Abstract
Filamins are a family of actin-binding proteins composed of filamin A, B and C. Besides of their ability to induce perpendicular branching of F-actin filaments via their actin binding domains near the N-terminus, filamins can regulate multiple cellular functions because of their unique ability to recruit more than 90 protein binding partners to their primary sequences which are having highly diversified cellular functions. However, this family of proteins has not been examined in the testis until recently. Herein, we highlight recent findings in the field regarding the role of these proteins in cell epithelia, and based on recent data in the testis regarding their role on spermatogenesis, this review provides the basis for future functional studies.
Introduction
Filamin A [formerly known as actin-binding protein 280 (ABP280)] is a non-muscle actin filament cross-linking protein first identified in macrophages in 1975.Citation1 Since then, three isoforms of filamins, known as filamin A, B and C, which are products of distinct genes have been identified in different mammalian epithelia.Citation2,Citation3 Studies from the past 36 years have shown that filamins play multiple cellular roles, serving as organizers of cell structure (e.g., cytoskeleton) and function, regulating cell signaling, transcription, cell adhesion, focal adhesion assembly, cell apoptosis and organ development.Citation4-Citation8 A recent study has demonstrated that filamin A serves as a central mechanotransduction element of the cytoskeleton.Citation9 In short, filamin A working with FilGAP (an filamin A-binding GTPase-activating protein specific for Rac GTPase) and β-integrin acts as a “molecular switch” that converts mechanical stimuli into chemical signalsCitation9 to elicit cellular responses in response to changes in environment, growth and/or development. While the filamin protein family is composed of only three proteins, however, each filamin is known to serve as scaffolds for multiple proteins, and more than 90 binding partners of filamins have been identified to date, ranging from cell adhesion proteins (e.g., β1-, β3- and β7-integrin, ICAM-1), cytoskeletons (e.g., F-actin, vimentin), GTPases (e.g., Cdc42, Rho, Rac), GTPase regulatory proteins (e.g., FilGAP), cytokines (e.g., interferon-α), adaptors (e.g., vinculin), ion channels (e.g., K+ channel), receptors (e.g., interferon receptor, dopamine receptor, insulin receptor), signaling proteins (e.g., MEKK1, MKK4, JNK), protein kinases (e.g., PKCα, ROCK, p21 activated kinase 1 or Pak1), endocytic vesicle-mediated protein trafficking-related proteins (e.g., caveolin-1), proteases (e.g., caspase), polarity proteins (e.g., 14–3-3) and even transcription factors (e.g., androgen receptor, Smads).Citation5,Citation8 Interestingly, while many of these molecules are intimately related to spermatogenesis (e.g., vinculin, 14–3-3, JNK, ROCK, PKC, Pak1, Smads, caspase, caveolin-1), there is no report in the literature, investigating the role of filamins on spermatogenesis and testicular function except a recent study.Citation10 Herein, we provide an update on filamins, in particular filamin A and how this protein relates to cell adhesion function at the ectoplasmic specialization (ES) at the Sertoli cell-elongating spermatid interface (known as apical ES) and at the Sertoli-Sertoli cell interface at the blood-testis barrier (BTB) (known as basal ES),Citation11,Citation12 and how filamins can likely be working with other actin binding (e.g., drebrin E)Citation13,Citation14 and regulatory proteins (e.g., Arp2/3 complex,Citation15 N-WASP,Citation15,Citation16 Eps8Citation17).Citation18,Citation19 This information should be helpful to investigators in the field seeking to study the impact of actin dynamics on different cellular events of spermatogenesis, including spermatogonial stem cell/spermatogonial renewal, germ cell differentiation, meiosis, spermiogenesis and spermiation.Citation20-Citation24
Structure of Filamins
Each mammalian filamin is composed of two polypeptide chains of ~280 kDa that self-associate to form a V-shaped dimeric protein,Citation25 with these two polypeptides being non-covalently linked via their dimerizing domain at the C-terminus (), such that each filamin subunit binds to only one F-actin ().Citation4 Each monomer of filamins is composed of an F-actin-binding domain (ABD) at its N-terminus and a rod segment consisting of 24 homologous repeats of ~96 amino acid residues in each repeat [Repeats 1–8 are known to bind vimentin and PKCCitation26; Repeats 9–15 that binds F-actin; Repeats 16–23 that binds dopamine receptor, GTPases, β-integrins and Pak1, and Repeat 24 (the dimerizing domain that also binds ROCK) at the C-terminus] that adopts an immunoglobulin-like fold (Ig repeatsCitation27) (). Two calpain-sensitive hinge domain regions that separate the 24 Ig repeats into two large rod domains (Rod 1: Repeats 1–15 and Rod 2: Repeats 16–23) between Repeats 15 and 16 (known as Hinge 1, H1) and between Repeats 23 and 24 at the C-terminus (known as Hinge 2, H2) (). Thus, the binding of a V-shaped dimeric filamin moleculeCitation25 to two filamentous actin (F-actin) filaments favors perpendicular (i.e., at 90°) branching of F-actin (). Rod 1 domain is mostly used for actin-binding (see ) while Rod 2 domain associate mostly with other partner proteinsCitation28 (see ) for filamins.
Figure 1. A schematic molecular model of filamin A illustrating its role as a F-actin cross-linker. The actin-binding domain (ABD) of filamin A is located at its N-terminus, which is followed by the 9–15 immunoglobulin (Ig) repeats, constituting the Rod 1, which is capable of binding one F-actin filament. The hing region (H1), the Rod 2 region and the H2 region are also shown. The 24 Ig repeat is the dimerizing domain where two subunits of filamin A are dimerized via the two 24 Ig repeats through non-covalent interactions. The shaded “gray” area between the two Rod 2 domains of two filamin A subunits is the region where filamin A interacts with its binding partners, such as integrins, Rho, Rac, Cdc42, ROCK, Pak1, FiGAP, dopamine receptor and others.
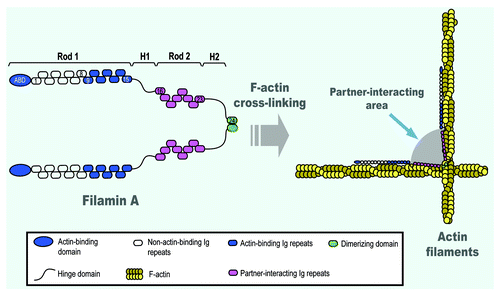
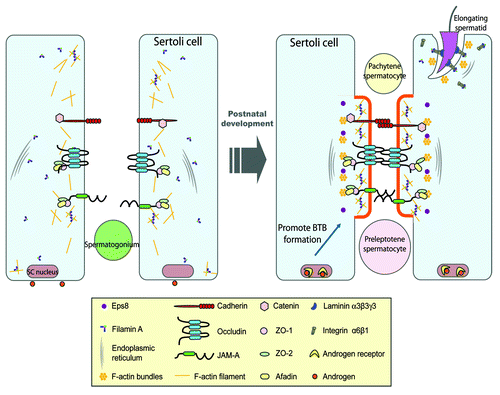
Figure 2. A schematic drawing illustrating the role of filamin A in the assembly of a functional BTB during postnatal development. In immature rat testes, cell adhesion protein complexes (e.g., occludin-ZO-1, cadherin-catenin, JAM-A-ZO-1) cannot be recruited to the BTB site to assemble the functional TJ-permeability barrier (see left panel). At age 17–25 d postpartum, the expression of filamin A increases, the functional filamin A recruits the assembly of actin filament network at the BTB site, which in turn, recruits cell adhesion protein complexes. This process is facilitated by androgen, which induces cross-linking of F-actin filaments mediated by filamin A to form rigid scaffold underneath cell membrane, which can lead to membrane protrusion at cell-cell interface to facilitate adhesion formation (see right panel). Also, during spermiogenesis, the assembly of cell adhesion protein complexes (e.g., integrin-laminin) at the Sertoli-spermatid interface, namely the apical ectoplasmic specialization (apical ES), is likely facilitated by the recruitment of integrins to the apical ES via interactions between integrins (e.g., α6-integrin, β1-integrin) and filamin A (right panel).
Filamin A, Ectoplasmic Specialization (ES) and Cell Adhesion
ES is an atypical adherens junction (AJ) type uniquely found in the mammalian testis.Citation11,Citation29-Citation32 It is limited to the interface of Sertoli cells and spermatids (steps 8–19 spermatids in rats) during spermiogenesis known as the apical ES, but once it appears, it is the only anchoring junction that supersedes desmosome and gap junction (restricted to steps 1–7 spermatids in rats) that anchor developing spermatids to the Sertoli cell in the seminiferous epithelium until it undergoes degeneration at spermiation to allow the release of spermatozoa into the tubule lumen.Citation11,Citation24,Citation30,Citation33,Citation34 However, ES is also found at the Sertoli-Sertoli cell interface at the BTB, known as the basal ES.Citation30 But unlike the apical ES, the basal ES coexists with tight junction (TJ) and gap junction (GJ); and together with desmosome, all these junctions constitute the BTB (since the endothelial TJ-barrier of the microvessels located in the interstitium contribute relatively little to the barrier function of the BTB in the testis), so that post-meiotic spermatid development takes place in the adluminal (or apical) compartment behind the BTB in an immune-privileged site,Citation30,Citation35-Citation37 segregated from the host immune system in the mammalian body.Citation30,Citation38,Citation39
ES is typified by the presence of tightly packed actin filament bundles sandwiched between cisternae of endoplasmic reticulum and the opposing plasma membranes of either the Sertoli cell and the elongating/elongated spermatid (at the apical ES) or the two adjacent Sertoli cells (at the basal ES),Citation11,Citation30 which is also the hallmark ultrastructure of the ES, making this anchoring junction type in the testis different from all other anchoring junctions in the mammalian body. However, at the apical ES, this typical ultrastructural features, namely, the actin filament bundles that lie perpendicular to the plasma membrane is limited only to the Sertoli cells without comparable ultrastructures visible in the spermatid, but the actin filament bundles are found on both sides of the Sertoli cells at the basal ES.Citation30,Citation33,Citation40 Recent studies using a mechanical device to pull attached spermatids from the Sertoli cell epithelium in order to estimate the strength of the apical ES have shown that this is one of the strongest anchoring junctions, significantly stronger than desmosome in the testis,Citation41,Citation42 and it is noted that desmosome is considered to be a very strong adhesion junction.Citation43,Citation44 Based on these findings, it was postulated that the unusual adhesive strength of the ES (e.g., apical ES) is the result of these actin filament bundles.Citation30,Citation40 Indeed, the BTB is one of the tightest blood-tissue barriers in the mammalian body, which is also largely contributed by the tightly packed actin filament bundles at the basal ES coexisting with TJ.Citation30 Since filamins (e.g., filamin A) induce perpendicular branching of F-actin filaments, the presence of filamin A in Sertoli cells of the rat testisCitation10 seemingly suggest that it is being used to induce changes in the conformation of the tightly packed actin filament bundles which are necessary to maintain the morphology of apical and basal ES in the seminiferous epithelium, to a “branched” state, facilitating ES restructuring during spermiogenesis. This possibility is physiologically necessary since spermatids are not anchored statically in a specific location in the epithelium during spermiogenesis. Instead, developing spermatids are moving “up-and-down” the epithelium during the epithelial cycle, perhaps to “acquire” necessary signals and nutrients from the Sertoli cells at the cell-cell interface, namely the apical ES, for their development during spermiogenesis which is composed of a series of dynamic changes, both in cell shape, morphology, biochemically, and at the molecular level. On the other hand, the basal ES at the BTB is also not a static ultrastructure even the barrier function conferred by the BTB cannot be compromised, event transiently, during spermatogenesis. This is because preleptotene spermatocytes connected in “clones” via intercellular bridges must traverse the BTB at stage VIII of the epithelial cycle to enter the adluminal compartment to continue their development, such as meiosis I and II, and spermiogenesis. Thus, the basal ES is also a highly dynamic ultrastructure. In short, ES provides unusual adhesive strength to the developing spermatids during spermiogenesis via apical ES, and to the Sertoli cell at the BTB via basal ES, this ultrastructure requires intricate regulation so that the actin filament bundles can be “switched” on-and-off between the “bundled” and “branched” state such that the adhesive function can be constantly regulated during the epithelial cycle. The fact that filamins can rapidly induce branched actin filaments, it is likely that filamins are working in concert with the Arp2/3/N-WASP protein complex that is known to confer branced actin polymerization, these proteins thus provide an efficient molecular mechanism to alter the “fluidity” and “rigidity” of the actin filament bundles at the ES during spermatogenesis. In short, filamins are important cell adhesion regulators based on their intrinsic actin binding activity, along with their protein binding partners, so that multiple regulatory proteins can be recruited to the actin filament bundles at the ES during spermatid movement throughout spermatogenesis (). For instance, recent studies have shown that filamin A can recruit integrins to the specific cellular domains via a unqiue mechanism,Citation45 and β1-integrin is a known constituent cell adhesion protein at the apical ES;Citation46-Citation48 and a filamin A-integrin receptor complex has been identified in epithelial cells to elicit changes in cell adhesion,Citation49 and filamin B was found to be involved in the assembly of focal adhesion complex (or focal contact, a cell-matrix anchoring junction type).Citation8 These latter findings thus illustrate that filamins can regulate changes in cell adhesion via their indirect effects on cell adhesion proteins (e.g., integrins) at the apical ES (), besides the actin-based cytoskeleton and the vimentin-based intermediate filament cytoskeleton.
Filamin A and Blood-Testis Barrier Function in the Testis
In light of the findings regarding the role of filamins as a cell organizer and a regulator of cytoskeletal function, it is not unusual that filamin A is predominantly found in Sertoli cells in the testis since germ cells lack the extensive actin filament networks.Citation10 Interestingly, filamin A was predominantly expressed in developing testes, in particular at the BTB at ~15–25 d postpartum and tightly co-localized with the F-actin network,Citation10 at the time the BTB was being assembled.Citation50 Furthermore, a knockdown of filamin A by RNAi using specific siRNA duplexes in Sertoli cells was found to perturb the TJ-permeability barrier function due to a disruption of actin dynamics.Citation10 This result was consistent with a recent report using human coronary artery endothelial cells (HCECs) in which a knockdown of filamin A was found to reduce the vascular permeability in vitro.Citation51 Proteomics analysis using bovine brain capillary endothelial cells (BBCEC) also revealed the participation of filamin A in establishing the blood brain barrier phenotype.Citation52 More important, the knockdown of filamin A in vivo was found to significantly delay the BTB assembly in developing rat testes, which was caused, at least in part, by the inability of BTB proteins (e.g., occludin, N-cadherin) to be recruited to localize properly at the BTB site to induce necessary cell adhesion at the Sertoli-Sertoli cell interface (),Citation10 demonstrating for the first time that filamin A is a functionally significant actin-binding and cross-linking protein crucial for the assembly of the BTB, which is necessary for initiation of spermatogenesis, such as differentiation of type A spermatogonia to B type to initiate cell cycle progression in the testis.Citation50,Citation53
Concluding Remarks and Future Perspectives
The recent identification of filamin A in Sertoli cells and its involvement in regulating BTB assembly during postnatal development in ratsCitation10 has added a new member to the growing list of actin regulatory proteins in the testis, which include Eps8, Arp3, N-WASP and drebrin E.Citation13,Citation14,Citation16,Citation18 It is obvious that this list will be rapidly growing in the years to come. Furthermore, future investigations need to include studies to assess how these proteins are working with other protein partners in the testis to regulate spermatogenesis, such as polarity proteins (e.g., PAR6, 14–3-3).Citation19 Additionally, future studies should take advantage of what are known in the field regarding the roles of these actin binding and/or regulatory proteins in other epithelia, so that better functional experiments can be designed to understand the intricate actions of these proteins to regulate different distinctive cellular events in the seminiferous epithelium pertinent to spermatogenesis.
| Abbreviations: | ||
| Arp2/3 | = | actin-related protein 2/3 |
| N-WASP | = | neuronal Wiskott-Aldrich syndrome protein |
| Eps8 | = | epidermal growth factor receptor pathway substrate 8 |
| ICAM-1 | = | intercellular adhesion molecule-1 |
| JNK | = | c-Jun N-terminal kinase |
| MEKK1 | = | also known as MAP3K1, mitogen-activated protein kinase kinase kinase 1 |
| MKK4 | = | also known as MAP2K4, mitogen-activated protein kinase kinase 4 |
| Pak1 | = | also known as p21 activated kinase 1, p21 protein (Cdc42/Rac)-activated kinase 1 |
| PAR6 | = | partitioning-defective 6 |
| PKC | = | protein kinase C |
| ROCK | = | Rho-associated protein kinase |
| 14-3-3 | = | also known as PAR5, partitioning-defective 5 |
Acknowledgments
This work was supported in part by grants from the National Institutes of Health (U54 HD029990 Project 5 to C.Y.C.; R01 HD056034 to C.Y.C.), and a fellowship from the National Natural Science Foundation of China (81100462 to W.H.S.).
References
- Hartwig JH, Stossel TP. Isolation and properties of actin, myosin, and a new actinbinding protein in rabbit alveolar macrophages. J Biol Chem 1975; 250:5696 - 705; PMID: 124734
- Stossel TP, Condeelis J, Cooley L, Hartwig JH, Noegel A, Schleicher M, et al. Filamins as integrators of cell mechanics and signalling. Nat Rev Mol Cell Biol 2001; 2:138 - 45; http://dx.doi.org/10.1038/35052082; PMID: 11252955
- Gomer RH, Lazarides E. Switching of filamin polypeptides during myogenesis in vitro. J Cell Biol 1983; 96:321 - 9; http://dx.doi.org/10.1083/jcb.96.2.321; PMID: 6833359
- Zhou AX, Hartwig JH, Akyürek LM. Filamins in cell signaling, transcription and organ development. Trends Cell Biol 2010; 20:113 - 23; http://dx.doi.org/10.1016/j.tcb.2009.12.001; PMID: 20061151
- Nakamura F, Stossel TP, Hartwig JH. The filamins: organizers of cell structure and function. Cell Adh Migr 2011; 5:160 - 9; http://dx.doi.org/10.4161/cam.5.2.14401; PMID: 21169733
- Kim H, McCulloch CA. Filamin A mediates interactions between cytoskeletal proteins that control cell adhesion. FEBS Lett 2011; 585:18 - 22; http://dx.doi.org/10.1016/j.febslet.2010.11.033; PMID: 21095189
- Campbell ID. Studies of focal adhesion assembly. Biochem Soc Trans 2008; 36:263 - 6; http://dx.doi.org/10.1042/BST0360263; PMID: 18363570
- Whitmarsh AJ. Filamin B: a scaffold for interferon signalling. EMBO Rep 2009; 10:349 - 51; http://dx.doi.org/10.1038/embor.2009.44; PMID: 19305389
- Ehrlicher AJ, Nakamura F, Hartwig JH, Weitz DA, Stossel TP. Mechanical strain in actin networks regulates FilGAP and integrin binding to filamin A. Nature 2011; 478:260 - 3; http://dx.doi.org/10.1038/nature10430; PMID: 21926999
- Su WH, Mruk DD, Lie PPY, Lui WY, Cheng CY.. Filamin A is a regulator of blood-testis barrier assembly during postnatal development. 2012; In press
- Cheng CY, Mruk DD. A local autocrine axis in the testes that regulates spermatogenesis. Nat Rev Endocrinol 2010; 6:380 - 95; http://dx.doi.org/10.1038/nrendo.2010.71; PMID: 20571538
- Cheng CY, Mruk DD. An intracellular trafficking pathway in the seminiferous epithelium regulating spermatogenesis: a biochemical and molecular perspective. Crit Rev Biochem Mol Biol 2009; 44:245 - 63; http://dx.doi.org/10.1080/10409230903061207; PMID: 19622063
- Cheng CY, Mruk DD. Actin binding proteins and spermiogenesis: Some unexpected findings. Spermatogenesis 2011; 1:99 - 104; http://dx.doi.org/10.4161/spmg.1.2.16913; PMID: 22319657
- Li MWM, Xiao X, Mruk DD, Lam YL, Lee WM, Lui WY, et al. Actin-binding protein drebrin E is involved in junction dynamics during spermatogenesis. Spermatogenesis 2011; 1:123 - 36; http://dx.doi.org/10.4161/spmg.1.2.16393; PMID: 22319661
- Lie PPY, Chan AYN, Mruk DD, Lee WM, Cheng CY. Restricted Arp3 expression in the testis prevents blood-testis barrier disruption during junction restructuring at spermatogenesis. Proc Natl Acad Sci U S A 2010; 107:11411 - 6; http://dx.doi.org/10.1073/pnas.1001823107; PMID: 20534520
- Rotkopf S, Hamberg Y, Aigaki T, Snapper SB, Shilo BZ, Schejter ED. The WASp-based actin polymerization machinery is required in somatic support cells for spermatid maturation and release. Development 2011; 138:2729 - 39; http://dx.doi.org/10.1242/dev.059865; PMID: 21652648
- Lie PPY, Mruk DD, Lee WM, Cheng CY. Epidermal growth factor receptor pathway substrate 8 (Eps8) is a novel regulator of cell adhesion and the blood-testis barrier integrity in the seminiferous epithelium. FASEB J 2009; 23:2555 - 67; http://dx.doi.org/10.1096/fj.06-070573; PMID: 19293393
- Cheng CY, Mruk DD. Regulation of spermiogenesis, spermiation and blood-testis barrier dynamics: novel insights from studies on Eps8 and Arp3. Biochem J 2011; 435:553 - 62; http://dx.doi.org/10.1042/BJ20102121; PMID: 21486226
- Cheng CY, Lie PPY, Wong EWP, Mruk DD, Silvestrini B. Adjudin disrupts spermatogenesis via the action of some unlikely partners: Eps8, Arp2/3 complex, drebrin E, PAR6 and 14-3-3. Spermatogenesis 2011; 1:291 - 7; http://dx.doi.org/10.4161/spmg.1.4.18393; PMID: 22332112
- Lie PPY, Cheng CY, Mruk DD. The biology of interleukin-1: emerging concepts in the regulation of the actin cytoskeleton and cell junction dynamics. Cell Mol Life Sci 2012; 69:487 - 500; http://dx.doi.org/10.1007/s00018-011-0760-0; PMID: 21744066
- Lie PPY, Cheng CY, Mruk DD. The biology of the desmosome-like junction a versatile anchoring junction and signal transducer in the seminiferous epithelium. Int Rev Cell Mol Biol 2011; 286:223 - 69; http://dx.doi.org/10.1016/B978-0-12-385859-7.00005-7; PMID: 21199783
- Phillips BT, Gassei K, Orwig KE. Spermatogonial stem cell regulation and spermatogenesis. Philos Trans R Soc Lond B Biol Sci 2010; 365:1663 - 78; http://dx.doi.org/10.1098/rstb.2010.0026; PMID: 20403877
- Caires K, Broady J, McLean D. Maintaining the male germline: regulation of spermatogonial stem cells. J Endocrinol 2010; 205:133 - 45; http://dx.doi.org/10.1677/JOE-09-0275; PMID: 20147357
- O’Donnell L, Nicholls PK, O’Bryan MK, McLachlan RI, Stanton PG. Spermiation: The process of sperm release. Spermatogenesis 2011; 1:14 - 35; http://dx.doi.org/10.4161/spmg.1.1.14525; PMID: 21866274
- Seo MD, Seok SH, Im H, Kwon AR, Lee SJ, Kim HR, et al. Crystal structure of the dimerization domain of human filamin A. Proteins 2009; 75:258 - 63; http://dx.doi.org/10.1002/prot.22336; PMID: 19137608
- Kim H, Nakamura F, Lee W, Hong C, Pérez-Sala D, McCulloch CA. Regulation of cell adhesion to collagen via beta1 integrins is dependent on interactions of filamin A with vimentin and protein kinase C epsilon. Exp Cell Res 2010; 316:1829 - 44; http://dx.doi.org/10.1016/j.yexcr.2010.02.007; PMID: 20171211
- Gorlin JB, Yamin R, Egan S, Stewart M, Stossel TP, Kwiatkowski DJ, et al. Human endothelial actin-binding protein (ABP-280, nonmuscle filamin): a molecular leaf spring. J Cell Biol 1990; 111:1089 - 105; http://dx.doi.org/10.1083/jcb.111.3.1089; PMID: 2391361
- Nakamura F, Osborn TM, Hartemink CA, Hartwig JH, Stossel TP. Structural basis of filamin A functions. J Cell Biol 2007; 179:1011 - 25; http://dx.doi.org/10.1083/jcb.200707073; PMID: 18056414
- Mruk DD, Cheng CY. Sertoli-Sertoli and Sertoli-germ cell interactions and their significance in germ cell movement in the seminiferous epithelium during spermatogenesis. Endocr Rev 2004; 25:747 - 806; http://dx.doi.org/10.1210/er.2003-0022; PMID: 15466940
- Cheng CY, Mruk DD. The blood-testis barrier and its implications for male contraception. Pharmacol Rev 2012; 64:16 - 64; http://dx.doi.org/10.1124/pr.110.002790; PMID: 22039149
- Mruk DD, Cheng CY. Cell-cell interactions at the ectoplasmic specialization in the testis. Trends Endocrinol Metab 2004; 15:439 - 47; PMID: 15519891
- Wong EWP, Mruk DD, Cheng CY. Biology and regulation of ectoplasmic specialization, an atypical adherens junction type, in the testis. Biochim Biophys Acta 2008; 1778:692 - 708; http://dx.doi.org/10.1016/j.bbamem.2007.11.006; PMID: 18068662
- Vogl AW, Vaid KS, Guttman JA. The Sertoli cell cytoskeleton. Adv Exp Med Biol 2008; 636:186 - 211; http://dx.doi.org/10.1007/978-0-387-09597-4_11; PMID: 19856169
- Mruk DD, Silvestrini B, Cheng CY. Anchoring junctions as drug targets: role in contraceptive development. Pharmacol Rev 2008; 60:146 - 80; http://dx.doi.org/10.1124/pr.107.07105; PMID: 18483144
- Fijak M, Bhushan S, Meinhardt A. Immunoprivileged sites: the testis. Methods Mol Biol 2011; 677:459 - 70; http://dx.doi.org/10.1007/978-1-60761-869-0_29; PMID: 20941627
- Hedger MP. Immunophysiology and pathology of inflammation in the testis and epididymis. J Androl 2011; 32:625 - 40; http://dx.doi.org/10.2164/jandrol.111.012989; PMID: 21764900
- Kaur G, Long CR, Dufour JM. Genetically engineered immune privileged Sertoli cells - a new road to cell based gene therapy. Spermatogenesis 2012; In press
- Franca LR, Auharek SA, Hess RA, Dufour JM, Hinton BT. Morphofunctional and immunological aspects of the blood-testis and blood-epididymal barriers. In: Biology and Regulation of Blood-Tissue Barriers. Ed. Cheng C. Y. Austin, TX, Landes Bioscience and Springer Science + Business Media, LLC. http://www.landesbioscience.com/curie/chapter/5148/ (2012).
- Pelletier RM. The blood-testis barrier: the junctional permeability, the proteins and the lipids. Prog Histochem Cytochem 2011; 46:49 - 127; http://dx.doi.org/10.1016/j.proghi.2011.05.001; PMID: 21705043
- Russell LD, Peterson RN. Sertoli cell junctions: morphological and functional correlates. Int Rev Cytol 1985; 94:177 - 211; http://dx.doi.org/10.1016/S0074-7696(08)60397-6; PMID: 3894273
- Wolski KM, Perrault C, Tran-Son-Tay R, Cameron DF. Strength measurement of the Sertoli-spermatid junctional complex. J Androl 2005; 26:354 - 9; http://dx.doi.org/10.2164/jandrol.04142; PMID: 15867003
- Wolski KM, Mruk DD, Cameron DF. The Sertoli-spermatid junctional complex adhesion strength is affected in vitro by adjudin. J Androl 2006; 27:790 - 4; http://dx.doi.org/10.2164/jandrol.106.000422; PMID: 16809272
- Green KJ, Getsios S, Troyanovsky S, Godsel LM. Intercellular junction assembly, dynamics, and homeostasis. Cold Spring Harb Perspect Biol 2010; 2:a000125; http://dx.doi.org/10.1101/cshperspect.a000125; PMID: 20182611
- Green KJ, Simpson CL. Desmosomes: new perspectives on a classic. J Invest Dermatol 2007; 127:2499 - 515; http://dx.doi.org/10.1038/sj.jid.5701015; PMID: 17934502
- Pentikäinen U, Ylänne J. The regulation mechanism for the auto-inhibition of binding of human filamin A to integrin. J Mol Biol 2009; 393:644 - 57; http://dx.doi.org/10.1016/j.jmb.2009.08.035; PMID: 19699211
- Palombi F, Salanova M, Tarone G, Farini D, Stefanini M. Distribution of β 1 integrin subunit in rat seminiferous epithelium. Biol Reprod 1992; 47:1173 - 82; http://dx.doi.org/10.1095/biolreprod47.6.1173; PMID: 1283530
- Salanova M, Stefanini M, De Curtis I, Palombi F. Integrin receptor α 6 β 1 is localized at specific sites of cell-to-cell contact in rat seminiferous epithelium. Biol Reprod 1995; 52:79 - 87; http://dx.doi.org/10.1095/biolreprod52.1.79; PMID: 7711187
- Siu MKY, Cheng CY. Interactions of proteases, protease inhibitors, and the β1 integrin/laminin γ3 protein complex in the regulation of ectoplasmic specialization dynamics in the rat testis. Biol Reprod 2004; 70:945 - 64; http://dx.doi.org/10.1095/biolreprod.103.023606; PMID: 14645107
- Gehler S, Baldassarre M, Lad Y, Leight JL, Wozniak MA, Riching KM, et al. Filamin A-β1 integrin complex tunes epithelial cell response to matrix tension. Mol Biol Cell 2009; 20:3224 - 38; http://dx.doi.org/10.1091/mbc.E08-12-1186; PMID: 19458194
- Mok KW, Mruk DD, Lee WM, Cheng CY. A study to assess the assembly of a functional blood-testis barrier in developing rat testes. Spermatogenesis 2011; 1:270 - 80; http://dx.doi.org/10.4161/spmg.1.3.17998; PMID: 22319674
- Griffiths GS, Grundl M, Allen JSI 3rd, Matter ML. R-Ras interacts with filamin a to maintain endothelial barrier function. J Cell Physiol 2011; 226:2287 - 96; http://dx.doi.org/10.1002/jcp.22565; PMID: 21660952
- Pottiez G, Sevin E, Cecchelli R, Karamanos Y, Flahaut C. Actin, gelsolin and filamin-A are dynamic actors in the cytoskeleton remodelling contributing to the blood brain barrier phenotype. Proteomics 2009; 9:1207 - 19; http://dx.doi.org/10.1002/pmic.200800503; PMID: 19206108
- Mok KW, Mruk DD, Lee WM, Cheng CY. Spermatogonial stem cells alone are not sufficient to re-initiate spermatogenesis in the rat testis following adjudin-induced infertility. Int J Androl 2012; 35:86 - 101; http://dx.doi.org/10.1111/j.1365-2605.2011.01183.x; PMID: 21696392