamster, estrogens are able to induce initiation of spermatogenesis, independently of FSH in the photo-regressed adult male.Citation24 In roe deer (Capreolus capreolus), estrogens were shown to be implicated in sperm production and in spermatozoal maturation.Citation25 In the wild male ground squirrel (Citellus dauricus Brandt), a positive immunoreactivity for aromatase has been detected in Leydig and Sertoli cells and all types of spermatogenic cells but only during the breeding season. These authors concluded that estrogens play an important role in spermatogenesis, testicular recrudescence and regression.Citation26 Similar observations have been published in the bank vole by the group of Bilinska.Citation27,Citation28
Moreover, treatment of adult monkeys with an aromatase inhibitor have suggested the involvement of estrogens in spermatid differentiation.Citation29,Citation30 The study conducted by the group of Balasinor in rats suggested that the process of spermatid elongation from steps 8 to 19 is androgen-dependent, whereas differentiation of round spermatids from steps 1 to 6 is regulated by estrogen.Citation31 The observation of abnormal acrosome development in the ArKO mouse suggests that acrosome biogenesis may be an estrogen-dependent process.Citation22 This hypothesis is in agreement with the observation that a high level of aromatase is detected in the Golgi complex of the developing spermatid,Citation9 as well as by the presence of ERs in rat spermatids.Citation14,Citation16 Estrogens can also induce rapid effects via non-genomic pathways, that is, they bind onto membrane receptors to activate different signaling pathways via the production of second messengers (e.g., Ca2+, cAMP, NO) and the activation of tyrosine kinase receptors (e.g., EGFR, IGF-1R) and protein/lipid kinases (e.g., PI3kinase, Akt, members of the MAP kinase family or Src kinases, PKA, PKC).Citation32,Citation33
Recent data have illustrated that the seven transmembrane receptor associated to a G protein (GPR30) is involved in the proliferation-inducing effects of estrogens in a GC1 cell lineCitation34 and that GRP30 is present in human and rodent testicular cells.Citation8 GPR30 is notably involved in the induction of expression of apoptotic markers (e.g., BAX, FAS and FASL) by estradiol in rat pachytene spermatocytes and round spermatids.Citation15,Citation16 Studies conducted in rodents and primates have shown that spermatogenesis is under the control of estrogens at different levels. For example, in the immature bank vole, exposure to a low dose of 17β-estradiol was shown to accelerate the onset of spermatogenesis, which was blocked by the injection of the antiestrogen, ICI 182,780 ().Citation28 Other studies have demonstrated an improvement in the recrudescence of spermatogenesis in estradiol-treated rodents.Citation24,Citation35 Neonatal administration of estrogen to rats induced an increase in the number of spermatogonia at day 16 post-coitus.Citation36 Similarly, studies from Culty's group have shown that rat gonocyte multiplication is partly under estradiol control,Citation37,Citation38 except that endogenous estradiol can inhibit gonocyte development in mice.Citation39 There is also evidence for the direct involvement of estrogens in preventing germ cell apoptosis as demonstrated by the effects of estradiol in human adult seminiferous tubule cultures.Citation40
Conversely, exposure of adult male rats to a high phytoestrogen diet disrupts spermatogenesis by increasing germ cell apoptosis.Citation20 A significant decrease of the numbers of round and elongated spermatids, but not spermatogonia and spermatocytes, in ArKO mice clearly demonstrates a role of estrogens in regulating survival factor(s) in spermatids.Citation22
It is also well known that estrogens can trigger pachytene cells to undergo apoptosis before further differentiation.Citation45 Thus, estrogens are important to maintain the proper Sertoli:germ cell ratio in the seminiferous epithelium. Additionally, estradiol also stimulates spermatozoal motility in golden hamstersCitation41 and humans.Citation42,Citation43 In addition, Fraser and collaborators have reported a positive effect of estrogenic xenobiotic genestein on capacitation in human and mouse spermatozoa.Citation44
In contrast to androgen receptors, which are localized mostly in somatic cells, estrogen receptors are found in most testicular cells, including germ cells. The ERα gene is highly expressed in the adult testis, in particular at stages VII to XIV. In addition, the steady-state mRNA levels of both ERs are higher in purified round spermatids than in pachytene spermatocytes, suggesting a putative role of estrogens in the haploid steps of spermatogenesis, namely spermiogenesis.Citation14
As already demonstrated in ArKO miceCitation22 and from data obtained from the ERαKO, estrogens do play an important role in germ cell development.Citation8 Nevertheless, it has been claimed that GPR30 is not involved in estrogenic responses in reproductive organs in mice.Citation46 However, these authors generated Gpr30 deficient mice and showed that mutant males and females are both fertile; it is noteworthy that data were not provided for any functional aspects of spermatogenesis. Conversely, in zebrafish the identification of a functionally-active membrane ER has been reported with a high homology when compared to mammalian GPER, which was expressed in spermatogonia, spermatocytes and Sertoli cells.Citation47 Moreover, it has been reported that in immature rat Sertoli cells, besides ERs, a functional GPER was expressed and involved in anti-apoptotic control.Citation48
Estrogens, Human Fertility and Future Perspectives
Idiopathic infertility is a worldwide health issue, and no efficient treatment is available to date. While the role of estrogens in spermatogenesis is still a matter of debate, but from the observations of reduced sperm number and motility in men genetically deficient in aromatase,Citation18,Citation19 which coupled with our data and those of literature as briefly discussed above, these findings suggest a role for aromatase/estrogens not only during the development and maintenance of spermatogenesis but also in spermatozoa maturation via spermiogenesis since not only aromatase transcript but also a biologically active protein namely Gpr30 as well as ERs have been demonstrated.Citation49 In humans, the main source of estrogens is the Leydig cell.Citation4,Citation50 However, adult Sertoli cells are also able of synthesizing estradiol in vitro,Citation51 and estrogens are found in ejaculated spermatozoa.Citation52 Aromatase mRNA was also reported in immature germ cells and in ejaculated spermatozoa,Citation53 and estrogen seems to be related to the quality (such as motility) of sperm cells.Citation54,Citation55
In short, it is increasingly clear that estrogens, ERs and aromatase are found and/or produced by immature germ cells including spermatocytes and spermatids, as well as ejaculated spermatozoa in humans and many other species. Recent studies have demonstrated their involvement in male gamete maturation, in particular spermiogenesis, and the final steps of sperm maturation including capacitation and/or acrosome reaction. More importantly, ERs are found throughout the spermatozoon and localized intensely within mitochondria, an organelle found in the mid-piece of spermatozoa that provides the energy source for flagellar movement in the female reproductive tract. In addition, aromatase and ER mRNA transcripts could be relevant markers for assessing infertility in men and may serve also as diagnostic tools for clinicians to evaluate the sperm quality.Citation49 In short, this is an emerging concept that post-meiotic haploid spermatids play an unexpected role in regulating their development during spermiogenesis as well as spermatozoal function via their ability to produce estrogens locally in the seminiferous epithelium.
Figures and Tables
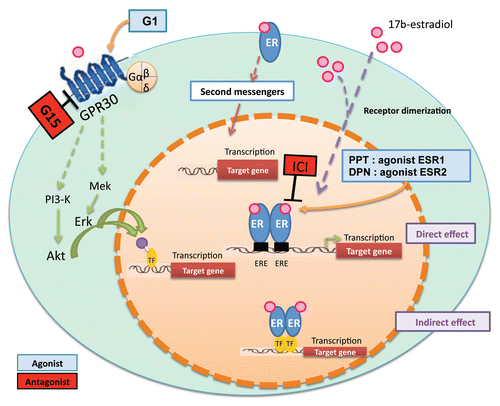
Figure 1 A schematic illustrating estrogen targeting in a testicular cell via genomic and non-genomic pathways involving estrogen receptors (ERs) and GRP30, respectively. G1, agonist of GPR30; G15, antagonist of GPR30; PPT, agonist of ESR1; DPN, antagonist of ESR2; TF, transcription factors; ER, estrogen receptors; ICI, selective estrogen inhibitor (ICI 182780).
Acknowledgments
The authors are indebted to their students (J. Levallet, D. Silandre, C. Bois and F. Mohammadi-Dubois). In addition, the financial support of the French Ministry of Education and Research, INRA, to our laboratory for the past decade is gratefully acknowledged.
References
- Zondek B. Mass excretion of oestrogenic hormone in the urine of the stallion. Nature 1934; 133:209 - 210
- Dorrington JH, Fritz IB, Armstrong DT. Control of testicular estrogen synthesis. Biol Reprod 1978; 18:55 - 64
- Carreau S, Genissel C, Bilinska B, Levallet J. Sources of oestrogen in the testis and reproductive tract of the male. Int J Androl 1999; 22:211 - 223
- Carreau S. Payne AH, Hardy MP. Leydig cell aromatase: from gene to physiological role. The Leydig cell 2007; Totowa NJ, USA Human Press Inc. 189 - 195
- Papadopoulos V, Carreau S, Szerman-Joly E, Drosdowsky MA, Dehennin L, Scholler R. Rat testis 17beta-estradiol: identification by gas chromatography-mass spectrometry and age related cellular distribution. J Steroid Biochem 1986; 24:1211 - 1216
- Levallet J, Bilinska B, Mittre H, Genissel C, Fresnel J, Carreau S. Expression and immunolocalization of functional cytochrome P450 aromatase in mature rat testicular cells. Biol Reprod 1998; 58:919 - 926
- Bouraïma-Lelong H, Vanneste M, Delalande C, Zannatta L, Wolczynski S, Carreau S. Aromatase gene expression in immature rat Sertoli cells: age-related changes in the FSH signalling pathway. Reprod Fertil Dev 2010; 22:508 - 515
- Carreau S, Hess RA. Oestrogens and spermatogenesis. Philos Trans R Soc Lond B Biol Sci 2010; 365:1517 - 1535
- Nitta H, Bunick D, Hess RA, Janulis L, Newton SC, Millette CF, Osawa Y, Shizuta Y, Toda K, Bahr JM. Germ cells of the mouse testis express P450 aromatase. Endocrinology 1993; 132:1396 - 1401
- Levallet J, Carreau S. 1 In vitro gene expression of aromatase in rat testicular cells. C R Acad Sci III 1997; 320:123 - 129
- Boitani C, Ritzén EM, Parvinen M. Inhibition of rat Sertoli cell aromatase by factor(s) secreted specifically at spermatogenic stages VII and VIII. Mol Cell Endocrinol 1981; 23:11 - 22
- Levallet J, Mittre H, Delarue B, Carreau S. Alternative splicing events in the coding region of the cytochrome P450 aromatase gene in male rat germ cells. J Mol Endocrinol 1998; 20:305 - 312
- Silandre D, Delalande C, Durand P, Carreau S. Three promoters PII PI.f, and PI.tr direct the expression of aromatase (cyp19) gene in male rat germ cells. J Mol Endocrinol 2007; 39:169 - 181
- Bois C, Delalande C, Nurmio M, Parvinen M, Zanatta L, Toppari J, Carreau S. Age- and cell-related gene expression of aromatase and estrogen receptors in the rat testis. J Mol Endocrinol 2010; 45:147 - 159
- Chimento A, Sirianni R, Delalande C, Silandre D, Bois C, Ando S, et al. 17β-estradiol induces expression of apoptotic markers through activation of GPR30 pathway in rat pachytene spermatocytes. Mol Cell Endocrinol 2010; 320:136 - 144
- Chimento A, Sirianni R, Zolea F, Bois C, Delalande C, Ando S, et al. GPR30 and ERs are expressed in rat round spermatids and mediate estrogen-dependent rapid pathways modulating expression of cyclin B1 and Bax. Int J Androl 2011; http://dx.doi.org/10.1111/j.1365-2605.2010.01100.x
- Saunders PT, Millar MR, Macpherson S, Irvine DS, Groome NP, Evans LR, et al. ERβ1 and the ERβ2 splice variant (ERβcx/β2) are expressed in distinct cell populations in the adult human testis. J Clin Endocrinol Metab 2002; 87:2706 - 2715
- Rochira V, Granata AR, Madeo B, Zirilli L, Rossi G, Carani C. Estrogens in males: what have we learned in the last 10 years?. Asian J Androl 2005; 7:3 - 20
- Rochira V, Carani C. Aromatase deficiency in men: a clinical perspective. Nat Rev Endocrinol 2009; 5:559 - 568
- Assinder S, Davis R, Fenwick M, Glover A. Adult-only exposure of male rats to a diet of high phytoestrogen content increases apoptosis of meiotic and post-meiotic germ cells. Reproduction 2007; 133:11 - 19
- Li X, Rahman N. Impact of androgen/estrogen ratio: lessons learned from the aromatase overexpression mice. Gen Comp Endocrinol 2008; 159:1 - 9
- Robertson KM, O'Donnell L, Jones ME, Meachem SJ, Boon WC, Fisher CR, et al. Impairment of spermatogenesis in mice lacking a functional aromatase (cyp 19) gene. Proc Natl Acad Sci USA 1999; 96:7986 - 7991
- Tsubota T, Howell-Skalla L, Nitta H, Osawa Y, Mason JI, Meiers PG, et al. Seasonal changes in spermatogenesis and testicular steroidogenesis in the male black bear Ursus americanus. J Reprod Fertil 1997; 109:21 - 27
- Pak TR, Lynch GR, Tsai PS. Estrogen accelerates gonadal recrudescence in photo-regressed male siberian hamsters. Endocrinology 2002; 143:4131 - 4134
- Schön J, Blottner S. Estrogens are involved in seasonal regulation of spermatogenesis and sperm maturation in roe deer (Capreolus capreolus). Gen Comp Endocrinol 2008; 159:257 - 263
- Zhang H, Sheng X, Hu X, Li X, Xu H, Zhang M, et al. Seasonal changes in spermatogenesis and immunolocalization of cytochrome P450 17α-hydroxylase/c17–20 lyase and cytochrome P450 aromatase in the wild male ground squirrel (Citellus dauricus Brandt). J Reprod Dev 2010; 56:297 - 302
- Bilinska B, Schmalz-Fraczek B, Kotula M, Carreau S. Photoperiod dependent capability of androgen aromatization and the role of estrogens in the bank vole testis visualized by means of immunohistochemistry. Mol Cell Endocrinol 2001; 178:189 - 198
- Gancarczyk M, Paziewska-Hejmej A, Carreau S, Tabarowski Z, Bilinska B. Dose- and photoperiod-dependent effects of 17β-estradiol and the anti-estrogen ICI 182,780 on testicular structure, acceleration of spermatogenesis and aromatase immunoexpression in immature bank voles. Acta Histochem 2004; 106:269 - 278
- Shetty G, Krishnamurthy H, Krishnamurthy HN, Bhatnagar S, Moudgal RN. Effect of estrogen deprivation on the reproductive physiology of male and female primates. J Steroid Biochem Mol Biol 1997; 61:157 - 166
- Shetty G, Krishnamurthy H, Krishnamurthy HN, Bhatnagar AS, Moudgal NR. Effect of long-term treatment with aromatase inhibitor on testicular function of adult male bonnet monkeys (M. radiata). Steroids 1998; 63:414 - 420
- D'Souza R, Gill-Sharma MK, Pathak S, Kedia N, Kumar R, Balasinor N. Effect of high intratesticular estrogen on the seminiferous epithelium in adult male rats. Mol Cell Endocrinol 2005; 241:41 - 48
- Prossnitz ER, Arterburn JB, Sklar LA. GPR30: a G protein-coupled receptor for estrogen. Mol Cell Endocrinol 2007; 265:138 - 142
- Prossnitz ER, Maggiolini M. Mechanisms of estrogen signaling and gene expression via GPR30. Mol Cell Endocrinol 2009; 308:32 - 38
- Sirianni R, Chimento A, Ruggiero C, De Luca A, Lappano R, Ando S, et al. The novel estrogen receptor, G protein-coupled receptor 30, mediates the proliferative effects induced by 17beta-estradiol on mouse spermatogonial GC-1 cell line. Endocrinology 2008; 149:5043 - 5051
- Ebling FJ, Brooks AN, Cronin AS, Ford H, Kerr JB. Estrogenic induction of spermatogenesis in the hypogonadal mouse. Endocrinology 2000; 141:2861 - 2869
- Walczak-Jedrzejowska R, Slowikowska-Hilczer J, Marchlewska K, Kula K. Maturation, proliferation and apoptosis of seminal tubule cells at puberty after administration of estradiol, follicle stimulating hormone or both. Asian J Androl 2008; 10:585 - 592
- Li H, Papadopoulos V, Vidic B, Dym M, Culty M. Regulation of rat testis gonocyte proliferation by platelet-derived growth factor and estradiol: identification of signaling mechanisms involved. Endocrinology 1997; 138:1289 - 1298
- Thuillier R, Mazer M, Manku G, Boisvert A, Wang Y, Culty M. Interdependence of platelet-derived growth factor and estrogen-signaling pathways in inducing neonatal rat testicular gonocytes proliferation. Biol Reprod 2010; 82:825 - 836
- Delbes G, Levacher C, Pairault C, Racine C, Duquenne C, Krust A, Habert R. Estrogen receptor β-mediated inhibition of male germ cell line development in mice by endogenous estrogens during perinatal life. Endocrinology 2004; 145:3395 - 3403
- Pentikainen V, Erkkila K, Suomalainen L, Parvinen M, Dunkel L. Estradiol acts as a germ cell survival factor in the human testis in vitro. J Clin Endocrinol Metab 2000; 85:2057 - 2067
- Jin W, Arai KY, Watanabe G, Suzuki AK, Takahashi S, Taya K. The stimulatory role of estrogen on sperm motility in the male golden hamster (Mesocricetus auratus). J Androl 2005; 26:478 - 484
- Idaomar M, Guerin JF, Lornage J, Moncharmont P, Czyba JC. Effects of estradiol and its antagonist tamoxifen on motility and metabolism of human spermatozoa. Adv Contracept Deliv Syst 1987; 3:337 - 345
- Idaomar M, Guerin JF, Lornage J, Czyba JC. Stimulation of motility and energy metabolism of spermatozoa from asthenozoospermic patients by 17β-estradiol. Arch Androl 1989; 22:197 - 202
- Fraser LR, Beyret E, Milligan SR, Adeoya-Osiguwa SA. Effects of estrogenic xenobiotics on human and mouse spermatozoa. Hum Reprod 2006; 21:1184 - 1193
- Perrard M, Durand P. Redundancy of the effect of TGFβ1 and β-NGF on the second meiotic division of rat spermatocytes. Microsc Res Tech 2009; 72:596 - 602
- Otto C, Fuchs I, Kauselmann G, Kern H, Zevnik B, Andreasen P, et al. GPR30 does not mediate estrogenic responses in reproductive organs in mice. Biol Reprod 2009; 80:34 - 41
- Liu X, Zhu P, Sham KW, Yuen JM, Xie C, Zhang Y, et al. Identification of a membrane estrogen receptor in zebrafish with homology to mammalian GPR30 and its high expression in early germ cells of the testis. Biol Reprod 2009; 80:1253 - 1261
- Lucas TFG, Royer C, Siu ER, Lazari MF, Porto CS. Expression and signaling of G protein-coupled estrogen receptor 1 (GPER) in rat Sertoli cells. Biol Reprod 2010; 83:307 - 317
- Carreau S, Wolczynski S, Galeraud-Denis I. Aromatase, oestrogens and human male reproduction. Philos Trans R Soc Lond B Biol Sci 2010; 365:1571 - 1579
- Payne AH, Kelch RP, Musich SS, Halpern ME. Intratesticular site of aromatization in the human. J Clin Endocrinol Metab 1976; 42:1081 - 1087
- Carreau S. Paracrine control of human Leydig cell and Sertoli cell functions. Folia Histochem Cytobiol 1996; 34:111 - 119
- Gunasegaram R, Loganath A, Peh KL, Ratnam SS. Aromatization of [4-14C]testosterone to [14C]estradiol-17beta by testicular tissue from male-to-female transsexuals on estrogen therapy. Arch Androl 1995; 35:127 - 133
- Lambard S, Galeraud-Denis I, Saunders PT, Carreau S. Human immature germ cells and ejaculated spermatozoa contain aromatase and oestrogen receptors. J Mol Endocrinol 2004; 32:279 - 289
- Aquila S, Sisci D, Gentile M, Carpino A, Middea E, Catalano S, et al. Estrogen receptor (ER)alpha and ERbeta are both expressed in human ejaculated spermatozoa: evidence of their direct interaction with phosphatidylinositol-3-OH kinase/Akt pathway. J Clin Endocrinol Metab 2004; 89:1443 - 1451
- Carreau S, Wolczynski S, Galeraud-Denis I. Mammalian sperm quality and aromatase expression. Microsc Res Tech 2009; 72:552 - 557