Abstract
Until recently, the histology and ultrastructural events of spermatogenesis in reptiles were relatively unknown. Most of the available morphological information focuses on specific stages of spermatogenesis, spermiogenesis, and/or of the mature spermatozoa. No study to date has provided complete ultrastructural information on the early events of spermatogenesis, proliferation and meiosis in class Reptilia. Furthermore, no comprehensive data set exists that describes the ultrastructure of the entire ontogenic progression of germ cells through the phases of reptilian spermatogenesis (mitosis, meiosis, and spermiogenesis). The purpose of this review is to provide an ultrastructural and histological atlas of spermatogenesis in reptiles. The morphological details provided here are the first of their kind and can hopefully provide histological information on spermatogenesis that can be compared to that already known for anamniotes (fish and amphibians), birds, and mammals. The data supplied in this review will provide a basic model that can be utilized for the study of sperm development in other reptiles. The use of such an atlas will hopefully stimulate more interest in collecting histological and ultrastructural data sets on spermatogenesis that may play important roles in future nontraditional phylogenetic analyses and histopathological studies in reptiles.
Introduction
Spermatogenesis is a complex process in which progenitor cells (spermatogonia) mitotically divide, undergo meiosis, and then differentiate into one of the few motile cells, spermatozoa, within the male vertebrate body. It is not surprising that a multitude of books and reviews have been devoted to this topic.Citation1–Citation5 These reviews concern birds and mammals in terms of amniotic sperm development. Only one review has looked specifically at the major steps of spermatogenesis (light and electron microscopy) in a reptilian taxon, the Ophidia (snakes), but even here the ultrastructure is provided only for spermiogenesis and the mature spermatozoon.Citation6 The ultrastucture of the early stages of spermatogenesis have not been explored in the Reptilia. Thus, this original review provides the first complete account of the entire process of spermatogenesis in a morphological context that includes ultrastructure of all the major generational types (stages and steps) of sperm development in reptilies. The class Reptilia is a major taxon within Amniota and in the context of this review includes: Chelonia, Squamata, Crocodylia, Rhynchocephalia, thus excluding the class Aves paraphyletically from the archosaurs.
The last decade has produced a large number of manuscripts that focus on the light and electron microscopy of the process, progression, morphology, germ cell cycle, and developmental strategies of spermatogenesis within the class Reptilia (for example: spermatogenesis in the turtle, Trachemys scripta and OphidiaCitation6,Citation7). Spermatogenesis is required of all vertebrate males, whose main means of passing on genetic material is via sexual reproduction. In amniotes (reptiles, birds and mammals), a shift in testicular architecture and germ cell arrangement occurred within the seminiferous tubules and epithelia that are morphologically unique to Amniota () among vertebrates.Citation6 The significance of which is most likely associated evolutionarily with the adaptations of the amniotic egg and complete reliance of amniotes on internal fertilization, which frees them completely from water for breeding.Citation8,Citation9 The differences morphologically and ultrastructurally within the germ cell development cycle, spermiogenesis, and the spermatozoa between both Reptilia and other amniotic clades may in time provide powerful nontraditional information that could be important in understanding the phylogenetics within reptiles, which at best is considered highly controversial at the present time.Citation10
Seminiferous Epithelium Organization and Germ Cell Development Strategy
The anamniotic (fish and amphibians) testis consists of lobules or tubules that are lined with cysts () where germ cells progress as a single population through the main stages of spermatogenesis: proliferation (mitosis), meiosis and spermiogenesis.Citation11–Citation14 Once the population of germ cells has completed maturation, they undergo spermiation and are released from the seminiferous epithelium as spermatozoa.Citation15,Citation16 In order for this to occur, cysts rupture and sperm are often shed as a unit, which in many cases compromises Sertoli cell integrity and the blood testis barrier that is often seasonal in temperate and some tropical anamniotic species.Citation17,Citation18
Reptiles have a permanent population of Sertoli cells and a more stable blood testis barrier than anamniotic taxa.Citation6,Citation17,Citation19–Citation21 With this morphological shift in the amniotic seminiferous epithelia, the germ cells are also arranged differently than within the cysts of fish and amphibians. Within all amniotes, the testis is composed of germ cell generations that are associated with the Sertoli cells () and are arranged in a consistent pattern between the basal lamina and the lumen of the seminiferous epithelium (). Reptiles also appear to have a regular histological arrangement of germ cell generations ( and ) within their seminiferous epithelia similar to mammals. No study until the beginning of this decade had determined that reptiles lacked a consistent spatial germ cell development like the birds and mammals. Birds and mammals always have the same spermatogonial and spermatocyte generations associated with each of the step spermatids within the seminiferous epithelium.Citation22–Citation25 The term step is adopted from the mammalian literature.Citation26 Spermatid steps refers to each morphological transition during spermiogenesis within the testis of a species (i.e., , steps 1–7). This spatial arrangement only allows for 3 to sometimes 4 spermatids to be observed within a cross section of seminiferous tubule within the testes of birds and mammals.Citation26 Studies devoted to the germ cell development strategy in reptiles representing different orders and exhibiting different seasonal patterns have now shown that reptiles no matter their seasonal cycle type exhibit a germ cell development very similar to that of amphibians and not like the consistent spatial development seen in other amniotes.Citation7,Citation27–Citation32
In reptiles, the majority of germ cell generations progress through the stages of spermatogenesis as a single population (). The mitotic and meiotic cells are restricted to the basal compartment (close to the basement membrane) and dividing spermatogonia are always present, except in turtles late in the summer/fall and winter when only resting spermatogonia are present and are quiescent.Citation7 Meiosis is also consistent during active spermatogenesis and large populations or cohorts of cells progress through meiosis together and then enter spermiogenesis as large populations (example: : Germ cell development in the turtle, Trachemys scripta, in the snake, Agkistrodon piscivorus).
During spermiogenesis, large cohorts of cells develop together through acrosome development, elongation and maturation ( and C) and the seminiferous epithelium expands into large columns that are separated by deep luminal crypts ( and B). These columns accumulate many sequential generations of spermatids at one time within the seminiferous epithelium. Five to six different spermatids can be observed within the seminiferous epithelium at the same time, which is much different than the usual three found consistently within birds and mammals. The elongating steps of spermiogenesis accumulate apically within cellular processes of Sertoli cells. The accumulation of these elongates that are finishing spermiogenesis are then released in a large single spermiation event that leads to dilations of the lumina and increased sizes or diameters of the seminiferous tubules ( and C). In seasonal breeders, such as those reptiles found in temperate environments, once this single major spermiation event occurs in the summer or late fall, the testis enters a quiescent period where spermatogenesis slows or ceases altogether. The histology of the seminiferous tubules at this time shows reduced epithelia with typically only spermatogonia present (). Within the lumina of the seminiferous tubules are pieces of the Sertoli cells with various generations of germ cells that have been sloughed from the epithelia and are thought to be recycled and reabsorbed along the length of the reproductive tract.
In continuous breeding reptiles, such as Sceloporus bicanthalisCitation33 and Anolis lineatopusCitation31 (), once a cycle of spermatogenesis is complete the basal compartment shows an increased rate of mitosis and meiosis, which amplifies the seminiferous epithelial height. These new generations of germ cells then enter spermiogenesis and spermiation again as a single wave or cohort. These steps are repeated over and over again leading to bimonthly sequential increases and decreases of the seminiferous epithelium and tubule diameter (). Notice from that when the diameter is the widest in this bimonthly cycle the epithelium height is the lowest. Thus, when the seminiferous tubule diameter width is high then spermiation has occurred increasing the size of the lumen and tubule overall. In contrast, the spermiation event has caused a loss of germ cells and Sertoli cell columns therefore decreasing the overall seminiferous epithelial height.
Understanding the morphology and progression of germ cells within the seminiferous epithelium of reptiles might provide better insight on how the reptilian testis functions during seasonal cycles. For example, endocrinologists know that in mammals FSH (Follicle Stimulating Hormone) stimulates type A spermatogonial division during early spermatogenesis.Citation34 FSH has been found in reptiles,Citation35,Citation36 and all present data suggest that it causes recrudescence of spermatogenesis in temperate reptiles either before or after mating.Citation37,Citation38 However, it is not known whether type A spermatogonia with the ability to divide are present within the seminiferous epithelium at all times of the year or whether the population of dividing spermatogonia is temporal (may be responsible for refractory period for example, see ref. Citation39) and only available for FSH activation during certain times of the reptilian spermatogenic cycle. Understanding the basic cytological events of spermatogenesis in reptiles may help resolve the existing controversies and inconsistencies with regard to refractory periods, temperature effects, and hormonal stimulation within the testicular cycles of reptiles. Furthermore, since a temporal germ cell development strategy is employed by all major taxa of reptiles studied to date, they may provide a feasible model for histopathological studies of the testis in response to pesticides or heavy metals. The simplified germ cell development strategy allows for easier observation of germ cell types because they all progress together through the stages of spermatogenesis. Thus, morphological abnormalities, morphometric differences, and absences or overabundance of germ cell types can potentially be discernable in reptilian models.
Germ Cell Cycle and Germ Cell Morphologies during Spermatogenesis
Spermatogenesis is the process of sperm development and begins with spermatogonia that divide, undergo meiosis, and then differentiate into spermatozoa. Sperm development is typically broken down into three phases based upon functional and morphological criteria.Citation26
(1) The proliferative phase involves the spermatogonia, which undergo rapid successive incomplete (cytoplasmic) mitotic divisions that lead to a large clonal population of germ cells that will allow the testis to produce immense numbers of spermatozoa. (2) The meiotic phase includes the spermatocytes, which undergo the reduction divisions, segregation and recombination of genetic material. The final aspect of spermatogenesis is the maturational or spermiogenic phase, (3) where haploid undifferentiated spermatids transform into specialized hydrodynamic sperm cells equipped and capable of reaching and fertilizing eggs. Germ cells, particularly the mitotic and meiotic cell types, within these phases have very specific morphologies that are highly conserved among vertebrates.Citation1,Citation2 Russell et al.Citation26 provides a thorough review of the process of spermatogenesis and the morphology of the individual germ cell types for mammals. Once all germ cell morphologies for each phase of spermatogenesis are known, then a germ cell cycle map can be produced that allows the visualization of the transformation of spermatogonia to mature spermatozoa (). Because of the conserved nature of especially the morphologies of the earlier germ cells of spermatogenesis (), within this review only one representative example will be provided for each cell type to prevent redundancy. However, within spermiogenesis, two major morphological themes play out within reptiles, which will be equally represented within the figures provided here. Also, it should be noted that all cell types were taken at the same magnification (for both light and electron microscopy) whenever possible throughout this manuscript unless stated within the figure legend. Thus, scale bars that represent magnifications for all the micrographs within a figure are often provided for just one of the sets of images within a plate. At all times, caution should be taken when interpreting the information provided in this review, although the figures represent the normal morphologies of cell types, differences within reptiles may exist, especially among species not studied to date.
Proliferative stage: spermatogonia morphology.
Regardless of seasonal cycle (i.e., prenuptial, postnuptial, mixed) type within reptiles, all species produce large numbers of cells in the beginning of each spermatogenic cycle, this is true of most amniotes studied to date.Citation26 One of the major functions of the spermatogonia is to produce a large population of clonal germ cells that can then enter the other phases of spermatogenesis. Since large numbers of spermatozoa are typically needed for successful fertilization,Citation1,Citation2 the rapid and successive division of the spermatogonia population fulfils this requirement.
Three major types of spermatogonia can be recognized within the reptilian seminiferous epithelium. Spermatogonia are typically restricted to the basal compartment within the seminiferous epithelium in close association to the basement membrane of the Sertoli cells (, Sp). The resting spermatogonia () have only been described to date in the turtle, Trachemys scripta.Citation7 Spermatogonia of similar morphology have been observed from other histological studies of turtles (Gribbins KM, unpublished data). All temperate turtles studied to date are considered postnuptial in the seasonal pattern of spermatogenesis.Citation39 This means that turtles start spermatogenesis after spring mating and spermiation occurs in the late summer or early fall. Most temperate snakes are also postnuptial and only one postnuptial snake has complete spermatogenic data (light microscopy) that details the germ cell cycle.Citation29 In this study, no resting spermatogonia were reported. However, upon re-examination for the present review, resting spermatogonia are actually observed in October testes within the snake, Seminatrix pygaea, similar in morphology to that described here for Trachemys scripta (). Thus, to date, resting spermatogonia have only been observed within postnuptial breeding reptiles, where an extended quiescent period may be necessary during late summer to prevent another round of spermatogenesis from progressing into the late fall or winter months.Citation7
Resting spermatogonia have dark staining chromatin that are tightly packed within the nuclei and lack visible nucleoli (). They also have few to no major organelles present within their cytoplasms except for mitochondria. The function of these spermatogonial cells is unknown, as little is understood of the mechanics behind division and differentiation of spermatogonia in reptilian testes. We hypothesize that they are stem cells with similar function to those types of spermatogonia described in mammals. Stem cell spermatogonia in mammals have been reported to be resistant to stress, damage, and are often non-dividing.Citation26,Citation40,Citation41 Resting spermatogonia in reptiles have tightly packed DNA and thus might be more resistant to damage because the availability of cleavage sites of the DNA in this condensed form is restricted. Furthermore, resting spermatogonia are rarely seen dividing in the testes of turtles and snakes. Reptilian resting spermatogonia most likely provide a resilient population of stem cells that can survive insult and hibernation to be utilized in the following early/late spring, early summer months to repopulate the spermatogonia populations within the testes of temperate snakes and turtles.
The second type of spermatogonia seen within the testis of reptiles is the type A spermatogonia (). Spermatogonia A in reptiles are very similar in function and morphology to those described in mammals (Apaired and Aaligned type spermatogonia).Citation26 These spermatogonia are known as proliferative spermatogonia in mammals and within reptiles provide much of the mitotic power of the seminiferous epithelium. They also are typically found juxtapositioned to the basement membrane within the basal compartment of the reptilian seminiferous epithelium. However, type A and B (described next) spermatogonia can produce several layers of spermatogonia that extend out into the interior of the seminiferous epithelium during the early divisions of spermatogenesis in reptiles. Type A spermatogonia are ovoid in shape with typically one cell membrane surface resting on the basement membrane of the seminiferous epithelium. Their chromatin is diffuse except next to the inner leaflet of the nuclear envelope and nucleoli are present. The organelles responsible for protein production are also present within the cytoplasm, which suggests these cells are anabolically active. Spermatogonia A are the most abundant spermatogonia seen within the reptiles studied to date.Citation6
Type B spermatogonia are the last type of spermatogonia observed within the seminiferous epithelium of reptiles (). They are similar to the differentiating spermatogonia (A1,2,3,4, B and Intermediate types) illustrated within the mammalian testis.Citation26,Citation42 Spermatogonia B have more heterochromatin within the nucleoplasm and associated with the inner nuclear envelope compared to spermatogonia A. They are also more round compared to the ovoid-shaped spermatogonia A. Spermatogonia B still possess the nucleoli and organelle machinery responsible for protein production and are thus anabolically active cells. They in some case have much more rough ER and larger Golgi complexes to that of other spermatogonia in reptiles.
Meiotic stage: spermatocyte morphology.
The spermatogonia B divide to form the first cell type of meiosis. Prophase 1 of meiosis is exceptionally long in reptiles (roughly 4 weeks) like that of other vertebrates.Citation26 There are gradual morphological transitions that are easily recognizable with histological practice during this prolonged phase of meiosis (: PL-DI). A common theme among all reptiles and other amniotes is that spermatocyte size increases dramatically during prophase 1. Thus, the largest and most recognizable germ cells within the seminiferous epithelium are the terminating spermatocytes of prophase 1. This elongated prophase allows plenty of time for recombination of paired chromosomes to occur before the first nuclear division of meiosis. The morphology of pre-leptotene spermatocytes is very similar to spermatogonia B. The diagnostic character that separates the two cell types is size and location. Pre-leptotene spermatocytes () typically have less heterchromatin and are smaller than B type spermatogonia. Pre-leptotene cells are not only roughly 30% smaller than spermatogonia B but are also found further away from the basement membrane. Rarely do pre-leptotene cells make contact with the basement membrane of the seminiferous epithelium in reptiles.
Leptotene spermatocytes represent the true beginning of prophase. Heterochromatin common in the pre-leptotene phase has now disappeared and when underexposed the “wispy” threads of the condensing chromosomes can be visualized ( and black arrows). The chromosomes however are not yet paired into homologs. Zygotene spermatocytes are often hard to differentiate from leptotene cells especially via the light microscope. The only real differences are a slight size increase in zygotene cells and a thickening of chromosomal fibers. The continued condensation and repackaging of the chromosomes during the zygotene phase of meiosis leads to the initial pairing of homologous chromosomes into tetrads and the formation of synaptonemal complexes seen only by the electron microscope ( and black arrows). Since the homologous chromosomes are now paired along most of their length, the condensing chromosomes appear thicker in light microscope sections of zygotene spermatocytes.
Pachytene spermatocytes have fully paired homologous chromosomes with very thick chromosomal fibers and large areas of open nucleoplasm separating the chromosome clumps when observed via the light microscope (). Pachytene cells are also the most abundant and longest phase of prophase 1 in reptiles and most amniotes studied to date. Synaptonemal complexes are abundant and robust within the thinning nuclear membrane of pachytene cells ( and black arrows). These structures are where the actual crossing over events occur within regions called chiasmata between the homologs of the tetrads. This pairing apparatus appears as an elongated tripartite (two thick lateral elements and one thin central element) structure that is only recognizable by the electron microscope ( and inset). The arms of the chromosomes can also be easily distinguished radiating out from the synapses. The time germ cells spend in the pachytene phase lends itself to the importance of genetic recombination and its significance to genetic diversity and reproductive success within species of vertebrates. Pachytene spermatocytes within the reptilian testis, like that of mammals,Citation43,Citation44 become highly synthetic toward the middle and end of their tenure, which lends to their great size. From the ultrastructure, one can see the enlarged nucleoli and tremendous amount of rough endoplasmic reticula. However, unlike mammals, a prominent sex vesicleCitation26 (represents the sex chromatin) that is juxtapositioned to the inner leaflet of the nuclear envelope does not arise in reptilian pachytene cells in the species studied to date.
The diplotene phase () of meiosis 1 in reptiles is very brief as are metaphase 1 and the rest of the division stages of the 1st and 2nd parts of meiosis. Typically cross-sections of seminiferous tubules will show the diplotene, metaphase 1 (), metaphase 2 (), and secondary spermatocytes ( and ) all in the same area of seminiferous epithelium. In this phase, synaptonemal complexes dissipate and chromosomal condensation and packaging is nearly complete. Like mammals,Citation26,Citation45 reptilian diplotene cells possess an electron dense body with a central clear area when observed under the electron and light microscopes ( and black arrows). Diplotene spermatocytes are also easier to recognize in cross section in reptiles than within mammals because condensed chromosomes under the light microscope appear near the inner nuclear membrane resulting in a spoke-like pattern within the diplotene nucleus. Diplotene cells progress into metaphase, anaphase and telophase very rapidly. Usually all of these phases except metaphase () are hard to differentiate between one another and in most cases are absent in cross-sections of seminiferous tubules in reptiles. The completion of the reduction division of meiosis results in the formation of secondary spermatocytes ().
Secondary spermatocytes () are also very short lived during spermatogenesis in reptiles. Like mammals, they are hard to discern from the first step of spermiogenesis and are very rare within the seminiferous epithelium. Secondary spermatocytes are about 30% to 40% larger than step 1 spermatids ( and inset) and have a bit more heterochromatin and very prominent nucleoli. A feature that both cell types share is a prominent form of nuage called the chromatoid body, a feature they also share with mammals.Citation26 Nuage is defined as a prominent inclusion that is amorphic and found within the cytoplasm of spermatids during spermiogenesis.Citation45–Citation47 The form of this material can be variable. In many cases it is electron dense and granular ( and black arrows) or can be more translucent and less dense ( and inset: white arrowhead). Nuage should not be confused with structures that resemble intracellular residual bodiesCitation26 ( and inset; white arrow; , turtle, black arrowhead) or deteriorating membrane bound organelles. Another ultrastructural characteristic of secondary spermatocytes are their vacuolated mitochondria ( and black arrowheads). Metaphase 2 cells can be differentiated from metaphase 1 cells based on size and amount of heterochromatic material located within the cytoplasm. Metaphase 2 cells are smaller and have about half the amount of heterochromatin () compared to metaphase 1 germ cells. This size difference in cytoplasm and genetic material portrays nicely the reduction division and the production of haploid cells.
Spermiogenic stage: spermatid morphology.
The resulting haploid and undifferentiated round step 1 spermatid within the reptilian testes undergoes one of the most dramatic changes of any cell type within the adult vertebrate body. This process takes about 5 to 8 weeks in reptiles (), which is longer than the typical 4 weeks observed in most mammals studied to date.Citation26 This transformation occurs without cell division and several cytological events occur at the same time during spermiogenesis; it is easier to concentrate on one histological process at a time. In order to simplify what is occurring within spermatids, three major morphogenic themes will be presented in this review for spermiogenesis: acrosome development, elongation of the nucleus/condensation of DNA, and development of the flagellum. The end result of these three morphological courses is a highly hydrodynamic motile cell, the spermatozoon, which has three major structures: the acrosome complex, the nucleus, and the flagellum ().
Even though spermiogenesis is historically broken down into distinct morphological steps,Citation1,Citation26 it is best to think of the process as a continual progression or transition and that spermatids can easily be observed between stages or what some have called early and late steps of spermiogenesis.Citation26 Thus, cycle maps and spermatid step morphological descriptions for a given species are not set in stone. The number of spermatid steps varies among mammalian species, for example: 19 in the rat,Citation5,Citation46 16 in the mouse,Citation5,Citation48 12 in the dog.Citation42 In contrast, reptiles are more consistent in the number of ontogenic steps required to complete spermiogenesis. All the species studied to date have 7 or 8 steps (, s1–s7) and include reptiles representing multiple orders within class Reptilia.Citation7,Citation27–Citation31,Citation33
Detailed ultrastuctural studies of the complete process of spermiogenesis within the Reptilia are rare. Many studies have incomplete descriptions of different aspects of spermiogenesis within the testis of reptiles. They are too numerous to list here and the concentration of this review is on complete descriptions of spermiogenesis in order to better understand spermatogenesis as a whole morphological mechanism within reptiles. Comprehensive studies of spermiogenesis, as well as the morphology of the spermatozoa have been shown to be important data as non-traditional characters used in phylogenetic research of Reptilia over the last decade (for example, see references Citation49–Citation51). In Lepidosauria (snakes, lizards and tuataras), only 8 studies have entire ultrastructural descriptions of spermiogenesis: the skink, Chalcides ocellatus;Citation52 the tuatara, Sphenodon punctatus;Citation53 the lizard, Tropidurus torquatus;Citation54 the iguana, Iguana iguana;Citation55 the skink, Scincella lateralis;Citation56 the snake, Agkistrodon piscivorus;Citation57 the anole, Anolis lineatopus;Citation58 and the Gecko, Hemidactylus turcicus.Citation59 Even less information on spermiogenesis exists in turtles and crocodilians. Four studies that provide entire ultrastructure details of spermatid development occur in the turtles Trachemys scriptaCitation60 and Pelodiscus sinensis,Citation61 and in the crocodilians Caiman crocodylusCitation62 and Alligator mississippiensis.Citation63
The round spermatids (, S1–S4) are uniformly consistent in morphology and development across Reptilia. They along with all other steps of spermiogenesis develop within the columns of the apical regions of the seminiferous epithelia ( and ) closest to the lumina of the seminiferous tubules. The major events of acrosome formation occur during the tenure of the round spermatid stage. Step 1 spermatids () are the result of the 2nd division of meiosis. They are not only smaller than secondary spermatocytes but also have an enlarged Golgi complex ( and black arrows) and typically a small developing acrosome vesicle (called the proacrosome in mammalsCitation64) ( and B, white arrows) that does not made contact with the nucleus. As Step 1 spermatids progress through their development this vesicle grows in size and makes contact with the apex of the nucleus ( and C). The size of the developing acrosome increases due to membrane and vesicular materials ( and black arrowhead) gained from transport vesicles ( and white arrowheads) that originate from the Golgi complex. In all reptiles studied to date (reviewed in ref. Citation6) only one Golgi complex is associated with the developing acrosome, except in the snake, Agkistrodon controtrix, which has two complexes aiding the development of the acrosome ( and black arrows). The significance of possessing two Golgi complexes is not known but this morphological setup does allow for early formation and observation of the acrosome granule ( and white*). Of course Scincella lateralisCitation56 has the earliest developing acrosome granule in the reptiles studied to date. This lizard's granule within the acrosome vesicle develops before the vesicle even contacts the nucleus, and this species only has one Golgi complex. Thus, a species of reptile does not need two Golgi complexes to acquire an early developing acrosome granule. Also note, as stated earlier, that because of incomplete mitotic divisions during proliferation all developing cohorts of germ cells within the reptilian testis are connected via cytoplasmic bridges ( and black*). These bridges are thought to allow for cytoplasmic sharing and are most likely involved with coordinated communication that controls the simultaneous development of the reptilian temporal germ cell development strategy.Citation6
During the remainder of the round spermatid stage, the acrosome vesicle continues to develop and enlarge (). Within this vesicle (, S1, ac) an electron dense acrosome granule (, S2, black arrowhead) forms most likely from the materials delivered from the transport vesicles of the Gogli complex. The cytoplasm at this time is packed with mitochondria and a large amount of rough and smooth endoplasmic reticula. As the acrosome grows in size, it first indents the apex of the nucleus so that the newly forming acrosome complex resides in a deep nuclear fossa. Then late in the development of the round spermatid, a cytoplasmic shift occurs, forcing most the organelles and cytoplasm caudally within the round spermatid (, S3, black*). This results in the outer acrosome membrane becoming directly juxtapositioned to the cellular membrane ( and inset, black arrowheads) and causes the acrosome to collapse and flatten on to the apical surface of the round spermatid nucleus. The force produced from the meeting of acrosome and cellular membrane eventually causes the acrosome to completely flatten the apical nucleus, which also has thin electron dense subacrosome space (, S4, black arrow) adhering the inner acrosome membrane to the apical nuclear envelope. This shift from a centrally located nucleus to an eccentrically located nucleus becomes even more pronounced during the elongation phase of spermiogenesis (). The exact mechanism that controls the cytoplasmic shift and the beginning stages of elongation are not completely understood in amniotes. It has been suggested that actin components of the cytoskeleton play a major role in the cytoplasmic shift and early events of elongation.Citation65,Citation66 Later events of elongation and head formation are most likely controlled by the manchette,Citation67 a cytoskeletal scaffolding of microtubules associated with the body of the elongating nucleus.
The continued elongation (, S4) of the nucleus caudally forces the distal cytoplasm to push the apex of the nucleus further against the cell membrane, which most likely results in the enveloping of the acrosome complex around the now rounded nuclear head ( and ). In many instances during reptilian spermiogenesis the squeezing of the enveloping cell membrane, acrosome membranes and nuclear membranes causes prominent nuclear shoulders on the caudal lateral boards of the nuclear apex ( and ). The complete progression and transition of the acrosome vesicle and the nuclear head is illustrated in .
When combining the known complete morphological data on the process of elongation during spermiogenesis, two major ultrastructural themes separate the squamates (lizard, snakes) from the rest of extant reptiles (turtles, crocodilians and tuataras). Thus for simplicity and ease of understanding, most the of morphology on elongating spermatids presented here will show a squamate representative (several different lizards and snakes) and Alligator mississippiensis or Trachemys scripta for the non-squamate examples. As elongation continues, the nuclear body lengthens leading to a more cylindrical appearance to the nucleus (). Elongation during both themes results in similarly shaped spermatid nuclei. Also, the manchette ( and Scincella lateralis, Inset, ma) is found in all species of reptile studied to date during elongation except in Anolis lineatopus, which lacks a manchette all together.Citation58 The manchette is thought to aid in the elongation of the nucleus when present and is also represented in almost every mammal and bird.Citation2,Citation26,Citation56,Citation64,Citation68 The manchette has long been thought to be a major player in the elongation and constriction of the nuclear width during amniote spermiogenesis,Citation64,Citation66 but the exact mechanism is not known. It also seems that a functioning manchette is not necessarily needed for elongation to occur, as it does not exist in A. lineatopus although normal elongation does take place.
As the nucleus elongates, it also becomes restricted in width. As you compare the size of the nucleus of a late elongating spermatid to the larger spermatocytes one can see the disproportionate size difference in favor of the spermatocytes. The chromatin of these germ cells must be tightly packed together in order for that amount of DNA to fit within the nucleus of the hydrodynamic spermatozoa. Condensation is the process by which the DNA is packaged within the body of the nucleus. It occurs in two ways and roughly follows the two themes of elongation between squamate and non-squamate reptiles. Non-squamate reptiles have a granular type of condensation pattern. Chromatin is packed into larger and ever tightening granules as the nucleoplasm shrinks within the elongating spermatid nucleus ( and B). In contrast, the squamates typically have a filamentous type of condensation of the their chromatin (). The filamentous pattern can condense into more or less straight fibers ( and F) as it does in Scincella lateralisCitation56 spermatids or actually concentric fibers () that wrap in ever tightening coils within cross sections of elongating spermatids () as in Agkistrodon piscivorus.Citation57 Also open areas of nucleoplasm can occur, often termed lacunae ( and black arrow),Citation49,Citation56 within the body of the nucleus during and after elongation have been completed. Many squamate elongating spermatids have two prominent translucent areas ( and white arrows) within the nucleoplasm on either side of the caudal nuclear fossa ( and white arrowhead) where the flagellum (proximal and distal centrioles, , pc and dc) attaches to the caudal nuclear body. Reptilian elongating spermatids not only have the manchette (, ma) along the length of the body of the nucleus but also in some cases possess a large amount of smooth endoplastic reticula ( and black arrowheads) running in juxtaposition to the body of the nucleus during middle elongation.
The acrosome and flagellar development is complicated in reptiles and many of the differences between species within and among orders of reptiles occur in the compartmentalized acrosome complex or within such parts of the flagellum as the midpiece. Therefore, these variations within these parts of the spermatozoa and/or spermatids are the focus of recent nontraditional phylogenetic studies in reptiles.Citation6,Citation49,Citation50,Citation53 The plates that are represented within this review are at the climax of acrosome and flagellar development as seen within the anatomy of the step 7 or 8 spermatids, which are the stages right before spermiation. This high complexity of the acrosome complex in reptilesCitation53,Citation57,Citation69,Citation71 is often absent in mammals and birds,Citation69,Citation70 and has been suggested to aid in the sequential release of hydrolytic enzymes during fertilization.Citation72
In turtles, tuataras and crocodiliansCitation53,Citation63 the major differences within their acrosome complexes are the presence of deep endonuclear canals (1 to 3) (, en) that house the perforatoria. These nuclear canals are absent in squamates () and their perforatoria are completely epinuclear and reside in the subacrosome spaces right under the proximal acrosome vesicles (, pf). The acrosome complex ( and B, ac) consists of the acrosome superficially, which envelops the entire nuclear head. This enzymatic sac is typically rounded or slightly pointed at its most anterior end and has thin elongated shoulders ( and black arrowheads; B, as) that run at least a third of the way down the lateral aspects of the apical nucleus (). In both non-squamate and squamate reptiles the acrosome lumen can be striated, with a darker cortex and a light medulla as in Alligator mississippiensisCitation73 and Hemidactylus turcicus.Citation59 The acrosome is glued to the nuclear head by the paracrystalline subacrosome space that is typically made up of electron dense granules. The subacrosome space ( and B, sa) can be striated also as in the lizard, Barisia imbricata () (Gribbins KM, unpublished data). The basal part of the subacrosome space, with the epinuclear translucent zone (, ep), is separated from the rostrally located basal plate ( and white arrowhead) and perforatorium (, pf), which is right under the most proximal part of the acrosome head ( and B), by a thin white translucent line ( and white arrowheads) in most squamates. The nucleus penetrates the subacrosome space as a narrow pointed rostrum in almost all reptiles studied to date ( and B, nr). Surrounding the nuclear head and acrosome are several layers of Sertoli cell membrane ( and black arrows) that most likely aid in the continual attachment of the developing spermatid to the Sertoli cells of the seminiferous epithelium via desmosome (anchoring) junctions (, ds).
In the Reptilia, the flagellum becomes evident early in the round spermatid stage as a small principle piece growing from the distal centriole (, s3, white arrowhead). The 9 + 2 arrangement of the microtubules growing from the centrioles in cross section is termed the axoneme (, ax). The centrioles have a 9 + 3 arrangement of concentric peripheral tubules and a centrally located microtubule pair in cross section for the distal centriole (). The flagellum attaches to the caudal end of the nucleus within an implantation fossa ( and white arrowhead). Most non-squamate reptiles, like Alligator mississippiensis (), lack a prominent neck region of the flagella and the midpiece abuts directly to the caudal nucleus. In contrast, in many snakes and lizards () there is a neck region between the implantation (nuclear) fossa and the beginning of the midpiece. The laminar projects (, lp), pericentrioler matieral (, pe), dense collar (, dl), and the central doublet of the distal centriole all aid in the support, robustness of the neck, and to the future propulsional function of the reptilian sperm tail.
The midpiece of the flagellum is defined by the concentric rings of mitochondria surrounding the axoneme (). In non-squamate reptiles these mitochondria are large uniformly round with many peripheral concentrically layered cristae (, turtle). The number of mitochondria appears from the few studies done to date to be fairly consistent along the length of the midpiece in turtles,Citation60,Citation61 crocodilians,Citation62,Citation63 and the tuataraCitation53 and ranges from 8 to 10 in sagittal section. In squamates, the sperm tend to maintain slightly higher and more variable numbers of elongated mitochondria (on average around 12 in lizards) (, lizard) along the length of the midpiece (except some geckos, 2) with snakes having much higher numbers than that of non-squamate reptiles with 14 or more mitochondria in sagittal sections of the midpiece.Citation69 Snakes also tend to have many rows of dense bodies (presumably deteriorating mitochondria), which leads to very long midpieces (, snake). Non-squamate reptiles tend to lack dense bodies altogether within their midpieces (, turtle). The other major difference within the midpiece of non-squamate reptiles is the distal centriole runs along the entire length of the midpiece axoneme (, turtle, dc), whereas squamates have a short distal centriole that is typically restricted to the neck region only. Therefore, the principle piece penetrates up into the midpiece region (, snake and lizard, pp) of the flagellum in Squamata. Associated with microtubule triplets within the distal centriole are peripheral axonemal fibers (, lizard, pf) within most reptiles studied to date. These fibers again are hypothesized to aid in the strengthening of the proximal attachment point of the flagella to the body of the nucleus. These peripheral fibers continue into the principle piece but tend to dissipate further from the terminating point of the midpiece, which is marked by the annulus ( and white arrowheads) in reptiles and other amniotes. Of these fibers numbers 3 and 8 tend to stay enlarged in turtles, some crocodilians, and in most squamates (, lizard, ).Citation69
The principle piece () has an axomene that is surrounded by a fibrous sheath, which tends to be thicker proximally and thins as you move distally along the principle piece's length (, fs). The fibrous sheath is made up of uniform annulated fibrous rings that are stacked sequentially along the length of the principle piece in reptiles similar to that of all amniotes.Citation69 The endpiece () in reptiles is easily discernable from the principle piece as it has lost the fibrous sheath and all that remains is the axoneme and the cell membrane (, lizard, cm and ax).
Conclusions
The purpose of this review is to provide basic information on the histology and ultrastucture of spermatogenesis in reptiles. This qualitative study may encourage other scientists to begin to more thoroughly explore sperm development in reptiles. The studies that have been done to date provide relatively large but incomplete data sets that could be important or useful in phylogenetic analyses if and only if more ultrastructural information is collected for reptilian representatives across this class. Studying both spermatogenesis and spermatozoon ultrastructure within many new species representing families across Reptilia will only add to the robustness of such data. Ultrastructural spermatogenic characters collected from such studies will provide for better phylogenetic analysis overall and enable a better understanding of the evolution of sperm development and morphology within reptiles and across amniotes.
These data also provide a basic model that could be used for histopathological studies of the testis in amniotes. In order to understand how toxins or heavy metals affect sperm development, a thorough understanding of the histology of spermatogenesis has to be understood.Citation26 Reptiles provide the perfect model for these types of studies because the temporal development of germ cells as they progress through spermatogenesis provides a simplistic and straightforward series of germ cell morphologies that can be easily delineated during the major phases of spermatogenesis. This should make it easier to discern abnormalities among different generations of germ cells within the testis and the absence or disruptions of particular stages of sperm development. Also, many reptiles are semi-aquatic and relatively abundant and thus could be sentinel species for the studies of aquatic pesticides and their toxicological impacts on germ cell development. Chemicals such as atrazine have been shown to alter sperm development in amphibians and fishes.Citation74 Reptiles could impact these studies even further as they have a testis more similar to those of other amniotes, including humans, than to anamniote fish and amphibians.
Figures and Tables
Figure 1 (A) Sagittal section of entire testis and anterior excurrent ducts of Carphophis vermis (Western Worm Snake). Bar = 0.2 cm. Testis (T), efferent ducts (black arrowhead), epididymis (black arrows). (B) Low power view of seminiferous tubules in cross and sagittal sections near the tunica albuginea (white arrowheads) of the testis of Carphophis vermis. Bar = 200 µm. Seminiferous tubules (ST), interstitial space (white*).
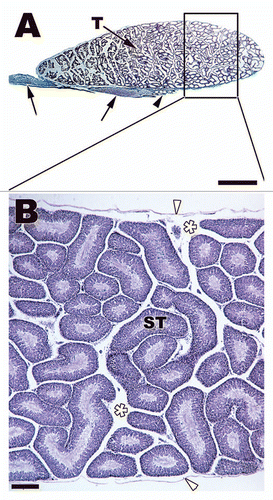
Figure 2 (A) Transverse section through a seminiferous tubule in Notophthalmus viridescens (Red-spotted Newt). The tubule has multiple cysts (C) resting on a prominent basal lamina (black arrow). Sertoli cell processes (black arrowheads) enclose the entire population of developing germ cells. The tubule has a lumen (L) and populations of germ cells within cysts are all at the same stage of development, which is early mitosis and meiosis within this section of testis. (B) Transverse section of late developing cysts within the Notophthalmus viridescens. The germ cell population within each cyst (C) is completing spermiogenesis and is close to spermiation. The tails of the spermatozoa extend out into the lumen (L) of the tubule. Bars = 45 µm.
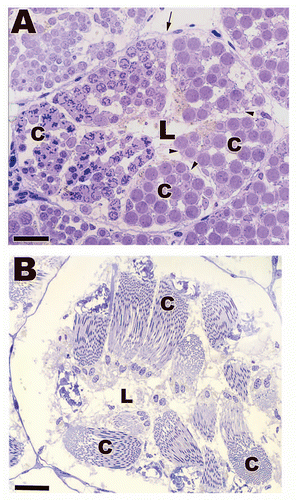
Figure 3 Light (A) and low power electron (B) micrographs depicting the seminiferous epithelia within Agkistrodon contortrix (northern Copperhead Snake) and Thamnophis sauritus (Garter Snake) testes. Sertoli cells sit on a prominent boundary layer (black arrows) made up of myofibroblasts (black arrowheads) and the Sertoli cell nuclei (white arrow) rest near the basement membrane of the seminiferous epithelium. The Sertoli cell processes (black *) wrap around developing germ cells. Sp, spermatogonia A; Pl, preleptotene spermatocyte; Pa, pachytene spermatocyte; Rd, round spermatid; Sd, elongating spermatid; L, lumen; Li, Lipid droplet. (A) Bar = 25 µm; (B) Bar = 15 µm.
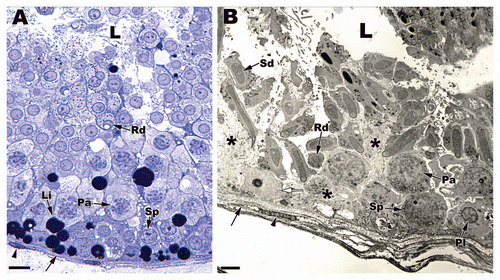
Figure 4 A cross section of June seminiferous epithelium within Seminatrix pygaea (Black Swamp Snake) and higher magnifications of represented cell types. Bar = 50 µm for seminiferous epithelium section and 10 µm for magnified germ cell types. S3, step 3 spermatid; S2, step 2 spermatid; S1, step 1 spermatid; SS, secondary spermatocyte; DI, diplotene; PA, pachytene; LP, leptotene; PL, pre-leptotene spermatocytes; SpB, type B spermatogonia; SpA, type spermatogonia.
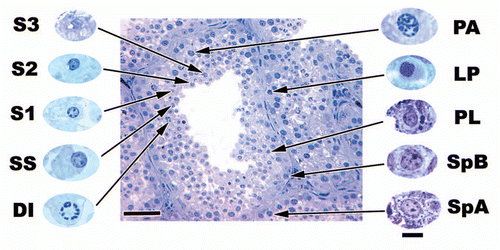
Figure 5 The appearance and duration of time that germ cell types are seen within the seminiferous epithelium of the Red-eared Slider Turtle, Trachemys scripta. Resting, resting spermatogonia; Sp. A and B spermatogonia A and B; Pre-Lept., preleptotene spermatocyte; Lept., leptotene spermatocyte; Zyg., zygotene spermatocyte; Pach., pachytene spermatocyte; Dia., diakinesis; MI, meiosis 1; MII , meiosis 2; Steps 1–4 and 5–8, spermatid steps 1–8.
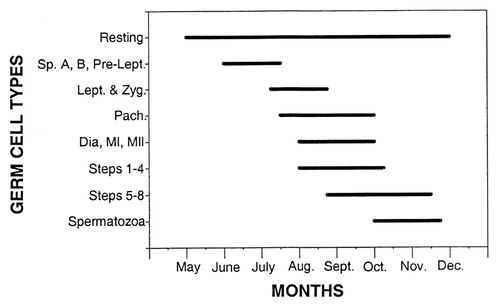
Figure 6 Specific monthly samples of seminiferous epithelia within the testis of Agkistrodon contortrix (northern Copperhead Snake). Bars = 20 µm. (A) May epithelium showing high lipid (white arrowheads) content and the following cell types: spermatogonia A (SpA) and B (SpB), preleptotene spermatocytes (PL), pachytene spermatocytes (PA). (B) July epithelium showing fewer lipids and the cell types: spermatogonia B (SpB), preleptotene spermatocytes (PL), zygotene spermatocytes (ZY), pachytene spermatocytes (PA), steps 1–3 spermatids (s1–s3). (C) Early August epithelium showing almost no lipids and the cell types: spermatogonia A (SpA), spermatogonia B (SpB), preleptotene spermatocytes (PL), steps 1–6 spermatids (s1–s6), mature spermatozoa (ms). (D) Late August epithelium showing more lipids and the cell types: spermatogonia A (SpA), spermatogonia B (SpB) and shed generations of older cell types (white arrows).
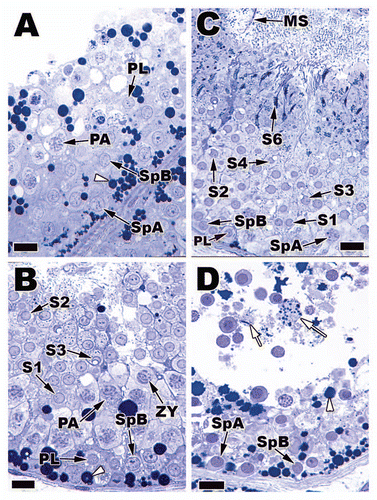
Figure 7 Top: Variation in seminiferous tubule diameter across months of the year in the testes of Sceloporus bicanthalis (Trans Volcanic Bunchgrass Lizard). Values represented on this graph are means ± 1 standard error. A and B on the graph indicate significant differences (p ≤ 0.05; Dunn-Sidak multiple range test). Micrographs from left to right represent seminiferous tubules from March, July and November. Bars = 40 µm. Bottom: Variation in seminiferous epithelial height during every month of the year in Sceloporus bicanthalis testes. Values represented on this graph are means ± 1 standard error. Note that bimonthly the tubular diameter increases (blue arrow, Top) as the epithelia height decreases (blue arrow, Bottom). Thus, the release of sperm lowers the height of the epithelium while increasing the luminal size as sperm fills the luminal space of the seminiferous tubules.
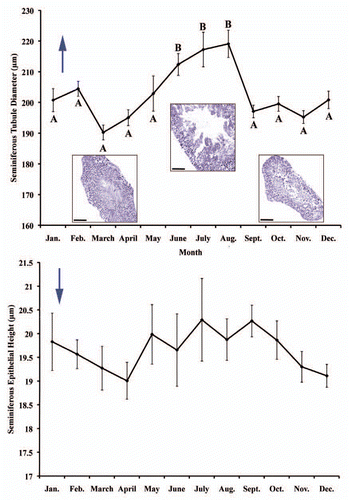
Figure 8 Germ cell types found within the seminiferous epithelia of testes of Seminatrix pygaea (Black Swamp Snake). Bar = 15 µm. Type A spermatogonia (SpA), type B spermatogonia (SpB), pre-leptotene spermatocytes (PL), leptotene spermatocytes (LP), zygotene spermatocytes (ZY), pachytene spermatocytes (PA), diplotene spermatocytes (DI), meiosis I (M1), secondary spermatocytes (SS), meiosis II (M2), step 1 spermatid (S1), step 2 spermatid (S2), step 3 spermatid (S3), (black arrow: acrosome granule), step 4 spermatid (S4) (black arrow: acrosome granule), step 5 spermatid (S5), step 6 spermatid (S6) (black arrowhead: apical extension with acrosome), step 7 spermatid (S7), mature sperm (MS).
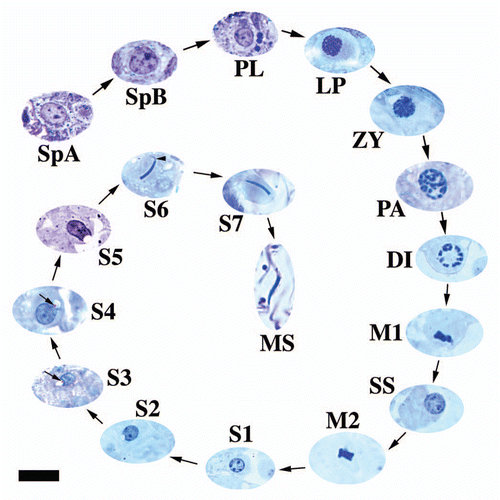
Figure 9 Spermatogonia representatives from the alligator (Alligator mississippiensis), lizard (Podarcis muralis) and snake (Seminatrix pygaea) at the light and electron microscope level. Notice how similar is morphology the cell types are at both levels of microscopy. The have similar nuclear morphology, nucleoli present (black arrow), and the same organelle distribution cytoplasmically: mitochondria, mi; endoplasmic reticula, er. Light: Bar = 20 µm, TEM: Bar = 5 µm.
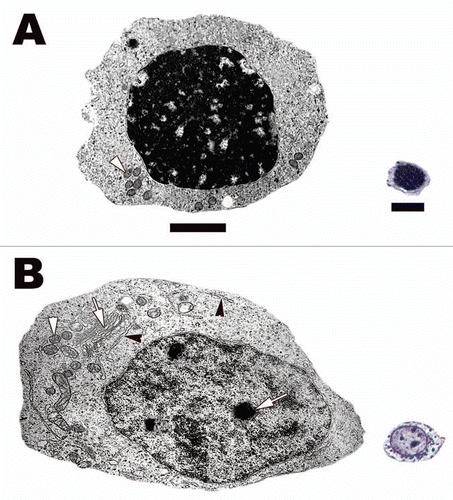
Figure 10 (A) Resting spermatogonia from the seminiferous epithelium of the Red-eared Slider Turtle (Trachemys scripta). Note tight dark staining heterochromatin and lack of organelles cytoplasmically. (B) Spermatogonia A from the seminiferous epithelium of the Black Swamp Snake (Seminatrix pygaea). Note the oval shape, prominent nucleoli (white arrow), and cellular organelles responsible for protein synthesis and ATP production within its cytoplasm: mitochondria, white arrowheads; rough endoplasmic reticula, black arrowheads; Golgi apparatus, white arrow. Light: Bar = 10 µm, TEM: Bar = 5 µm.
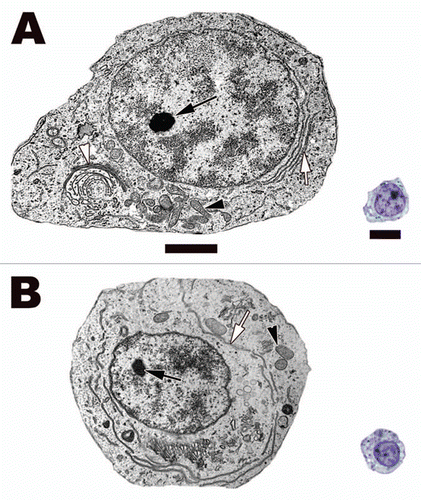
Figure 11 Spermatogonia B (A) and preleptotene spermatocyte (B) from the seminiferous epithelium of the Black Swamp Snake (Seminatrix pygaea). Notice that both cell types' nuclei are more round that speramtogonia A and they have very similar morphology except for size. Rough endoplasmic reticula, white arrows; nucleoli, black arrows; mitochondria, black arrowheads. Light: Bar = 15 µm, TEM: Bar = 5 µm.
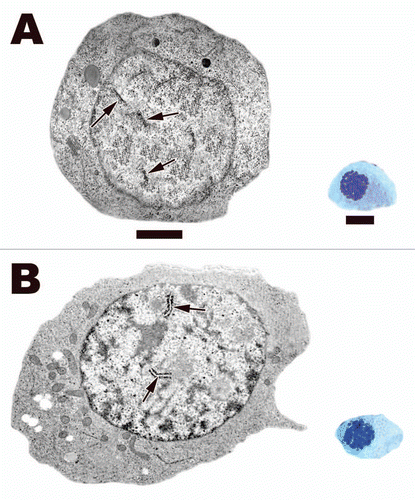
Figure 12 (A) Leptotene spermatocyte within the testis of the Black Swamp Snake (Seminatrix pygaea). The nucleus and the entire cell is now round as it has traveled away from the basal lamina. Linear densities (beginning of chromosome condensation) (black arrows) are common within the nucleus under the electron microscope and they appear as bundles of filaments when viewed via light microscopy. (B) Zygotene spermatocyte within the seminiferous epithelium of the European Wall Lizard (Podarcis muralis). Synaptonemal complexes (black arrows) start to form during this phase of meiosis. These complexes are where crossing over takes place. The formation of these complexes along with the progression of DNA condensation leads to thicker chromatin fibers within these spermatocytes under the light microscope. Light: Bar = 15 µm, TEM: Bar = 5 µm.
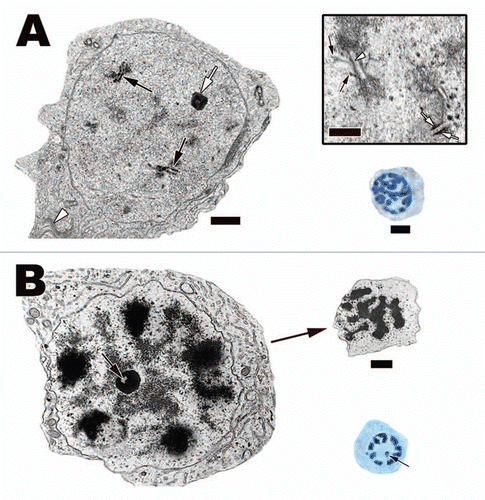
Figure 13 (A) A pachytene spermatocyte in the Cottonmouth Snake (Agkistrodon piscivorus) testis. Completed synaptonemal complexes (black arrows) can be seen in cross sections of the nucleus. These complexes are a tripartite structure with two lateral thick components (Inset, white arrows) and one centrally located thin component (Inset, white arrowhead). Also visible under the electron microscope are the radiating individual chromosomal arms (Inset, black arrows). The thickness of these structures and the increase in protein synthesis (indicated by a prominent nucleolus [white arrow] and large Golgi complex [white arrowhead]) in these cells translates into large open areas of nucleoplasm and thick chromatin filaments under the light microscope. Light: Bar = 15 µm, TEM: Bar = 5 µm, Inset bar = 1 µm. (B) Diplotene spermatocyte in the testis of the Plateau Imbricated Alligator Lizard (Barisia imbricata). These spermatocytes have large almost fully condensed chromosomes near the nuclear membrane, which give the nucleus a spoke-like appearance under the light microscope. A prominent nucleolus with a centrally located lucent spot (black arrows) is a diagnostic character of these spermatocytes. As the chromosomes start to gather near the center of the dividing spermatocytes, the cells enter the first stages of the actual division of chromosomes known as prometaphase 1 (right top corner of B). Bar = 15 µm.
![Figure 13 (A) A pachytene spermatocyte in the Cottonmouth Snake (Agkistrodon piscivorus) testis. Completed synaptonemal complexes (black arrows) can be seen in cross sections of the nucleus. These complexes are a tripartite structure with two lateral thick components (Inset, white arrows) and one centrally located thin component (Inset, white arrowhead). Also visible under the electron microscope are the radiating individual chromosomal arms (Inset, black arrows). The thickness of these structures and the increase in protein synthesis (indicated by a prominent nucleolus [white arrow] and large Golgi complex [white arrowhead]) in these cells translates into large open areas of nucleoplasm and thick chromatin filaments under the light microscope. Light: Bar = 15 µm, TEM: Bar = 5 µm, Inset bar = 1 µm. (B) Diplotene spermatocyte in the testis of the Plateau Imbricated Alligator Lizard (Barisia imbricata). These spermatocytes have large almost fully condensed chromosomes near the nuclear membrane, which give the nucleus a spoke-like appearance under the light microscope. A prominent nucleolus with a centrally located lucent spot (black arrows) is a diagnostic character of these spermatocytes. As the chromosomes start to gather near the center of the dividing spermatocytes, the cells enter the first stages of the actual division of chromosomes known as prometaphase 1 (right top corner of B). Bar = 15 µm.](/cms/asset/1e282251-3de9-43f9-b1f8-7d628dba48a2/kspe_a_10918092_f0013.gif)
Figure 14 (A) Electron and light micrographs of meiosis metaphase 1 in the testis of the Northern Copperhead Snake (Agkistrodon contortrix). Note the thick spindle fiber microtubules (white arrows) and the aligned chromosomes (black arrow). The light micrographs on the right represent the size difference between meiosis metaphase 1 (M1) and meiosis metaphase 2 cells (M2). There is not only a size difference but also about half the amount of chromatin can be seen in the M2 cell compared to the M1 phase. ss, secondary spermatocyte. Light: Bar = 15 µm, TEM: Bar = 5 µm. (B) Electron and light micrographs of secondary spermatocytes within the seminiferous epithelium of the American Alligator (Alligator mississippiensis). These spermatocytes are large with vacuolated mitochondria (black arrowheads), and a small nuage that is visible via both light and electron microscopy (black arrows). Nucleolus, white arrow. The inset (Bar = 5 µm) shows size differences between secondary spermatocytes (SS) and Step 1 spermatids (S1). Note that both cell types share the common feature of a nuage (black arrows), which is also found in the other steps of spermiogenesis. Also labeled in the inset are: step 5 spermatid, S5; electron lucent nuage, white arrowhead; and a developing residual body, white arrow.
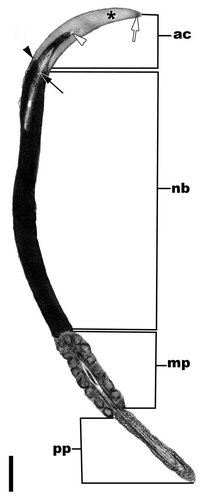
Figure 15 A sagittal section through the head and proximal flagellum of a spermatozoon from the lumen of a seminiferous tubule of the American Alligator (Alligator mississippiensis). The major parts of the spermatozoa in reptiles and other amniotes include the acrosome complex (ac), nuclear body (nb), and the flagellum, which here includes the midpiece (mp) and the principle piece (pp). Bar = 5 µm. Acrosome cortex, white arrow; acrosome medulla, asterisk; epinuclear lucent zone, white arrowhead; nuclear shoulders, black arrowhead, endonuclear canal, black arrow.
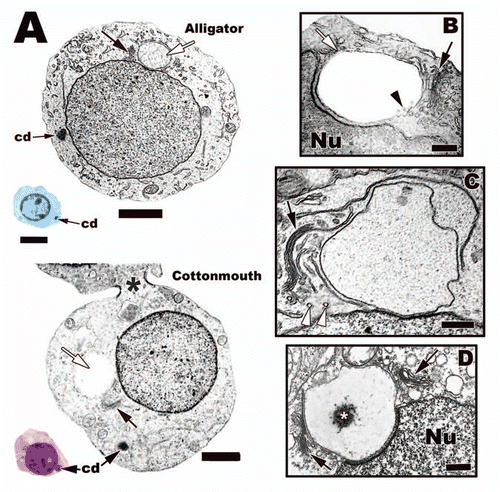
Figure 16 (A) Light and electron micrographs of step 1 spermatids from the testes of the American Alligator (Alligator mississippiensis) and the Cottonmouth Snake (Agkistrodon piscivorus). Note the characters that define this step, chromatoid body (cd), the large Golgi complex (black arrows), and acrosome vesicle (white arrows). Also, there are cytoplasmic bridges that allow cytoplasmic communication between cohorts of germ cells during spermatogenesis in reptiles (black *). Light: Bar = 15 µm, TEM: Bar = 5 µm. (B–D) Early acrosome development in step 1 speramtids from different reptiles. (B) Jamaican Anole, Anolis lineatopus; (C) American Alligator, Alligator mississippiensis; (D) Northern Copperhead Snake, Agkistrodon contortrix. There are transport vesicles (white arrowheads) from the Golgi (black arrows) that deliver materials (black arrowhead) and membrane to the growing acrosome vesicle (white arrow) that rests either near or on the nucleus (Nu). Note that the Copperhead has two Golgi that feed the developing acrosome vesicle (black arrows) and granule (white*). Bars = 200 nm.
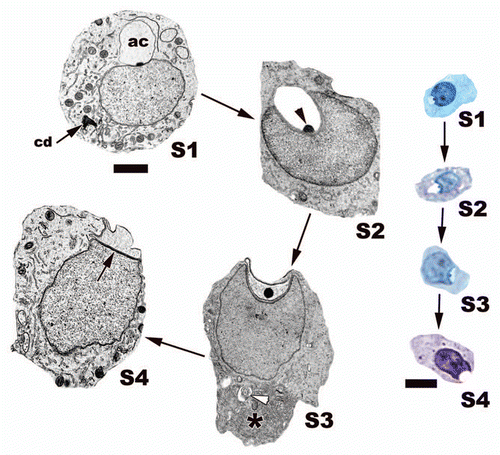
Figure 17 The major steps of the round stage of development during spermiogenesis within the seminiferous epithelium of the American Alligator (Alligator mississippiensis). The acrosome (ac) not only grows in size but a prominent granule (black arrowhead) is observed throughout this developmental stage. The acrosome causes a deep nuclear fossa to form during development. Late in this stage a cytoplasmic shift forces most cytoplasm (black*) within these spermatids toward the caudal end of the cell. This results in the acrosome making contact with the cell membrane, which flattens both the acrosome complex and the apical head of the nucleus. Note the electron dense subacrosome space (black arrow) between the nucleus and the developing acrosome complex. Developing flagellum, white arrowhead, chromatoid body, cd. Light: Bar = 15 µm, TEM: Bar = 5 µm.
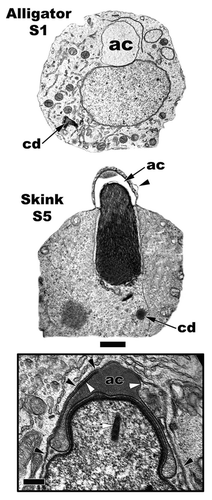
Figure 18 Comparison of a step 1 spermatid (American Alligator, Alligator mississippiensis) nuclear location to that of a step 5 spermatid location (Ground Skink, Scincella lateralis; Inset: Red-eared Slider Turtle, Trachemys scripta) location. The step 1 nucleus is more centrally located and the developing acrosome vesicle (ac) is large and round. The step 5 spermatid has been pushed against the cell membrane (black arrowheads) and is eccentrically placed, thus leading to contact between the cell membrane and the outer acrosome membrane (white arrowheads), which flattens it. Chromatoid body, cd; endonuclear canal (white arrow). Bar = 5 µm and Inset bar = 200 nm.
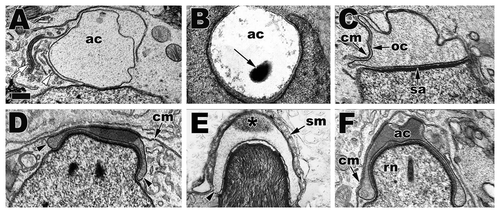
Figure 19 Progression of acrosome development across reptile taxa showing the similarities and conserved ontogenic morphology of the acrosome during its development. (A) S1, American Alligator (Alligator mississippiensis); (B) S2, Transvolcanic Bunchgrass Lizard (Sceloporus bicanthalis); (C) S4, American Alligator (Alligator mississippiensis); (D) early S5, Ground Skink (Scincella lateralis); (E) late S5, Northern Copperhead Snake (Agkistrodon contortix); (F) late S5, Red-eared Slider Turtle (Trachemys scripta). Note the acrosome shape changes as development progress from a round vesicle to a flattened vesicle against the cell membrane that eventually envelops the entire apical nuclear head. In crocodilians and turtles, this enveloping early in elongation causes the constriction of the nuclear head into the rostrum (rn). Acrosome vesicle, ac; golgi complex, white arrow; transport vesicle, white arrowhead; acrosome granule, black arrow; cell membrane, cm; outer acrosome membrane, oc; subacrosome space, sa; acrosome vesicle shoulders, black arrowheads; Sertoli cell membrane layers, sm; diffusion of the acrosome granule, black *. Bar = 200 nm.
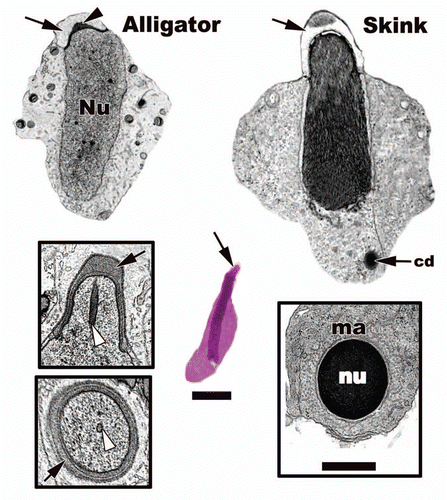
Figure 20 Light (Ground Skink step 5) and electron microscopy of mid-elongation in the American Alligator (Alligator mississippiensis) and the Ground Skink (Scincella lateralis) from the seminiferous epithelium. Acrosome, black arrows; acrosome granule, black arrowhead; nucleus, nu; chromatoid body, cd; endonuclear canal, white arrowheads; manchette, ma. Light: Bar = 15 µm, TEM: Bar = 5 µm.
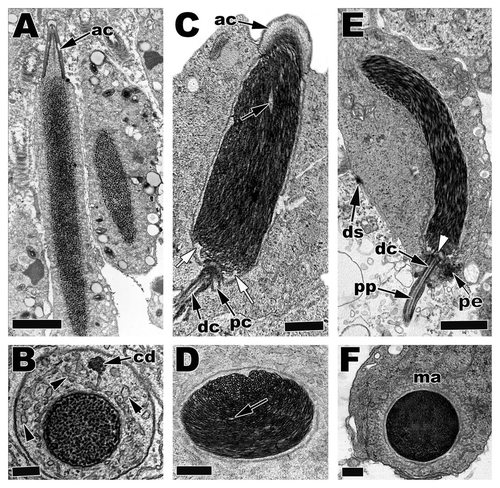
Figure 21 Electron micrographs of Step 5 and Step 6 spermatids in (A and B) S5, American Alligator, Alligator mississippiensis; (C and D) S5, Cottonmouth Snake, Agkistrodon piscivorus; (E and F) S6, Ground Skink, Scincella lateralis. Within the Alligator (A and B) and other non-squamate reptiles DNA condenses in a granular fashion. In squamates (snakes and lizards), DNA condenses in a filamentous fashion that can either have roughly parallel filaments like in (E) or circular or concentric filaments like in (C and D). acrosome, ac; chromatoid body, cd; endoplasmic reticula, black arrowheads; proximal centriole, pc; distal centriol, dc; translucent zones, white arrows; lacuna, black arrows; caudal nuclear fossa, white arrow; pericentriolar material, pe; principle piece, pp; manchette, ma; desmosome junction, ds. (A and E) Bars = 5 µm, (C) Bar = 3 µm, (B, D and F) Bars = 200 nm.
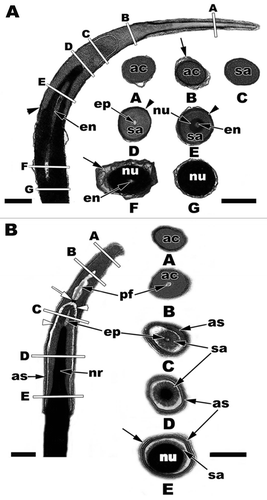
Figure 22 (A) The nuclear head with acrosome complex of the American Alligator (Alligator mississippiensis) in sagittal and cross sections. The letters A–G of the sagittal section correspond to the same letters of each cross section. Acrosome vesicle, ac; subacrosome space, sa; epinuclear lucent zone, ep; endonuclear canal, en; nucleus, nu; Sertoli cell membrane layers, black arrows; acrosome shoulder, black arrowheads. (B) The nuclear head with acrosome complex of the Imbricate Alligator Lizard (Barisia imbricata) in sagittal and cross sections. The letters A–E of the sagittal section correspond to the same letters of each cross section. Acrosome vesicle, ac; subacrosome space, sa; epinuclear lucent zone, ep; nucleus, nu; Sertoli cell membrane layers, black arrow; acrosome shoulders, as; nuclear rostrum, nr; perforatorium, pf; basal plate of the perforatorium, white arrow; thin white lucent zone, white arrowheads. Sagittal bars = 0.5 µm, cross section bars = 200 nm.
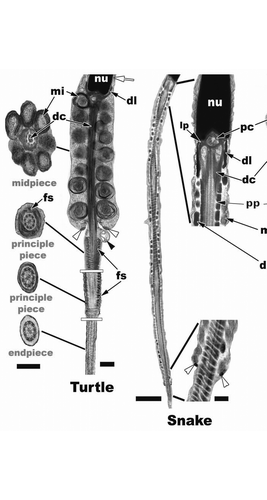
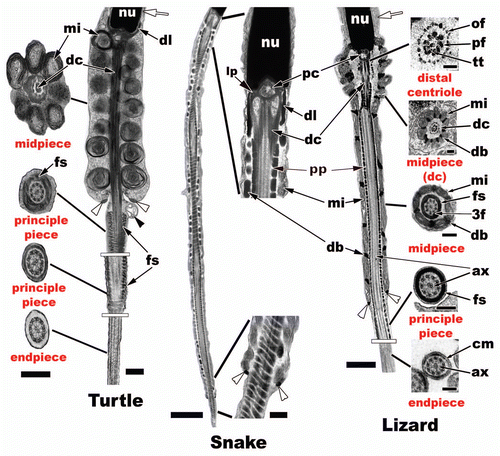
Figure 23 Turtle (Trachemys scripta): Step 7 spermatid flagellar morphology. The mitochondria (mi) of the midpiece have conspicuous outer concentric layers of membrane (cristae) and are large and round compared to the elongated mitochondria of the snake and lizard. There is no neck region between the mitochondria of the midpiece and the nuclear fossa. The dense collar (dl) points upward and surrounds the most caudal portion of the nucleus, which is in direct contrast to what is seen in squamates. The midpiece is made up of 7 to 8 concentric rings of mitochondria and the distal centriole (dc) runs the length of the midpiece, which is terminated by the annulus (white arrowheads). The principle piece extends away from the midpiece and is surrounded by the fibrous sheath (fs). The endpiece is the terminal end of the flagellum as it is in all reptiles and amniotes and has no fibrous sheath. Nucleus, nu; manchette, white arrow; residual body, black arrowhead. Sagittal and cross section bar = 0.5 µm. Snake (Seminatrix pygaea) (low mag. sagittal bar = 2 µm/high mag. sagittal bar = 200 nm) and Lizard (Hemidactylus turcicus) (sagittal bar = 1 µm, cross section bars = 200 nm): Step 7 spermatids have a distinct neck that houses the proximal centriole (pc), dense collar (dl), bilateral laminar projections (lp), large peripheral fibers (pf), an outer fiber ring (of) (when present: lizard) and the distal centriole (dc) in squamates. The principle piece (pp) starts within the midpiece at mitochondria tier 2 in the representative snake and lizard spermatids. Note the extremely long midpiece in the snake compared to other reptiles. Within the midpiece there are also dense bodies (db), which are not seen in the turtle spermatids. The axoneme (ax) of the principle piece is surrounded by the fibrous sheath (fs) and the enlarged peripheral fibers, 3 and 8 (3f), which remain along the length of the principle piece. Triplet microtubules, tt, cell membrane, cm.
References
- Courot M, Hochereau-de Rivers MT, Ortavant R. Johnson AD, Gomes WR, Van Demark NL. Spermatogenesis. The Testis 1970; 1:New York, NY Academic Press 339 - 432
- Roosen-Runge EC. The Process of Spermatogenesis in Animals 1977; Cambridge, England Cambridge University Press 5 - 250
- Ewing LL, Davis JC, Zirkin BR. Greep RO. Regulation of testicular function: A spatial and temporal view. International Review of Physiology 1980; 22:Baltmore, MD University Park Press 41 - 115
- Stienberger E, Steinberger A. Hamilton DW, Greep RO. Spermatogenic function of the testis. Handbook of Physiology. Male reproductive system 1975; 5:Washington, DC American Physiological Society 1 - 18
- Russell LD, Ettlin RA, Sinha Hikim AP, Clegg ED. Histological and histopathological evaluation of the testis 1990; Clearwater, FL Cache River Press
- Gribbins KM, Rheubert JL. Aldridge RD, Sever DM. The ophidian testis, spermatogenesis and mature spermatozoa. Reproductive Biology and Phylogeny of Snakes 2011; 9:Enfield, NH Science Publishers 183 - 264
- Gribbins KM, Gist DH, Congdon JH. Cytological evaluation of spermatogenesis and organization of the germinal epithelium in the male Slider Turtle, Trachemys scripta. J morphol 2003; 255:337 - 346
- Pudney J. Russell LD, Griswold MD. Comparative cytology of the non-mammalian vertebrate Sertoli cell. The Sertoli Cell 1990; Clearwater Fl Cache River Press 611 - 657
- Gist DH, Turner TW, Congdon JD. Chemical and thermal effects on the motility of sperm from the turtle epididymis. J Reprod Fert 2000; 119:271 - 277
- Modesto SP, Anderson JS. The phylogenetic definition of Reptilia. Syst Bio 2004; 53:815 - 821
- Van Oordt PGWJ. Regulation of the speramtogenic cycle in the frog, Rana temporaria. Mem Soc Endocrinol 1955; 4:25 - 38
- Van Oordt PGWJ, Brands F. The Sertoli cell in the testis of the common frog, Rana temporaria. Proc Soc Endocrinology 119th meeting. J Endocrinol 1970; 48:100
- Lofts B. Seasonal changes in the functional activity of the interstitial and spermatogenetic tissues of the Green Frog, Rana esculenta. Gen Comp Endo 1964; 4:550 - 562
- Burgos MH, Vitale-Calpe R. Histochemistry of the testis in normal and experimentally treated frogs, Rana rana. J Ultrastruct Res 1967; 19:221 - 237
- Lofts B, Boswell C. Cyclic changes in the distribution of the testis lipids in the common frog, Rana temporaria. Nature 1960; 187:708 - 709
- Lofts B. The Sertoli cell. Gen Comp Endo Suppl 1972; 3:636 - 648
- Bergmann M, Schindelmeiser J, Greven H. The blood-testis barrier in vertebrates having different testicular organizations. Cell Tiss Res 1984; 238:145 - 150
- Bergmann M, Greven H, Schindelmeiser J. The blood-testis barrier in a frog and salamander. Cell Tiss Res 1983; 232:189 - 200
- Baccetti B, Bigliardi E, Talluri MV, Burrini AG. The Sertoli cel in lizards. J Ultrastruct Res 1983; 168:268 - 275
- Morales A, Cavicchia JC. Seasonal changes of the blood-testis barrier in Viscacha (Lagostomus maximus maximus): A freeze fracture and lanthanum tracer study. Anta Rec 1993; 236:459 - 464
- Gribbins KM. The cytological evaluation of spermatogenesis and ultrastructure of the junctional complexes within the germinal epithelium of reptiles 2003; Cincinati OH Unviersity of Cincinnati Doctoral Thesis
- Yamamoto S, Tamate H, Itikawa O. Morphological studies of the sexual maturation in the male Japanese Quail (Coturnix coturnix japonica). Tohuku J Agric Res 1967; 18:27 - 37
- Taite AJ, Johnson E. Spermatogenesis in the grey squirrel (Sciurus carolinensis) and changes during sexual regression. J Reprod Fert 1982; 65:53 - 56
- Tsubota T, Kanagawa H. Annual changes in serum testosterone levels and spermatogenesis in the Hokkaido Brown Bear, Ursus arctos esoensis. J Mamm Soc Jpn 1989; 14:11 - 17
- Foreman D. Seminiferous tubule stages in the Prairie Dog (Cynomys ludovicianus) during the annual breeding cycle. Anat Rec 1997; 247:355 - 367
- Russell LD, Hikim SAP, Ettlin RA, Clegg ED. Histological and histopathological evaluation of the testis 1990; Clearwater Fl Cache River Press
- Gribbins KM, Elsey R, Gist DH. Cytological evaluation of spermatogenesis in the germinal epithelium of the American Alligator, Alligator mississippiensis. Acta Zool 2006; 87:59 - 69
- Gribbins KM, Gist DH. The cytological evaluation of the germinal epithelium and thegerm cell cycle in an introduced population of European Wall Lizard, Podarics muralis. J Morphol 2003; 256:296 - 306
- Gribbins KM, Happ CS, Sever DM. Ultrastructure of the reproductive system of the Black Swamp Snake (Seminatrix pygaea) V. The temporal germ cell development strategy of the testis. Acta Zool 2005; 86:223 - 230
- Gribbins K, Rheubert J, Collier M, Siegel D, Sever D. Histological analysis of spermatogenesis and the germ cell development strategy within the testis of the male western Cottonmouth Snake, Agkistrodon piscivorus. Annal Anat 2008; 190:461 - 476
- Gribbins KM, Rheubert JL, Poldemann EH, Collier MH, Wilson B, Wolf K. Continuous spermatogenesis and the germ cell development strategy within the testis of the Jamaican Gray Anole, Anolis lineatopus. Theriogenology 2009; 72:54 - 61
- Rheubert JL, McHugh HH, Collier MH, Sever DM, Gribbins KM. Temporal germ cell development strategy during spermatogenesis within the testis of the Ground Skink, Scincella lateralis (Sauria: Scincidae). Theriogenology 2009; 72:484 - 492
- Gribbins K, Anzalone M, Collier M, Granados-Gonzalez G, Villagran-Santa Cruz M, Hernandez-Gallegos O. Temporal germ cell development strategy during continuous spermatogenesis within the montane lizard, Sceloporus bicanthalis (Squamata; Phrynosomatidae). Theriogenology In press
- Waits GMH, Setchell BP. Lemming GE. Physiology of the mammalian testis. Marshall's Physiology of Reproduction Reproduction in the Male 1990; 2:4th Ed London, England Churchill Livingstone 1 - 105
- Licht P. Reproductive endocrinology of reptiles and amphibians: Gonadotropins. Ann Rev Phys 1979; 41:337 - 351
- Licht P, Famer SW, Bona-Gall A, Papkoff H. Pituitary gonadotropins in snakes. Gen Comp Endo 1979; 39:34 - 52
- Licht P, Denver RJ, Pavgi S. Temperature dependence of in vitro pituitary, testis and thyroid secretion in a turtle, Pseudemys scripta. Gen Comp Endo 1989; 76:274 - 285
- Masson GR, Guillette LJ Jr. FSH-induced gonadal development in juvenile lizards, Eumeces obsoletus. J Exp Zool 2005; 236:343 - 351
- Licht P. Laming GE. Reptiles. Marshall's Physiology of Reproduction. Reproductive cycles of vertebrates 1984; 1:New York, NY Churchhill Livingston 206 - 282
- Dym M, Clermont Y. Role of spermatogonia in the repair of the seminiferous epithelium following x-irradiation of the rat testis. Am J Anat 1970; 128:265 - 282
- Huckins C, Oakberg EF. Morphological and quantitative analysis of spermatogonia in mouse testes using whole mounted seminiferous tubules. II. The irradiated testes. Anat Rec 1978; 192:529 - 541
- Ewing LL, Davis JC, Zirkin BR. Greep RO. Regulation of testicular function: A spatial and temporal view. International Review of Physiology 1980; 22:Baltimore, MD University Park Press 41 - 115
- Russell LD, Frank B. Characterization of rat spermatocytes after plastic embedding. Arch Androl 1978; 1:5 - 18
- Monesi V. Synthetic activities during spermatogeneses in the mouse. RNA and protein. Exptl Cell Res 1965; 39:197 - 224
- de Kretser DM, Kerr JB. Knobil E, Neil J. The cytology of the testis. The Physiology of Reproduction 1988; New York, NY Raven Press 837 - 932
- Roosen-Runge EC. The Process of Spermatogenesis in Animals 1962; Cambridge England Cambridge Press
- Johnson AD, Gomes WR. The Testis. Advances in physiology, biochemistry and function 1977; New York, NY Academic Press 4
- Davis JR, Langford GA, Kirby RP. Johnson AD, Gomes WR, Van Demark NL. The testicular capsule 1970; 1:New York, NY Academic Press 281 - 337
- Jamieson BGM. Gagnon C. Spermatozoal phylogeny of the Vertebrata. The Male Gamete. From Basic Science to Clinical Applications 1999; Clearwater, Fl Cache River Press 303 - 331
- Teixeira RD, Colli GR, Bao SN. The ultrastructure of the spermatozoa of the lizard Micrablepharus maximiliani (Squamata, Gymnophthalmidae), with considerations in the use of sperm ultrastructure characters in phylogenetic reconstruction. Acta Zool 1999; 80:47 - 59
- Vieira GHC, Colli GR, Bao SN. The ultrastructure of the spermatozoon of the lizard Iguana iguana (Reptilia, Squamata, Iguanidae) and the variability of sperm morphology among iguanian lizards. J Anat 2004; 204:451 - 464
- Carcupino M, Corso G, Pala M. Spermiogenesis in Chalcides ocellatus tiligugu (Gmelin) (Squamata: Scincidae): an electron microscope study. Bollettino di Zoologia 1989; 56:119 - 124
- Healy JM, Jamieson BGM. The ultrastructure of spermatogenesis and epididymal spermatozoa of the tuatara, Sphenodon punctatus (Sphenodontidae, Amniota). Philo Trans Royal Soc Lon: Biol Sci 1994; 344:187 - 199
- Vieira GHC, Wiederhecker HC, Colli GR, Bao SN. Spermiogenesis and testicular cycle of the lizard Tropidurus torquatus (Squamata, Tropiduridae) in the Cerrado of central Brazil. Amphibia-Reptilia 2001; 22:217 - 223
- Ferreira A, Dolder H. Ultrastructural analysis of spermiogenesis in Iguana iguana (Reptilia: Sauria, Iguanidae). Euro J Morphol 2002; 40:89 - 99
- Gribbins K, Mills E, Sever D. The ultrastructure of spermiogenesis within the testis of the Ground Skink, Scincella lateralis (Scincidae, Squamata). J Morphol 2007; 268:181 - 192
- Gribbins K, Rheubert J, Anzalone M, Siegel D, Sever D. Ultrastructure of spermiogenesis in the Cottonmouth, Agkistrodon piscivorus (Squamata: Viperidae, Crotalinae). J Morphol 2010; 271:293 - 304
- Rheubert JL, Wilson BS, Wolf KW, Gribbins KM. Ultrastructural study of spermiogenesis in the Jamaican Gray Anole, Anolis lineatopus (Reptilia, Polychrotidae). Acta Zool 2010; 91:484 - 494
- Rheubert JL, Siegel DS, Venable KJ, Sever DM, Gribbins KM. Ultrastructural description of spermiogenesis within the Mediterranean Gecko, Hemidactylus turcicus (Squamata: Gekkonidae). Micron 2011; 42:680 - 690
- Spando RL, Russell LD. Spermiogenesis in the Red-Ear Turtle (Pseudemys scripta) and the Domestic Fowl (Gallus domesticus): A study of cytoplasmic events including cell volume changes and cytoplasmic elimination. J Morphol 1988; 198:95 - 118
- Zhang L, Xiang-Kun H, Mei-Ying L, Hui-Jun B, Qiu-Sheng C. Spermiogenesis in the Soft-Shelled Turtle, Pelodiscus sinensis. Anat Rec 2007; 290:1213 - 1222
- Saita A, Comazzi M. Electron microscope study of spermiogenesis in Caiman crocodylus. Boll Zool 1987; 4:307 - 318
- Gribbins KM, Siegel DS, Anzalone ML, Jackson DP, Venable KJ, Rheubert JL, Elsey RM. Ultrastructure of spermiogenesis in the American Alligator, Alligator mississippiensis (Reptilia, Crocodylia, Alligatoridae). J Morphol 2010; 271:120 - 1271
- Leblonde CP, Clermont Y. Spermiogeneses of rat mouse, hamster and guinea pig as revealed by the periodic acid-fuchsin sulfurous acid technique. Am J Anat 1952; 90:167 - 215
- Russell LD, Weber JE, Vogl AW. Characterization of filaments within the subacrosomal space of rat spermatids during spermiogenesis. Tissue Cell 1986; 8:887 - 898
- Fawcett DW, Anderson WA, Phillips DM. Morphogenetic factors influencing the shape of the sperm head. Dev Biol 1971; 26:220 - 251
- Wolosewick J, Bryan JHD. Ultrastructureal characterization of the manchette microtubules in the seminiferous epithelium of the mouse. Am J Anat 1977; 150:301 - 332
- Aire TA. Jamieson BGM. Anatomy of the testis and male reproductive tract. Reproductive biology and phylogeny of birds 2007; 6:Enfield, NH Science Publishers 37 - 113
- Jamieson BGM. Jamieson BGM, Ausio J, Justine JL. The ultrastructure of spermatozoa of the Squamata (Reptilia) with phylogenetic considerations. Advances in spermatozoal phylogeny and taxonomy 1995; 166:Paris, France Mem Mus Nat Hist Nat, Editions du Museum 359 - 383
- Jamieson BGM. Jamieson BGM. Avian spermatozoa: Structure and phylogeny. Reproductive biology and phylogeny of birds 2007; 6:Enfield, NH Science Publishers 349 - 511
- Harding HR, Aplin KP, Mazur M. Jamieson BGM, Ausio J, Justine JL. Ultrastucture of spermatozoa of Australian Blindsnakes, Ramphotyphlops spp (Typhlopidae, Squamata): first observations on the mature spermatozoon of scolecophidian snakes. Advances in spermatozoal phylogeny and taxonomy 1995; 166:Paris, France Mem Mus Nat Hist Nat, Editions du Museum 385 - 396
- Talbot P. Bacetti B. Compartmentalization in the acrosome. Comparative Spermatology-20 years after 1991; New York, NY Raven Press 225 - 259
- Gribbins KM, Touzinsky KF, Siegel DS, Venable KJ, Hester GL, Elsey RM. Ultrastructure of the spermatozoon of the American Alligator, Alligator mississippiensis (Reptilia Alligatoridae). J Morphol In Press
- Solomon KR, Carr JA, Du Preez LH, Giesy JP, Kendall RJ, Smith EE, Van Der Kraak GJ. Effects of atrazine on fish, amphibians and aquatic reptiles: A critical review. Critical Reviews in Toxicology 2008; 38:721 - 772