Abstract
Drosophila melanogaster spermatids undergo dramatic morphological changes as they differentiate from small round cells approximately 12 μm in diameter into highly polarized, 1.8 mm long, motile sperm capable of participating in fertilization. During spermiogenesis, syncytial cysts of 64 haploid spermatids undergo synchronous differentiation. Numerous changes occur at a subcellular level, including remodeling of existing organelles (mitochondria, nuclei), formation of new organelles (flagellar axonemes, acrosomes), polarization of elongating cysts and plasma membrane addition. At the end of spermatid morphogenesis, organelles, mitochondrial DNA and cytoplasmic components not needed in mature sperm are stripped away in a caspase-dependent process called individualization that results in formation of individual sperm. Here, we review the stages of Drosophila spermiogenesis and examine our current understanding of the cellular and molecular mechanisms involved in shaping male germ cell-specific organelles and forming mature, fertile sperm.
Introduction
Drosophila melanogaster is a powerful system for studying spermiogenesis, as the stages of sperm development are easy to examine and a variety of molecular genetic techniques permit dissection of the cellular processes involved. Drosophila spermiogenesis was first described at an ultrastructural level 40 y ago by Tates, Tokuyasu and others.Citation1-Citation12 The stages of spermiogenesis are readily identified in live squashed preparations by phase-contrast microscopy (reviewed in ref. Citation13), and differentiation can be observed for up to one week in live testis preparations cultured in vitro.Citation14-Citation16 Cell morphogenesis during spermiogenesis largely depends on stored mRNAs that are transcribed during meiotic prophase and translated at later stages of sperm development (reviewed in refs. Citation17, Citation18). Screens for male-sterile mutants have identified numerous genes involved in spermiogenesis,Citation19-Citation25 and newer techniques such as targeted gene disruption and RNA interference promise to reveal additional factors that are required (reviewed in ref. Citation26). For previous excellent reviews of Drosophila spermatogenesis, see refs. Citation13, Citation27–Citation29. Here, we present a discussion of the stages of spermiogenesis, followed by a description of molecular and cellular mechanisms involved in formation and morphogenesis of sperm-specific organelles and mature, individual sperm.
Early stages of spermiogenesis
Spermiogenesis begins immediately after meiosis, when clonally related groups of 64 interconnected spermatids begin to undergo the morphological changes required for sperm development. At normal cultivation temperatures (25°C), the entire process takes approximately five days, or half of the time it takes to go from the initial stem cell division to production of mature sperm (reviewed in ref. Citation28). At a cellular level, the first change observed is coalescence of the mitochondria around the basal body at one side of the nucleus. This is followed by mitochondrial agglomeration and migration of the basal body near the nuclear envelope (). Subsequently, the mitochondria fuse to form the nebenkern (), which consists of two enlarged mitochondrial derivatives that wrap around each other in a manner resembling an onion.Citation3,Citation11 During mitochondrial fusion, the basal body () becomes embedded in the nuclear envelope, and the nuclear envelope itself becomes asymmetrical, with nuclear pores being restricted to the side where the basal body docks (). Also in this region, the dense body, a microtubule (MT) and actin-rich structure involved in transport and nuclear shaping, begins to form (). The acroblast, a Golgi-derived organelle, develops on the side of the nucleus opposite the basal body (), where it serves as the site of formation of the acrosome (), a specialized membrane bound organelle required for fertilization.
Figure 1. Diagrams illustrating stages of spermiogenesis (A-C) as well as nuclear shaping and chromatin condensation (D) in Drosophila. (A) In early round spermatids, mitochondria aggregate and fuse together to form the nebenkern, positioned near the haploid nucleus. The nebenkern consists of two mitochondrial derivatives wrapped around each other in a manner resembling an onion. The basal body, with a short axoneme surrounded by the ciliary sheath, embeds in the nuclear envelope. On the side of the nucleus opposite the basal body, Golgi bodies aggregate to form an acroblast and an acrosomal granule, from which will derive the acrosome. A dark, dense structure named the protein body forms in the nucleus, which is devoid of a nucleolus. Spermatids remain connected to each other via ring canals throughout most of spermiogenesis. (B) Each elongating spermatid contains an axoneme and two mitochondrial derivatives. Axoneme elongation occurs at the growing end, in the region ensheathed by the ciliary membrane. A ring of pericentriolar material called the centriolar adjunct forms around the basal body. Some of the proteins associated with the basal body, such as Unc, also localize to the ring centriole, a structure found at the edge of the ciliary cap that may be equivalent to a transition zone at the base of the cilium. Perinuclear microtubules organize at one side of the nucleus, forming the dense body. When the flagellar axoneme is about half its final length, the nucleus and the acrosome also start to elongate. (C) Each group of 64 spermatids is surrounded by two somatic cyst cells: a head cyst cell and a tail cyst cell. Elongated spermatid cysts are polarized, with all of the nuclei positioned in the head cyst cell, and the tails growing in the opposite direction. Fully elongated spermatids undergo individualization, a process in which F-actin containing investment cones form around the nuclei and then migrate in synchrony along the spermatids, stripping them of excess cytoplasm and unneeded organelles, and investing them with their own plasma membranes. As the cones move from head to tail, a cystic bulge forms around and in front of the cones. The excess cellular material is deposited in a waste bag at the end of the cyst. (D) As the nuclei elongate, they go through leaf, early canoe, late canoe and needle-shaped stages. The dense bodies and the acrosomes elongate together with the nuclei. Inside the nucleus, the chromatin is reorganized, and histones are replaced by transition proteins (Tpl) and then by protamines and Mst77F. During this histone to protamine transition, histones undergo various modifications (acetylation, ubiquitination), other proteins become sumoylated, transient breaks occur in the DNA strands and proteasome activity is high.
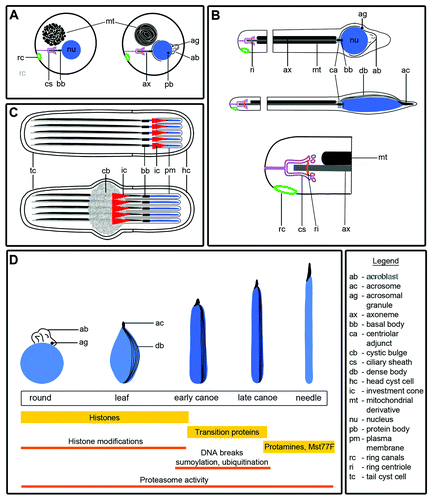
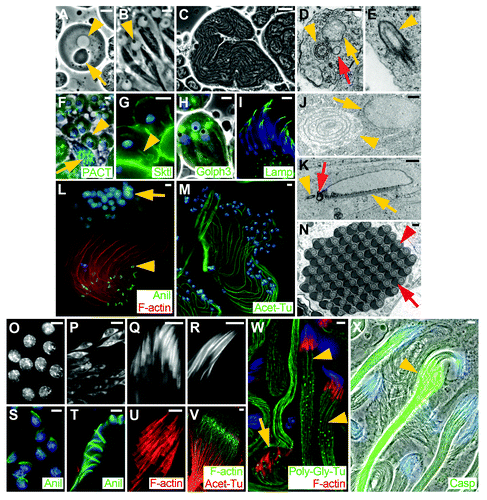
Figure 2. Micrographs illustrating selected stages of Drosophila spermiogenesis. (A) Round spermatid in onion stage with nucleus (arrowhead) and nebenkern (arrow). (B) Early elongated spermatids. Each nucleus contains a protein body (dark dot, arrowhead). (C) Mid elongated spermatid cyst. (D) Cross section of a mid elongated spermatid showing the axoneme (arrowhead), the major mitochondrial derivative starting to fill with paracrystalline material (arrow) and the minor mitochondrial derivative (red arrow). (E) Longitudinal section through a centriole near the cell periphery, showing the membrane cap (ciliary sheath, arrowhead). (F) Round spermatids (arrowhead) and late elongated cyst (arrow) showing basal bodies marked by GFP-PACT (green). (G) Round spermatids showing the ciliary membrane (arrowhead) contiguous with the plasma membrane marked by YFP-Skittles (green). (H) Early elongated spermatids showing the acroblast marked by Golph3-GFP (green). (I) Mature sperm nuclei showing the acrosome marked by GFP-Lamp (green). (J) Cross section through an onion stage spermatid. The nebenkern consists of two mitochondrial derivatives organized in concentric shells (arrowhead). The nucleus is only partially surrounded by a double membrane (arrow), where nuclear pores are located. (K) Longitudinal section showing a partially elongated nucleus, with basal body (arrowhead) and centriolar adjunct (red arrow) attached and with the dense body localized along one side of the nucleus (arrow). (L) Mid elongated cyst showing polarized spermatids, with nuclei at one end (arrow) and ring canals at the other (growing) end (arrowhead). Axonemes are marked by F-actin (phalloidin, red) and ring canals by anillin (green). (M) Mid elongated cyst showing polarized spermatids. Axonemes are marked by acetylated α-tubulin (green), which indicates stable microtubules. (N) Cross section through a cyst of individualized sperm, each with a nebenkern (black half circle, red arrow) and an axoneme (circle, red arrowhead). (O) Round spermatid nuclei. (P) Early elongated nuclei in leaf stage. (Q) Canoe stage nuclei. (R) Individualized nuclei. (S) Round nuclei showing dense bodies marked by anillin (green). (T) Canoe stage nuclei showing dense body marked by anillin (green). (U) Investment cones marked by F-actin (phalloidin, red). (V) Growing end of a late stage elongated spermatid cyst showing axonemes marked by acetylated α-tubulin (red) and the elongation complex marked by F-actin (phalloidin, green). (W) Elongated spermatid cysts in the individualization stage. Two cysts are at the beginning of individualization (arrowheads), whereas one cyst has just finished individualization and has a waste bag (arrow). Fully elongated axonemes are posttranslationally modified by polyglycylation (green). Investment cones are marked by F-actin (phalloidin, red). (X) Elongated cysts in various stages of individualization marked by activated Caspase 3 (green). The cystic bulge is visible in one of the cysts (arrowhead). (A-C) Phase-contrast images of live squashed preparations. (D, E, J, K, N) Transmission electron micrographs. (F, H) Merged phase-contrast and epifluorescence micrographs of live squashed preparations. (G, I, L, O, Q-T) Epifluorescence micrographs of fixed preparations. (M, P, U-V) Scanning confocal micrographs of fixed preparations. (W) Deconvolved confocal micrograph of fixed preparations. (X) Merged phase-contrast and epifluorescence micrograph of a fixed preparation. (F-I, L, M, S-T, W, X) Blue represents DNA stained with DAPI (in fixed preparations) or with Hoechst (in live squashed preparations). Scale bars = 1 μm in (J-K); = 5 μm in (A-B, F-I, L-M, O-X); = 20 μm in (C); = 200 nm in (D-E, N).
Spermatid elongation
Elongation of each group of 64 spermatids occurs within the syncytial cyst. During early stages of elongation, the cyst becomes polarized such that all of the nuclei localize to one end and the growing ends of the sperm tails are found at the other (; ). Ring canals composed of proteins found in the cleavage furrow during cytokinesis localize to the growing ends of elongating cysts (), as do membrane skeletal proteins.Citation30 The elongating spermatids are connected along their length by intercellular bridges, and plasma membrane deposition is required to provide sufficient membrane to enclose the individual sperm at the end of differentiation (reviewed in ref. Citation13). Elongation of the flagellar axoneme is coordinated with cyst elongation, and the distal portion of the axoneme is ensheathed in a ciliary membrane that is contiguous with the plasma membrane ( and ).Citation11,Citation31,Citation32 Throughout most of its length, the axoneme is surrounded by ER membranes that associate intimately with the two giant mitochondrial derivatives of the nebenkern as they unfurl and elongate along the flagellar axoneme ().
Nuclear shaping and chromatin condensation
Spermatid elongation is accompanied by dramatic changes in nuclear shape ( and ).Citation9 Following meiosis, onion stage spermatids contain spherical nuclei that are approximately 5 ∝m in diameter (). During sperm development, the nuclei become thinner and the chromatin condenses, until finally, at the end of spermiogenesis, the nuclei are needle-shaped and approximately 10 ∝m long (). Roughly halfway through elongation and nuclear shaping, a burst of post-meiotic transcription produces a set of mRNAs that are transported to the growing ends of the spermatid cysts.Citation33
Individualization and coiling of mature sperm
Following elongation and nuclear shaping, the mature sperm are invested with their own membranes in a process called individualization ().Citation5 Individualization requires formation of an individualization complex (IC) composed of 64 actin cones that form around the 64 needle shaped nuclei in a mature spermatid cyst (). The individualization complex moves processively down the length of the cyst, stripping away unneeded organelles and cytoplasm, and resolving intercellular bridges to encase each sperm cell in its own plasma membrane. Following individualization, each group of mature sperm becomes coiled in a process that brings the entire length of the nearly 2 mm long sperm bundle into the base of the testis.Citation6 After coiling, the sperm are released into the testis lumen and are transferred to the seminal vesicle, where they are stored until needed for fertilization.
The next sections provide a more in-depth view of the genes and processes involved in preparing each sperm-specific organelle for its role in fertilization and activation of development.
Mitochondrial Morphogenesis
Mitochondrial morphogenesis during insect spermiogenesis includes aggregation, fusion, membrane wrapping, unfurling and elongation.Citation34 At the end of meiosis II, mitochondria aggregate together near one side of the nucleus and fuse to form two mitochondrial derivatives (major and minor) that wrap around each other in an onion-shaped structure called the nebenkern ( and ).Citation3,Citation11 The mechanism that drives mitochondrial aggregation and positioning of the nebenkern in a specific location near the nucleus is not fully understood. However, treatment with high doses of colchicine, a MT inhibitor, suggests that aggregation, but not fusion, depends on MTs (reviewed in ref. Citation13). Consistent with this idea, mutants for the dynein-associated protein Lis-1 occasionally form multiple nebenkerns, suggesting that dynein motor activity is required for aggregation.Citation35
Mitochondrial fusion is mediated by Fuzzy onions (Fzo), a large, dynamin-related transmembrane GTPase, and the founding member of the mitofusin protein family.Citation36 fzo mutants show defects in mitochondrial fusion and form many small, enwrapped mitochondrial derivatives, rather than a single large nebenkern. At later stages, multiple small elongating mitochondria are observed, approximately half of which resemble the major mitochondrial derivative. Mitochondrial fusion also requires the intramembrane protease Rhomboid-7 and optic atrophy 1 (Opa1).Citation37 Mutations in rho-7 and opa1 affect mitochondrial morphology in a manner similar to loss of fzo.
Mitochondrial fission requires the dynamin-related protein Drp1, which is needed for normal clustering of mitochondria in primary spermatocytes and mitochondrial unfurling during elongation.Citation38 The Pink1/Parkin pathway, which includes genes (pink1, parkin, DJ-1) whose homologs are mutated in human Parkinson disease, regulates mitochondrial morphogenesis.Citation39,Citation40 In parkin mutant spermatids, both mitochondrial derivatives form, but only the major derivative unfurls from the nebenkern and subsequently exhibits abnormal shaping and condensation during spermatid elongation.Citation41 pink1 (PTEN-induced kinase-1) and DJ-1 mutant spermatids resemble parkin mutants,Citation39,Citation40,Citation42,Citation43 suggesting they share a common function. Genetic and biochemical data indicate Pink1 phosphorylates and activates Parkin, an E3 ubiquitin ligase, which in turn acts upstream of DJ-1.Citation39,Citation43,Citation44 Since Pink1 and Parkin mark damaged mitochondria for destruction by autophagy (reviewed in ref. Citation45), it is tempting to speculate that the Pink/Parkin pathway may provide a mark that identifies the minor mitochondrial derivative and ensures its proper differentiation.
Mitochondrial fusion and fission are intimately connected, and a balance between the two is required to maintain proper mitochondrial morphology (reviewed in ref. Citation46). Genetic interactions between pink1/parkin and fzo, opa1 and drp1 suggest Pink1/Parkin promotes mitochondrial fission or inhibits mitochondrial fusion in developing spermatids.Citation40 Recent experiments indicate Parkin ubiquitinates the Fzo-related Mfn protein to promote its degradation in Drosophila tissue culture cells.Citation44,Citation47 Since Fzo associates with spermatid mitochondria only around the time of fusion,Citation36 Parkin may promote fission by stimulating Fzo degradation.
Mitochondrial elongation was recently shown to drive spermatid elongation ().Citation48 Indeed, axoneme and nebenkern elongation can proceed independently. Spermatids that lack axonemes still undergo elongation.Citation49,Citation50 In contrast, mutants for fzo and no mitochondrial derivative (nmd), which have small mitochondrial derivatives, exhibit severe elongation defects.Citation48 Mitochondrial elongation occurs along cytoplasmic microtubules, and is mediated by Milton and dMiro.Citation38,Citation48 Milton, an adaptor protein, and dMiro, a Ca2+-binding regulator of Milton with two small GTPase domains, form a conserved complex that links mitochondria to kinesin motors.Citation51 Milton and dMiro anchor the two mitochondrial derivatives to the nucleus to facilitate their elongation.Citation38 Milton localizes to the growing ends of the sperm tails, where it promotes MT sliding.Citation48 In addition to Milton and dMiro, mitochondrial elongation requires the MT crosslinkers Nebbish (a homolog of human KIF14), Khc73 (a CAP-Gly MT bundler with a motor domain) and Fascetto (Feo, an anti-parallel MT bundler), as well as the MT depolymerizing kinesin Klp59D (a kinesin-13 family member). The source of the cytoplasmic MTs is unclear, but they appear to be nucleated by MT organizing centers that are tightly associated with the mitochondria.Citation48
During elongation, the major mitochondrial derivative fills with an electron dense paracrystalline material, whereas the minor mitochondrial derivative drastically reduces its size during individualization ().Citation3,Citation8 Mitoferrin, a mitochondrial iron importer with an established role in mitochondrial iron metabolism, is required for normal nebenkern elongation.Citation52 Mitoferrin mutants show reduced levels and aberrant distribution of para-crystalline material, which is thought to provide structural rigidity during sperm movement (reviewed in ref. Citation53). In addition, the mitochondrial derivatives form clumps along the elongating sperm tails. Thus, mitochondrial function appears tightly linked to mitochondrial morphogenesis.
Elimination of the majority of the minor mitochondrial derivative is accomplished by the comet tail-like motility of actin cones that strip away mitochondrial material during individualization.Citation54 Prior to individualization, mitochondrial DNA is removed in a process involving activation of endonuclease G.Citation55 DNA that is not removed by endonucleolytic cleavage is removed by the IC in a manner that likely involves a link between DNA in the mitochondrial matrix and actin cones in the surrounding cytoplasm.
Basal Body Development, Centriolar Adjunct Formation and Axoneme Initiation
Basal body development and axoneme initiation begin in primary spermatocytes, when the paired centrioles migrate to the cell periphery and act as basal bodies to template assembly of short cilia that extend from the cell surface.Citation3,Citation31 Immediately prior to metaphase of meiosis I, these elongated basal bodies internalize, together with caps of plasma membrane (), to form MT organizing centers at the poles of the dividing cell. Following meiosis, each haploid spermatid inherits a single elongated basal body whose short axoneme remains surrounded by a membrane cap, or ciliary sheath.Citation3,Citation11,Citation31 The basal body associates with cytoplasmic MTs during its movement toward the nucleus,Citation56 where it becomes surrounded by the centriolar adjunct (CA) (), an electron dense structure similar to the pericentriolar material (PCM) found in centrosomes.Citation57 Once embedded in the nuclear envelope, the basal body functions as a MT-organizing center, nucleating assembly of both the flagellar axoneme and perinuclear MTs.
Early defects in centriole formation and organization lead to spermatids lacking basal bodies and axonemes.Citation58 For example, mutations affecting Sas-6, Sak/Plk4, Sas-4/CPAP, Ana1 and Asterless/Cep152 abolish centriole duplication and flagellum assembly.Citation50,Citation58-Citation62 (For a review, see ref. Citation63.) Sak and Sas-6, but not Sas-4, are also required for assembly of a proximal centriole-like (PCL) structure recently identified in developing spermatids.Citation61 This immature centriole, which contains Ana1 and requires the conserved centriolar protein POC1 for its assembly, is dispensable for axoneme assembly and male fertility, but may contribute to producing the second centriole at fertilization.
Several centriolar proteins with roles in recruiting PCM proteins to centrosomes localize to basal bodies and may be important for CA assembly or function. For example, Sas-4, Drosophila pericentrin-like protein (D-Plp/CP309) and Spd-2 are required to recruit γ-tubulin, Centrosomin (Cnn) and other PCM proteins to centrosomes,Citation62,Citation64-Citation67 and may serve similar functions at the CA. However, these proteins have different effects on spermatid differentiation. D-plp mutants show fragmented centrioles and scattered basal bodies in the elongated cysts,Citation64 whereas cnn and spd-2 mutant axonemes show a variable lack of central pair MTs.Citation66,Citation68
Uncoordinated (Unc) localizes to distal end of the basal body and to the growing end of the sperm tail in a structure referred to as the ring centriole (; L.F. and J.A.B., unpublished observations).Citation34,Citation69,Citation70 Unc is needed for basal body and ciliary elongation in primary spermatocytes, and is required for robust accumulation of γ-tubulin at centrioles.Citation69 In addition, unc mutants show severe defects in flagellar axoneme organization.
Like Unc, Chibby (Cby), a conserved coiled-coil protein, is required for the centriole to basal body transition and normal axoneme assembly.Citation71 Following meiosis, Cby initially colocalizes with Unc at basal bodies. However, during elongation, Cby relocalizes from the basal body to the ring centriole at the growing end of the sperm tail. Unc distribution is aberrant in cby mutants, with Unc extending a variable distance beyond the normal centriole length. Interestingly, similar defects in Unc distribution and axoneme assembly are also observed in spermatids in which the plasma membrane lipid phosphatidylinositol 4,5-bisphosphate (PtdIns(4,5)P2) has been depleted by ectopic expression of a lipid phosphatase.Citation70 Hence, Cby and PtdIns(4,5)P2 may perform a common function in regulating Unc localization during spermatid elongation.
Mutants for Bld10/Cep135 share similarities with unc and cnn.Citation72,Citation73 bld10 mutants have short centrioles and basal bodies, like unc mutants, and axonemes that lack central pair MTs, like cnn mutants. Bld10 localizes in the lumen of the basal body, at both the proximal end, close to the nuclear membrane, and the distal end, at the site of axoneme assembly, and is also found in the PCL.Citation61,Citation72 Notably, Bld10 is dispensable for recruitment of γ-tubulin, Cnn and other PCM proteins.Citation72 Since Bld10 localizes to the transition zone, and this is the region where central pair MTs originate, it is possible that Cnn affects Bld10 activity in this region.
Basal Body Movement and Docking at the Nuclear Envelope
Docking and continued association of the basal body at the nuclear envelope is essential for formation of mature, motile Drosophila sperm. Movement of the basal body to the spermatid nucleus and attachment to the nuclear envelope requires proper microtubule and dynein organization. γ-tubulin appears important for this process, as defects in basal body docking at the nuclear envelope are seen in mutants for the γ-tubulin ring complex (γ-TuRC) subunits Grip75 and Grip128.Citation74 In grip75 and grip128 mutants, spermatid nuclei fail to associate with basal bodies, become scattered along the elongated cysts and exhibit defects in nuclear shaping, a MT-dependent process. Importantly, mature axonemes of normal architecture form in grip75 and grip128 mutants, indicating γ-TuRC is dispensable for axoneme assembly. Hence, γ-TuRC likely nucleates assembly of MTs involved in migration of the spermatid basal body to the nucleus or in attachment of the basal body to the nuclear envelope. The basal body proteins Unc and Dilatory (Dila/CEP131) are also required for tight association of basal bodies with spermatid nuclei.Citation69,Citation75 Since Unc and Dila are needed for robust CA accumulation of γ-tubulin, these mutants may be defective in γ-TuRC–dependent MT nucleation.
Dynein activity is also important for basal body docking, as several mutants with defects in basal body–nucleus attachment affect dynein-associated proteins. Null mutants for tctex-1, which encodes the 14kDa dynein light chain (Dlc90F), are male sterile and show nuclear scattering in later stages of elongation.Citation56 Most of the nuclei do not shape properly, especially those that are not associated with a basal body. Tctex-1 mediates cytoplasmic dynein localization at the nuclear membrane in early spermatids. In wild-type spermatids, dynein and its accessory factor dynactin are distributed in the region of the nuclear envelope where the basal body is attached. However, this dynein localization is lost in tctex-1 mutants. Asunder (Asun/Mat89Bb) colocalizes with dynein at the perinuclear surface in primary spermatocytes and spermatids, and has a role in recruitment of dynein–dynactin at the nuclear membrane.Citation76 Asun is dispensable for recruitment of centrosomal proteins. Nuclei are scattered in asun mutant cysts, similar to unc, and do not shape normally. Like γ-TuRC, dynein is dispensable for axoneme assembly, as axonemes can be nucleated from free, unattached, basal bodies in tctex-1 and asun mutants.Citation56,Citation76
It is not known whether dynein may play a direct role in anchoring the basal body at the nuclear envelope. However, recent data provide strong support for the idea that dynein mediates migration of the basal body toward the nuclear envelope prior to docking. Mutants for Lis-1, the Drosophila homolog of the human lissencephaly protein LIS1, show a defect in internalization of the elongated basal bodies from the cell surface in primary spermatocytes.Citation35 As a result, the meiotic spindle poles localize aberrantly at the plasma membrane, resulting in spindle defects. Similar phenotypes are also observed in tctex-1 mutants, indicating that these defects are likely caused by a loss of dynein activity. Indeed, Lis-1 is required for dynein–dynactin localization at the nuclear membrane during meiosis and in early spermatids. Lis-1 localization requires Asun, suggesting these proteins act together to regulate this process.
Several proteins are thought to mediate attachment of the basal body to the nuclear envelope. For example, Spag4, a conserved, testis-specific SUN domain protein, is required for basal body anchoring to the spermatid nucleus.Citation77 In early round spermatids, Spag4 localizes to the nuclear envelope in a hemispherical cap, similar to cytoplasmic dynein heavy chain (Dhc). During nuclear elongation, Spag4 associates with the basal body and the dense body. spag4 mutant males are sterile, with scattered nuclei and basal bodies. Similar defects are observed in mutants for Yuri Gagarin (Yuri), a coiled-coil cytoplasmic protein that colocalizes with Spag4 at the nuclear envelope cap, basal body and dense body.Citation77,Citation78 Interestingly, the two proteins strongly colocalize at the invagination in the nuclear envelope where the basal body embeds, suggesting they may anchor the basal body to the nucleus. Spag4 is required for dynein–dynactin localization at the basal body, whereas both Spag4 and dynein are needed for Yuri localization.Citation77 How Spag4, a predicted inner nuclear membrane protein, regulates cytoplasmic dynein is unclear. KASH domain proteins in the outer nuclear membrane typically link SUN domain proteins to cytoplasmic MTs (reviewed in ref. Citation79). However, the KASH domain proteins Klarsicht and MSP-300 are dispensable for basal body docking.Citation77 One possibility is that nuclear pores, which localize in the area where the basal body attaches to the nuclear envelope, help provide the missing link.
Two additional factors are required for basal body docking: the poly(A) polymerase Gld2 and the membrane lipid PtdIns(4,5)P2.Citation32,Citation70,Citation80 In gld2 mutants, γ-tubulin associates with basal bodies, but these are not consistently located near the scattered nuclei. It is unclear whether the primary defect is in basal body migration or docking, and polyadenylation of transcripts encoding proteins involved in these processes is unaffected.Citation80 Basal bodies also fail to associate consistently with nuclei in spermatids in which PtdIns(4,5)P2 has been severely or partially depleted.Citation32,Citation70 In cases where the basal body is found adjacent to the nuclear envelope, docking is defective, and axonemes extend from the unanchored basal bodies.Citation70 Severe depletion of PtdIns(4,5)P2 is accompanied by excess accumulation of the CA proteins Unc, Cnn and γ-tubulin and defects in axoneme assembly. It will be of interest to determine whether Gld2 and PtdIns(4,5)P2 regulate common targets involved in basal body docking.
Axoneme Assembly and Modifications
Drosophila has a canonical 9+2 flagellar axoneme, with nine outer doublet MTs and a central pair, the basic structure of most motile eukaryotic cilia and flagella. The outer doublet MTs associate with dynein “arms.” These consist of flagellar dyneins encoded by genes on the Y chromosome,Citation81,Citation82 which is required for male fertility.Citation83 A set of nine accessory MTs surrounds the axoneme (reviewed in ref. Citation84). Although the function of the accessory tubules is unknown, they may have a passive role in strengthening the flagellum and in amplifying the mechanical power generated by the flagellar dyneins.Citation85
Axoneme assembly in Drosophila requires specific isoforms of α- and β-tubulin (reviewed in refs. Citation84 and Citation86). α1-tubulin (αTub84B) functions throughout spermatogenesis,Citation87 whereas β1-tubulin (βTub56D) is found in the basal body and β2-tubulin (βTub85D) in the sperm axoneme.Citation88 Replacement of more than half of the α-tubulin pool with α2 causes defects in formation of central pair and accessory MTs, suggesting α1 contains sequences specifically required for formation of these MT structures.Citation89 When β1 replaces β2, only short 9+0 axonemes are formed.Citation90 Likewise, the somatic β3 isoform cannot substitute for β2.Citation49 The C-terminus of β2 is critical for axoneme formation, and hybrid β1 and β3 tubulins containing sequences from the β2 C-terminus can partially rescue axoneme assembly in β2 null flies.Citation88,Citation91,Citation92
Ciliary and flagellar axonemes are assembled at their distal tips within a specialized pocket of the plasma membrane termed the ciliary membrane, or sheath.Citation93 (For a review, see ref. Citation94.) In most cells, axoneme assembly is directed by intraflagellar transport (IFT), a process that employs MT motors and trafficking proteins to transport preassembled tubulin dimers and other proteins to and from the tip of the axoneme. Drosophila spermatids have a ciliary membrane that ensheaths the growing portion of the flagellar axoneme ().Citation11,Citation31,Citation32 However, assembly of the spermatid flagellar axoneme is independent of IFT,Citation95,Citation96 raising the question of how tubulin dimers access the growing tip. One possibility is that mRNAs encoding tubulin subunits and other axonemal proteins may be actively translated within the ciliary pocket. Consistent with this idea, ribosomes are present in the space between the tip of the elongating axoneme and the overlying ciliary membrane.Citation11
In addition to α- and β-tubulin, incorporation of axonemal dynein subunits is needed for normal axoneme architecture and sperm motility.Citation97,Citation98 The basal body and CA proteins Unc, Cnn and Cby (see above), as well as Touch-insensitive-larvaB (TilB) are also required for axoneme assembly.Citation68,Citation71,Citation99 Moreover, an important aspect of axoneme assembly and maturation is post-translational modification of axonemal MTs.
Axonemal MTs are post-translationally modified through tyrosination, polyglutamylation, polyglycylation and acetylation (reviewed in ref. Citation100). Polyglycylation, polyglutamylation and most of the other posttranslational modifications occur at the exposed, highly charged C-terminal tails of α- and β-tubulin, whereas α-tubulin acetylation occurs on lysine-40, which is found on the inner surface of the MTs (reviewed in ref. Citation101). Developing Drosophila flagellar axonemes contain acetylated α1-tubulin, as well as glutamylated and glycylated α- and β-tubulins.Citation102-Citation105 In Drosophila sperm, all α-tubulin is acetylated (), whereas only a subset of axonemal tubulins are glutamylated and glycylated.Citation89,Citation106 Glutamylation occurs during or shortly after axoneme assembly, and it precedes and is required for glycylation.Citation106 Polyglycylation occurs in late stages of spermiogenesis (),Citation103,Citation107 at the time of individualization. Axonemal MTs and accessory MTs are differentially post-translationally modified. The central pair and the accessory MTs are heavily polyglutamylated, whereas outer doublet MTs have only small amounts of glutamylation.
Tubulin modifications are important for axoneme assembly and formation of mature, fertile sperm. Mutations in β2-tubulin at position Gly56, where acetylation occurs, lead to defects in axoneme assembly.Citation108 Depletion of either of the two highly expressed glycylases, TTLL3A or TTLL3B, leads to strong reduction in polyglycylation, resulting in defects in sperm maturation and in male sterility.Citation109
Transport of glycine to the sperm flagellum might be regulated by the neurotransmitter transporter-like (Ntl) protein, a member of the NSS/SLC6 family.Citation110 Ntl is a spermatid-specific glycine transporter that localizes to the ER and is found along the ER-derived axial sheath that surrounds the axoneme. ntl mutants make sperm that are immotile and fail to be transferred to the seminal vesicle.Citation110 Post-translational glycylation of sperm tubulin is reduced in ntl mutants, supporting previous reports that glycylation is important for stability and motility of MT-based machinery.Citation107,Citation110
Nuclear Morphogenesis and Chromatin Reorganization
Nuclear morphogenesis during spermiogenesis includes two major aspects (): elongation of the nucleus (nuclear shaping) and chromatin reorganization (condensation). Nuclear shaping is driven by MTs that emanate from the basal body and associate with the nuclear envelope. Concomitantly, chromatin condensation occurs by switching from a histone-based chromatin configuration, present in early round spermatid nuclei, to a protamine-based configuration, present in mature sperm nuclei.
Nuclear shaping proceeds through several stages.Citation3 In early round spermatids, the nucleus first becomes flat on one side, near the basal body. This flattened surface is fenestrated with nuclear pores and associates with perinuclear MTs, which organize into parallel bundles, forming the dense body, a structure analogous to the vertebrate manchette.Citation3,Citation111 The dense body, which also contains filamentous actin and the actin-binding protein anillin (; L.F. and J.A.B., unpublished observations), is thought to provide support to the elongating nucleus. As elongation proceeds, the fenestrated portion of the nuclear envelope forms a cavity that fills with dense body MTs, which in turn associate with a layer of ER.Citation9 At this stage, the nucleus becomes canoe-shaped and a single layer of MTs associates with the convex side of the nucleus, where chromatin begins to condense.Citation3,Citation9 At later stages of nuclear shaping, the cavity disappears, and the dense body is disassembled and removed during individualization.
Several factors have been implicated in regulating nuclear shaping via dense body cytoskeletal proteins. For example, nuclear shaping is defective in mutants for CA proteins (e.g., Unc, Tctex-1, Grip75 and Grip128) where the basal body fails to embed in the nuclear envelope,Citation68,Citation69,Citation74 indicating a role for the CA in nucleating perinuclear MTs. Yuri and Spag4 participate in basal body docking, but Yuri also associates with the dense body and is responsible for actin accumulation in this structure.Citation77,Citation78 In spag4 and yuri mutants, the nuclei partially elongate, but they do not maintain their shape (they bend and curl). Similar nuclear shaping defects are observed in spermatids that have defects in basal body docking due to reduced levels of PtdIns(4,5)P2 (L.F. and J.A.B., unpublished observations).Citation32,Citation70 Like Yuri, Mst77F, a protein related to linker histone 1-like protein from mammals, localizes to the dense body.Citation112 Genetic interactions between mst77F and mutations in β2-tubulin suggest a role in MT organization (reviewed in ref. Citation113). Indeed, mutations in mst77F lead to defective nuclear shaping, small nuclei and male sterility.Citation114,Citation115 Nuclear shaping defects are also observed in mutants for the testis-specific proteasome subunit Prosα6T.Citation116 However, since Prosα6T localizes to the nucleus, these defects likely reflect a role in chromatin condensation rather than dense-body dependent nuclear shaping.
Chromatin reorganization in spermiogenesis involves replacement of histones first by transition proteins and then by protamines (). The histone to protamine switch starts in early canoe stage nuclei and takes approximately five hours to complete in cultured Drosophila spermatids.Citation117 Histones are probably degraded directly in the nucleus by the Prosα6T-containing testis-specific proteasome.Citation116 Before degradation, several histone modifications occur.Citation115 Histone H3 is methylated at lysine 9 and 27, which typically marks transcriptionally inactive chromatin, while H2A is mono-ubiquitinated and H4 is hyper-acetylated. Histone H4 hyperacetylation is required for the histone to protamine switch because inhibition of histone acetyltransferases prevents degradation of histones and the switch to a protamine-based chromatin.Citation117 However, a premature increase in the levels of histone H4 acetylation does not result in early onset of the histone to protamine switch, indicating that additional mechanisms might function to induce the switch.
Histone modifications likely facilitate access of chromatin remodeling proteins and enzymes, as the chromatin also becomes associated with the zinc finger protein CTCF, as well as small ubiquitin-related modifier (SUMO), the ubiquitin-conjugating enzyme UbcD6 and ubiquitin.Citation115 Following histone removal, the transition-like protein Tpl94D is incorporated. Tpl94D, together with DNA breaks, may facilitate chromatin unwinding and assembly of protamine and Mst77F on the condensing DNA. Several of these regulators appear to play conserved roles in nuclear shaping and chromatin condensation in mammalian spermatogenesis. For example, SUMO is involved in nucleation of spermatid MTs and nuclear shaping.Citation118 Ubiquitination of H2A is required during nuclear shaping in mice and may destabilize nucleosomes to promote the histone to protamine transition.Citation119,Citation120
Mature Drosophila sperm contain Mst77F, protamine A and protamine B as major chromatin components. mRNAs encoding Mst77F and protamines are translationally repressed until the elongated spermatid stage, after which these proteins accumulate in the nucleus.Citation114 Mst77F has a dual role in chromatin condensation and nuclear shaping through its association with perinuclear MTs.Citation112 Moreover, mst77F mutants are male sterile. In contrast, males with mutated protamines are fertile and have motile sperm, but about 20% of the sperm show defects in nuclear shaping. Since protamines are required in mice for production of fertile sperm,Citation121 this raises the possibility that Mst77F acts in parallel with protamines to promote chromatin condensation and male fertility in Drosophila.
Despite progress in identifying factors involved in nuclear shaping and chromatin reorganization, the relationship of these two processes with each other and with other aspects of spermatid morphogenesis is not well understood. For example, the physical mechanism that links dense body MTs with the outer nuclear membrane and with the chromatin is unclear, although the SUN domain protein Spag4 is a candidate to link chromatin to the inner nuclear membrane. Several other processes may also be coordinated with nuclear shaping and chromatin condensation. First, a burst of post-meiotic transcription occurs midway through nuclear shaping and spermatid elongation.Citation122,Citation123 Whether chromatin condensation is required for post-meiotic transcription, and whether the dense body participates in nuclear export of the resulting mRNAs is unknown. Second, incorporation of the specialized telomeric protein K81 into Drosophila sperm chromatin is essential for the chromosomes to participate in embryonic divisions and for inheritance of paternal DNA.Citation124,Citation125 (For a review, see ref. Citation126.) How K81 binding to telomeres is coordinated with protamine incorporation remains unclear. Third, maturation of the acroblast and acrosome and formation of the actin-based investment cones involved in individualization occur in the region of the perinuclear MTs, but the role of the MTs and the dense body in these processes is poorly understood.
Acroblast and Acrosome Formation
The acroblast, first described by Tates,Citation3 is composed of stacked Golgi cisternae adjacent to the spermatid nucleus (). The acroblast forms in onion stage spermatids by coalescence and fusion of Golgi-derived membranes. Several Golgi and endosomal trafficking regulators with roles in spermatocyte cytokinesis are required for acroblast formation. These include Four way stop (Fws), the Drosophila homolog of the conserved oligomeric Golgi (COG) tethering complex subunit Cog5;Citation127 Giotto (Gio), the Drosophila phosphatidylinositol transfer protein;Citation128 the recycling endosome regulator Rab11;Citation129 and Brunelleschi (Bru), the Drosophila homolog of the transport protein particle II (TRAPPII) complex subunit Trs120.Citation130 Similarly, in mice, the Golgi-associated protein GOPC is required for acroblast formation.Citation131 Little is known of the signals that promote acroblast formation, nor why the acroblast—unlike most Golgi bodies in Drosophila, which occur as paired individual stacks—resembles a Golgi ribbon (reviewed in ref. Citation132).
The role of the acroblast in secretory trafficking was initially suggested by the presence of glycosylation epitopes recognized by the lectin wheat germ agglutinin (WGA).Citation133 The acroblast resembles the Golgi at a molecular level, as demonstrated by the presence of the Golgins Lava lamp (Lva) and GCC88, the Golgi matrix protein GM130, the glycosylation enzyme Golgi α-mannosidase II (GMII),Citation127,Citation134 and Golgi phosphoprotein-3 (Golph3) (; L.F. and J.A.B., unpublished observations). Many of the proteins needed for acroblast formation, such as Fws, Rab11 and Bru and the Rab11 effector Nuclear fallout (Nuf), also localize at the acroblast,Citation127-Citation130,Citation134 as do coatomer (COP I) and the clathrin adaptors GGA and AP-1, which were recently shown to localize to this organelle.Citation134,Citation135 Hence, these proteins may regulate trafficking within the acroblast itself. However, the roles of these proteins within the acroblast have been difficult to examine because of the organelle’s small size. Moreover, the mechanism by which the condensed acrosomal granule, or acrosome, emerges from the acroblast is as yet unclear.
As in most metazoans, the acrosome () is critical for fertilization in Drosophila. Indeed, the acrosomal protein Sneaky (Snky), a polytopic transmembrane protein, is required for sperm plasma membrane breakdown during fertilization.Citation136 Similarly, the Drosophila ferlin Misfire (Mfr) is required for sperm plasma membrane breakdown,Citation137 but is dispensable for acrosome and acroblast assembly.Citation138 In mammals, the acrosome is an acidified lysosome-related secretory organelle. It is not yet known whether the acrosome is acidified in flies, but the lysosomal marker GFP-Lamp localizes to the mature acrosome (; L.F. and J.A.B., unpublished observations). Moreover, it is unclear whether the contents of the Drosophila acrosome are secreted. Upon fertilization, Snky-GFP and a secreted GFP remain within the acrosome.Citation136 However, it is possible that hemifusion or formation of a small fusion pore between the acrosome and plasma membrane induces plasma membrane breakdown. It will be interesting to determine whether Mfr localizes to the acrosome, and whether Mfr and Snky directly participate in membrane fusion.
Cyst Polarization
Cysts of 64 early round haploid spermatids initially appear unpolarized. However, by early stages of elongation, ring canals are observed to cluster along actin-rich cortical membranes at the distal (growing) ends of the elongating spermatids.Citation30 Real-time imaging of elongating spermatids cultured in vitro demonstrates that spermatid polarization is determined at the earliest stages of elongation and requires normal levels of the plasma membrane PtdIns(4,5)P2.Citation32
Polarization of spermatid cysts requires the PIP 5-kinase Skittles (Sktl); Funnel cakes (Fun; also called Sec8), a subunit of the octameric exocyst complex involved in targeted membrane delivery;Citation32 and Merlin (Mer), the Drosophila homolog of Neurofibromatosis-2.Citation139 Mutations in sktl, fun and mer result in elongated spermatid cysts that are bipolar, with spermatid nuclei found at both ends and sperm tails growing toward the middle, suggesting that these genes play a primary role in establishment of cyst polarity. Other mutants that show occasional defects in axoneme orientation include yuri and parkin,Citation41,Citation78 raising the possibility that basal body docking and mitochondrial morphogenesis influence spermatid polarization. In addition, a number of male-sterile mutants that are defective in elongation form non-polarized cysts. However, this is likely an indirect consequence of the failure in membrane addition.
Spermatid cyst polarization is germ cell intrinsic in that elongating spermatids can polarize in the absence of accompanying somatic cyst cells.Citation16,Citation32 However, the upstream signals that establish spermatid cyst polarity are currently unclear. One possibility is that this signal depends on activation of Rho family GTPases, which regulate cell polarity in multiple systems. In addition, PtdIns(4,5)P2 synthesis at the growing end may act in a positive feedback loop to maintain a high local concentration of Sktl and the exocyst, thereby reinforcing polarized growth during elongation.Citation32
Membrane Outgrowth
During elongation, Drosophila spermatids increase 150-fold in length, and the total surface area following individualization is estimated to be 5-fold greater than in early round spermatids.Citation5 Hence, it is not surprising that secretory trafficking is critical for spermatid cyst elongation. Indeed, the most dramatic defects in cyst elongation are generally observed in mutants that also affect membrane trafficking during male meiotic cytokinesis. For example, mutations affecting the Golgi SNARE Syntaxin-5 (Syx5), the Cog5 homolog Fws, and the exocyst subunits Sec8 and Exo84 have defects in both cytokinesis and spermatid elongation.Citation32,Citation127,Citation140,Citation141 In addition, mutations affecting peroxisome biogenesis, and therefore degradation of very long chain fatty acids (VLCFA), also fail cytokinesis and elongation,Citation142 indicating that membrane lipid composition is critical for plasma membrane growth. Proper levels of PtdIns(4,5)P2 are important for elongation, as expression of high levels of a PtdIns(4,5)P2 phosphatase blocks elongation, whereas expression of lower levels of the phosphatase or mutation of the PIP 5-kinase Sktl causes milder elongation defects.Citation32,Citation70
Other membrane trafficking mutants have more subtle defects. For example, mutations in fwd, which encodes Drosophila phosphatidylinositol 4-kinase IIIβ, (PI4KIIIβ) cause mild defects in elongation.Citation130,Citation143 These defects are dramatically enhanced when fwd is mutated in combination with the TRAPPII subunit bru,Citation130 indicating that these genes act redundantly to promote cyst elongation. Partial loss-of-function mutations affecting the clathrin uncoating factor Auxilin result in defects in membrane deposition due to failure to form Golgi-derived clathrin-coated vesicles.Citation144 However, elongation is only mildly affected, suggesting that other pathways can act redundantly with clathrin-mediated trafficking to promote plasma membrane expansion.
In addition to trafficking, other factors also promote spermatid cyst elongation. These include cytoplasmic MTs and mitochondria (see above), as well as cytoskeletal regulators, such as RacGAP84C (also called RotundRacGAP), a negative regulator of the Rho family GTPases Rac and Cdc42.Citation145,Citation146 Loss of RacGAP84C leads to formation of short cysts with bulbous ends that lack plasma membrane between the spermatids and contain elongated, disorganized flagella.Citation145 Similarly, loss of Drosophila dynein light chain-1 (Ddlc1) results in defects in maintenance of the spectrin-rich elongation complex at the growing end, and results in abnormal coiling of sperm tails and defects in membrane deposition that are enhanced by loss of one copy of cytoplasmic dynein heavy chain (Dhc) or dynactin (Glued).Citation147 The similarity of these mutant phenotypes suggests that regulation of Rho family GTPase signaling and dynein-dependent vesicular trafficking are required to coordinate flagellar outgrowth with plasma membrane deposition during spermatid cyst elongation.
Plasma Membrane Domains
One crucial aspect of spermiogenesis is proper localization of signaling proteins involved in fertility. In Drosophila, these proteins primarily localize to two specialized plasma membrane domains: the proximal region overlying the acrosome and the distal (caudal) region at the tip of the sperm tail. These regions stain with fluorescent lectins that bind distinct sugar residues. Several lectins bind strongly to both the acrosomal and tail regions [e.g., concanavalin A (ConA)], whereas others bind either the acrosomal region [Dolichos biflorus agglutinin (DBA)] or the end piece of the tail [(e.g., wheat germ agglutinin (WGA)].Citation133,Citation148 A lower level of lectin staining is present along the entire length of the sperm tail, but is absent from the plasma membrane overlying the sperm nucleus.
Among the sperm plasma membrane proteins stained by fluorescent lectins are several glycosidases: α-mannosidase, two β-hexosaminidase isoforms and α-L-fucosidase.Citation149-Citation152 These enzymes are concentrated in the acrosomal region as well as along the sperm tail. Staining with fluorescent markers reveals the presence of complementary sugar residues on the egg micropyle that may be critical for sperm binding and fertilization.Citation148,Citation151 Glycosylation is critical for sperm-egg interactions, as indicated by studies of the male sterile casanova mutant, which produces mature, motile sperm that are transferred to the female reproductive tract but are unable to fertilize eggs.Citation153 casanova mutant sperm exhibit normal levels of a-mannosidase activity, but only half the β-hexosaminidase activity found in normal sperm. In addition, β-hexosaminidase is absent from the plasma membrane overlying the acrosome. This type of sperm-egg interaction appears broadly conserved, as glycoprotein recognition is also critical for gamete binding interactions in mammals (reviewed in refs. Citation154, Citation155).
Another protein that localizes to a specialized membrane domain during spermiogenesis is the polycystic kidney disease protein polycystin 2 (Pkd2), the Drosophila homolog of the transient receptor potential channel TRPP2 [also called Almost there (Amo)], which is dispensable for sperm development, but required for proper sperm storage in the female reproductive tract.Citation156,Citation157 Pkd2 localizes to the ER in primary spermatocytes but localizes to the tail end piece in mature sperm.Citation157,Citation158 A version of Pkd2 that carries a mutation found in autosomal dominant polycystic kidney disease results in Pkd2 retention in the ER, thereby blocking tail tip localization and resulting in sperm failing to reach the storage organ in the female genital tract.Citation158
It will be of interest to learn where exactly Pkd2 localizes at the tip of the tail and whether its trafficking from the ER to the plasma membrane occurs during early or late stages of spermiogenesis. One possibility, based on the localization of mammalian Pkd2, is that Drosophila Pkd2 will be found to localize to the ciliary membrane that overlies the growing end of the flagellar axoneme.Citation11,Citation31,Citation32 Not much is known about the mechanism of formation of this specialized membrane domain. However, it is thought to originate with endocytosis of the short axonemes that are formed in mature primary spermatocytes (see above).Citation3,Citation11,Citation31 Following axoneme initiation, the centrioles are taken up into the cell, together with a cap of membrane. This membrane remains contiguous with the plasma membrane and covers the last ~4 ∝m at the growing end of the flagellar axoneme during spermatid elongation.Citation11,Citation31 Nothing is known of the protein composition of the ciliary membrane in Drosophila spermatids. However, Unc and Cby localize to the region of the ring centriole,Citation69-Citation71 which anchors the proximal portion of the ciliary membrane to the elongating axoneme. The membrane itself, which is located near the ring canals, can be visualized with YFP-Sktl () or a fluorescent PtdIns(4,5)P2 reporter (PLCδ-PH-GFP).Citation32
Individualization and Coiling
The last steps in Drosophila spermiogenesis are the individualization () and coiling of mature sperm. Individualization proceeds via formation of the individualization complex (IC), which consists of 64 investment cones rich in filamentous actin. These initially form around the mature needle-shaped nuclei and then translocate down the length of the elongated spermatids, stripping away material unneeded in mature sperm.Citation5,Citation159 Translocation of the IC results in formation of a cystic bulge containing organelles, membranes and cytoplasm to be discarded in the waste bag at the end of individualization. Following individualization, the sperm undergo a process of coiling in which the 64 sperm tails wind around each other and retract into the base of the testis. The mature sperm are then released into the testis and transferred to the seminal vesicle, where they are stored until copulation.
Three cytoskeletal motor proteins are known to regulate individualization. Formation of investment cones requires myosin V, an unconventional myosin that localizes to the acrosome in late stages of spermiogenesis.Citation160 Investment cone formation also requires Ddlc1, which localizes near the acrosome prior to cone formation and then to the cones once they are formed.Citation161 Ddlc1 is needed for tight bundling of the elongated nuclei and proper investment cone organization. IC translocation requires myosin VI, another unconventional myosin that localizes to the leading edge of the cones.Citation162 Surprisingly, myosin VI dimerization and processivity are not required for individualization, suggesting its motor activity is dispensable for IC movement.Citation163,Citation164 Instead, myosin VI appears to stabilize the investment cones as they progress.Citation163
The cones contain both unbranched (at the trailing end) and branched (at the leading edge) actin filaments and require regulators of actin polymerization for their formation.Citation165 Actin turns over throughout the cones, and the rate of turnover is faster in moving cones than in those that have not yet initiated movement.Citation15 Moreover, actin turnover appears important for movement, as actin but not MT inhibitors block IC progression. Actin bundling proteins (singed/fascin and quail/villin) colocalize with the unbranched filaments, whereas Arp2/3, Wasp, cortactin and capping protein localize with myosin VI at the leading edge.Citation166 Profilin, which regulates formin-dependent unbranched actin assembly, is required for formation and movement of the actin cones.Citation165 Arp2/3, which promotes branched actin formation, is also required. In the absence of Arp2/3, the meshwork is less dense and, although the cones move, cytoplasmic material is not removed and individualization fails.
Actin is linked to membrane remodeling during individualization. The endocytic regulator dynamin [also called Shibire (Shi) in flies] localizes to the plasma membrane overlying the cones.Citation166 Male germ cells lacking Vps28, an ESCRT I subunit involved in endosomal trafficking, are male sterile and exhibit defects in actin organization during individualization.Citation167 Nonetheless, two lines of evidence suggest endocytosis is not required for individualization. Staining of cellular membranes with the fluorescent dye FM1–43 reveals an absence of endocytic vesicles in the cystic bulge.Citation15 Moreover, dynamin is dispensable for individualization, but acts redundantly with myosin VI to promote actin cone assembly or stability.Citation166 The focal adhesion protein Lasp shows a similar localization to dynamin and is required for formation of robust actin cones during individualization.Citation168 The precise role of Lasp in individualization is complicated by its role in somatic cyst cells. However, one possibility is that dynamin and myosin VI, which are PtdIns(4,5)P2-binding proteins, act together with Lasp to help link actin filaments in the cones to the plasma membrane.
Successful individualization also requires activation of a caspase cascade, this being one of the first examples of a non-apoptotic role for activated caspases (reviewed in refs. Citation169–Citation171). Caspase activation occurs upon completion of spermatid morphogenesis and is independent of actin cone assembly or movement ().Citation172 Activation of the initiator caspase Dronc requires the Reaper-family protein Hid and Drosophila Apaf-1 (Dark) to relieve Drosophila inhibitor of apoptosis (Diap1) inhibition.Citation173 Dronc, in turn, activates the short prodomain caspases Dcp-1 and Drice.Citation174 Activated caspases are found in the cystic bulge and the waste bag and are important for individualization, as caspase inhibitors block IC movement.Citation172
Caspase activation is tightly controlled during spermiogenesis. The giant E2 ubiquitin ligase/inhibitor of apoptosis (IAP)-like protein dBruce prevents precocious caspase activation, as dBruce mutants exhibit scattered, round, degenerating nuclei in the elongating spermatid cysts.Citation172 dBruce is present in a gradient within elongating spermatid cysts, with the highest concentration at the distal end and the lowest concentration near the nuclei, where the IC forms, consistent with the observation that caspase activation is initially highest at the nuclear end.Citation175 As individualization proceeds, the highest levels of activated caspases are found associated with the cystic bulge and activated caspases are removed from the individualized portions of the maturing sperm.
Local activation of caspases near the nuclei and in the cystic bulge is achieved through the action of two different ubiquitin ligase complexes that are thought to target dBruce. The testis-specific Klhl10-Cul3 complex binds dBruce and is needed for caspase activation and IC formation.Citation176 Ubiquitination of dBruce by the Cul3 complex results in redistribution to form a gradient with the highest concentration of dBruce at the elongating end of the spermatid cyst. The activity of the Cul3 complex is regulated by the E3 ubiquitin ligase inhibitor Scotti (Soti).Citation175 Soti is transcribed postmeiotically and its mRNA localizes to the distal end of elongating spermatid cysts.Citation33 soti mRNA translation results in a gradient of Soti protein, with the highest concentration at the caudal end, similar to the gradient of dBruce.Citation175 Soti competes with dBruce for binding Klhl10, thereby allowing caspase activation in proximity to the developing actin cones. Within the cystic bulge, dBruce is regulated by a SCF ubiquitin ligase complex (SkpA-Cul1-Ntc), which also binds dBruce and promotes caspase activation.Citation177 Since the SCF complex localizes to the cystic bulge, it may trigger dBruce degradation and caspase activation as the IC moves down the individualizing cyst.
Despite steady progress in identifying factors involved in individualization, the signals involved in initiation of the process remain unclear. For example, it is unknown how actin cone assembly is stimulated at the nuclear end of the cyst in response to completion of elongation at the distal end. It seems likely that a signal that initiates at the caudal end is transduced to the nuclear end. Moreover, the signal is germ cell autonomous, rather than originating in the surrounding somatic cyst cells, as individualization and proceed in the absence of cyst cells in vitro.Citation15 Nonetheless, the nature of the initiating signal is unclear. It could be that calcium is involved, since several of the postmeiotically transcribed mRNAs that localize to the growing end of the sperm tails encode predicted calcium-binding proteins.Citation33 Alternatively, mitochondrial cytochrome-c (Cyt-c-d) activity may provide the necessary signal, as Cyt-c-d is required for caspase activation and initiation of IC movement.Citation178 Another possibility is that the signal involves polyglycylation of axonemal MTs, a process in which Ntl might be involved.Citation110
Conclusions and Future Outlook
The production of mature, fertile Drosophila sperm requires coordination of an array of morphogenetic processes affecting every organelle in the cell. Mitochondria fuse, unfurl, elongate, fill with crystalline material and degrade their DNA. Basal bodies migrate, dock at the nuclear envelope, form centriolar adjuncts and nucleate axoneme elongation. Axonemes assemble, acquire posttranslational modifications and become motile. Nuclei associate with dense bodies, elongate and condense their chromatin. Membrane trafficking becomes directed toward the acroblast and acrosome formation, as well as elongation of the spermatid cysts. The cysts acquire intrinsic polarity and specialized membrane domains overlying the acrosome and sperm tail tip. The mature sperm individualize and coil prior to transfer and storage in the seminal vesicle.
Beyond these morphological changes, mature sperm require specific signaling molecules, receptors and chromatin factors that are acquired during spermiogenesis and needed for fertilization and embryogenesis. The Pkd2 calcium channel localizes to the distal end of the sperm tail, where it promotes sperm storage in the female reproductive tract. Glycosidases present on the plasma membrane overlying the acrosome participate in sperm-egg recognition during fertilization. The acrosomal membrane protein Snky signals sperm plasma membrane breakdown upon entry into the oocyte. The presence of the K81 at telomeres provides protection of sperm chromatin and allows the paternal genome to participate in embryogenesis.
Drosophila sperm development has many parallels to spermiogenesis in other organisms, including mammals. During differentiation, mammalian spermatids develop in syncytial cysts and form specialized mitochondrial structures, perinuclear MT arrays, flagellar axonemes acrosomes and plasma membrane domains (reviewed in refs. Citation155, Citation179–Citation184). Mammalian spermatid nuclei undergo shaping and chromatin condensation (reviewed in refs. Citation185 and Citation186). Removal of excess cytoplasm and individualization also occurs during mammalian spermatid terminal differentiation, and the removed residual bodies (the mammalian equivalent of waste bags) also display characteristics of apoptotic bodies.Citation187 Moreover, mammalian sperm acquire signaling molecules and motility prior to participating in fertilization (reviewed in ref. Citation188). Hence, the molecular and cellular mechanisms uncovered by the study of Drosophila spermiogenesis will enhance our understanding of similar processes crucial to human male fertility and initiation of embryonic development.
| Abbreviations: | ||
| CA | = | centriolar adjunct |
| Cby | = | Chibby |
| Cnn | = | Centrosomin |
| IC | = | individualization complex |
| MT | = | microtubule |
| PCM | = | pericentriolar material |
| PtdIns(4,5)P2 | = | phosphatidylinositol 4,5-bisphosphate |
| Unc | = | uncoordinated |
Acknowledgments
J.A.B. is grateful to her mentors Minx Fuller and Barbara Wakimoto for inspiration. L.F. and J.A.B. thank Karen Hales, Tim Megraw and the reviewers for their helpful comments on the manuscript. Research in the Brill lab is funded by CIHR operating grant MOP-102546. We apologize to colleagues whose work we could not cite due to space limitations.
References
- Anderson WA. Cytodifferentiation of spermatozoa in Drosophila melanogaster: the effect of elevated temperature on spermiogenesis. Mol Gen Genet 1967; 99:257 - 73; http://dx.doi.org/10.1007/BF01797731; PMID: 5591274
- Shoup JR. Spermiogenesis in wild type and in a male sterility mutant of Drosophila melanogaster.. J Cell Biol 1967; 32:663 - 75; http://dx.doi.org/10.1083/jcb.32.3.663; PMID: 6034483
- Tates AD. Cytodifferentiation during spermatogenesis in Drosophila melanogaster: An electron microscope study. Leiden: Rijksuniversiteit, 1971.
- Stanley HP, Bowman JT, Romrell LJ, Reed SC, Wilkinson RF. Fine structure of normal spermatid differentiation in Drosophila melanogaster.. J Ultrastruct Res 1972; 41:433 - 66; http://dx.doi.org/10.1016/S0022-5320(72)90049-4; PMID: 4118303
- Tokuyasu KT, Peacock WJ, Hardy RW. Dynamics of spermiogenesis in Drosophila melanogaster. I. Individualization process. Z Zellforsch Mikrosk Anat 1972; 124:479 - 506; http://dx.doi.org/10.1007/BF00335253; PMID: 4622067
- Tokuyasu KT, Peacock WJ, Hardy RW. Dynamics of spermiogenesis in Drosophila melanogaster. II. Coiling process. Z Zellforsch Mikrosk Anat 1972; 127:492 - 525; http://dx.doi.org/10.1007/BF00306868; PMID: 4625686
- Rasmussen SW. Ultrastructural studies of spermatogenesis in Drosophila melanogaster Meigen. Z Zellforsch Mikrosk Anat 1973; 140:125 - 44; http://dx.doi.org/10.1007/BF00307062; PMID: 4199851
- Tokuyasu KT. Dynamics of spermiogenesis in Drosophila melanogaster. 3. Relation between axoneme and mitochondrial derivatives. Exp Cell Res 1974; 84:239 - 50; http://dx.doi.org/10.1016/0014-4827(74)90402-9; PMID: 4206336
- Tokuyasu KT. Dynamics of spermiogenesis in Drosophila melanogaster. IV. Nuclear transformation. J Ultrastruct Res 1974; 48:284 - 303; http://dx.doi.org/10.1016/S0022-5320(74)80083-3; PMID: 4210599
- Tokuyasu KT. Dynamics of spermiogenesis in Drosophila melanogaster. V. Head-tail alignment. J Ultrastruct Res 1975; 50:117 - 29; http://dx.doi.org/10.1016/S0022-5320(75)90013-1; PMID: 803563
- Tokuyasu KT. Dynamics of spermiogenesis in Drosophila melanogaster. VI. Significance of “onion” nebenkern formation. J Ultrastruct Res 1975; 53:93 - 112; http://dx.doi.org/10.1016/S0022-5320(75)80089-X; PMID: 810602
- Tokuyasu KT, Peacock WJ, Hardy RW. Dynamics of spermiogenesis in Drosophila melanogaster. VII. Effects of segregation distorter (SD) chromosome. J Ultrastruct Res 1977; 58:96 - 107; http://dx.doi.org/10.1016/S0022-5320(77)80011-7; PMID: 401895
- Fuller MT. Spermatogenesis. Cold Spring Harbor, New York: Cold Spring Harbor Press, 1993.
- Cross DP, Shellenbarger DL. The dynamics of Drosophila melanogaster spermatogenesis in in vitro cultures. J Embryol Exp Morphol 1979; 53:345 - 51; PMID: 119823
- Noguchi T, Miller KG. A role for actin dynamics in individualization during spermatogenesis in Drosophila melanogaster.. Development 2003; 130:1805 - 16; http://dx.doi.org/10.1242/dev.00406; PMID: 12642486
- Kawamoto T, Kawai K, Kodama T, Yokokura T, Niki Y. Autonomous differentiation of Drosophila spermatogonia in vitro. Dev Growth Differ 2008; 50:623 - 32; http://dx.doi.org/10.1111/j.1440-169X.2008.01060.x; PMID: 18657168
- White-Cooper H. Molecular mechanisms of gene regulation during Drosophila spermatogenesis. Reproduction 2010; 139:11 - 21; http://dx.doi.org/10.1530/REP-09-0083; PMID: 19755484
- White-Cooper H, Davidson I. Unique aspects of transcription regulation in male germ cells. Cold Spring Harb Perspect Biol 2011; 3:3; http://dx.doi.org/10.1101/cshperspect.a002626; PMID: 21555408
- Kiefer BI. Ultrastructural Abnormalities in Developing Sperm of X/0 DROSOPHILA MELANOGASTER.. Genetics 1966; 54:1441 - 52; PMID: 17248369
- Hess O, Meyer GF. Genetic activities of the Y chromosome in Drosophila during spermatogenesis. Adv Genet 1968; 14:171 - 223; http://dx.doi.org/10.1016/S0065-2660(08)60427-7; PMID: 4884781
- Kiefer BI. Phenotypic effects of Y chromosome mutations in Drosophila melanogaster. I. Spermiogenesis and sterility in KL-1- males. Genetics 1969; 61:157 - 66; PMID: 5802558
- Ayles GB, Sanders TG, Kiefer BI, Suzuki DT. Temperature-sensitive mutations in Drosophila melanogaster. XI. Male sterile mutants of the Y chromosome. Dev Biol 1973; 32:239 - 57; http://dx.doi.org/10.1016/0012-1606(73)90239-X; PMID: 4363872
- Hackstein JH. Spermatogenesis in Drosophila. A genetic approach to cellular and subcellular differentiation. Eur J Cell Biol 1991; 56:151 - 69; PMID: 1802704
- Castrillon DH, Gönczy P, Alexander S, Rawson R, Eberhart CG, Viswanathan S, et al. Toward a molecular genetic analysis of spermatogenesis in Drosophila melanogaster: characterization of male-sterile mutants generated by single P element mutagenesis. Genetics 1993; 135:489 - 505; PMID: 8244010
- Wakimoto BT, Lindsley DL, Herrera C. Toward a comprehensive genetic analysis of male fertility in Drosophila melanogaster.. Genetics 2004; 167:207 - 16; http://dx.doi.org/10.1534/genetics.167.1.207; PMID: 15166148
- White-Cooper H. Tissue, cell type and stage-specific ectopic gene expression and RNAi induction in the Drosophila testis. Spermatogenesis 2012; 2:11 - 22; http://dx.doi.org/10.4161/spmg.19088; PMID: 22553486
- Cooper KW. Normal spermatogenesis in Drosophila. In: Demerec M, ed. Biology of Drosophila. New York: Wiley, 1950:1-61.
- Lindsley D, Tokuyasu KT. Spermatogenesis. In: Ashburner M, Wright TR, eds. Genetics and Biology of Drosophila. New York: Academic Press, 1980:225-94.
- Renkawitz-Pohl R, Hollmann M, Hempel L, Schäfer M. Spermatogenesis. In: Gilbert LI, Iatrou K, Gill S, eds. Comprehensive Insect Physiology, Biochemistry, Pharmacology and Molecular Biology. Oxford: Elsevier, 2005:157-78.
- Hime GR, Brill JA, Fuller MT. Assembly of ring canals in the male germ line from structural components of the contractile ring. J Cell Sci 1996; 109:2779 - 88; PMID: 9013326
- Fritz-Niggli H, Suda T. Formation and significance of centrioles: A study and new interpretation of the meiosis of Drosophila. Cytobiologie 1972; 5:12 - 41
- Fabian L, Wei HC, Rollins J, Noguchi T, Blankenship JT, Bellamkonda K, et al. Phosphatidylinositol 4,5-bisphosphate directs spermatid cell polarity and exocyst localization in Drosophila. Mol Biol Cell 2010; 21:1546 - 55; http://dx.doi.org/10.1091/mbc.E09-07-0582; PMID: 20237161
- Barreau C, Benson E, White-Cooper H. Comet and cup genes in Drosophila spermatogenesis: the first demonstration of post-meiotic transcription. Biochem Soc Trans 2008; 36:540 - 2; http://dx.doi.org/10.1042/BST0360540; PMID: 18482002
- Phillips DM. Insect sperm: their structure and morphogenesis. J Cell Biol 1970; 44:243 - 77; http://dx.doi.org/10.1083/jcb.44.2.243; PMID: 4903810
- Sitaram P, Anderson MA, Jodoin JN, Lee E, Lee LA. Regulation of dynein localization and centrosome positioning by Lis-1 and asunder during Drosophila spermatogenesis. Development 2012; 139:2945 - 54; http://dx.doi.org/10.1242/dev.077511; PMID: 22764052
- Hales KG, Fuller MT. Developmentally regulated mitochondrial fusion mediated by a conserved, novel, predicted GTPase. Cell 1997; 90:121 - 9; http://dx.doi.org/10.1016/S0092-8674(00)80319-0; PMID: 9230308
- McQuibban GA, Lee JR, Zheng L, Juusola M, Freeman M. Normal mitochondrial dynamics requires rhomboid-7 and affects Drosophila lifespan and neuronal function. Curr Biol 2006; 16:982 - 9; http://dx.doi.org/10.1016/j.cub.2006.03.062; PMID: 16713954
- Aldridge AC, Benson LP, Siegenthaler MM, Whigham BT, Stowers RS, Hales KG. Roles for Drp1, a dynamin-related protein, and milton, a kinesin-associated protein, in mitochondrial segregation, unfurling and elongation during Drosophila spermatogenesis. Fly (Austin) 2007; 1:38 - 46; PMID: 18690063
- Clark IE, Dodson MW, Jiang C, Cao JH, Huh JR, Seol JH, et al. Drosophila pink1 is required for mitochondrial function and interacts genetically with parkin.. Nature 2006; 441:1162 - 6; http://dx.doi.org/10.1038/nature04779; PMID: 16672981
- Deng H, Dodson MW, Huang H, Guo M. The Parkinson’s disease genes pink1 and parkin promote mitochondrial fission and/or inhibit fusion in Drosophila. Proc Natl Acad Sci U S A 2008; 105:14503 - 8; http://dx.doi.org/10.1073/pnas.0803998105; PMID: 18799731
- Riparbelli MG, Callaini G. The Drosophila parkin homologue is required for normal mitochondrial dynamics during spermiogenesis. Dev Biol 2007; 303:108 - 20; http://dx.doi.org/10.1016/j.ydbio.2006.10.038; PMID: 17123504
- Greene JC, Whitworth AJ, Kuo I, Andrews LA, Feany MB, Pallanck LJ. Mitochondrial pathology and apoptotic muscle degeneration in Drosophila parkin mutants. Proc Natl Acad Sci U S A 2003; 100:4078 - 83; http://dx.doi.org/10.1073/pnas.0737556100; PMID: 12642658
- Hao LY, Giasson BI, Bonini NM. DJ-1 is critical for mitochondrial function and rescues PINK1 loss of function. Proc Natl Acad Sci U S A 2010; 107:9747 - 52; http://dx.doi.org/10.1073/pnas.0911175107; PMID: 20457924
- Ziviani E, Tao RN, Whitworth AJ. Drosophila parkin requires PINK1 for mitochondrial translocation and ubiquitinates mitofusin. Proc Natl Acad Sci U S A 2010; 107:5018 - 23; http://dx.doi.org/10.1073/pnas.0913485107; PMID: 20194754
- Ashrafi G, Schwarz TL. The pathways of mitophagy for quality control and clearance of mitochondria. Cell Death Differ 2012; http://dx.doi.org/10.1038/cdd.2012.81; PMID: 22743996
- Braschi E, McBride HM. Mitochondria and the culture of the Borg: understanding the integration of mitochondrial function within the reticulum, the cell, and the organism. Bioessays 2010; 32:958 - 66; http://dx.doi.org/10.1002/bies.201000073; PMID: 20824657
- Poole AC, Thomas RE, Yu S, Vincow ES, Pallanck L. The mitochondrial fusion-promoting factor mitofusin is a substrate of the PINK1/parkin pathway. PLoS One 2010; 5:e10054; http://dx.doi.org/10.1371/journal.pone.0010054; PMID: 20383334
- Noguchi T, Koizumi M, Hayashi S. Sustained elongation of sperm tail promoted by local remodeling of giant mitochondria in Drosophila. Curr Biol 2011; 21:805 - 14; http://dx.doi.org/10.1016/j.cub.2011.04.016; PMID: 21549602
- Hoyle HD, Raff EC. Two Drosophila β tubulin isoforms are not functionally equivalent. J Cell Biol 1990; 111:1009 - 26; http://dx.doi.org/10.1083/jcb.111.3.1009; PMID: 2118141
- Basto R, Lau J, Vinogradova T, Gardiol A, Woods CG, Khodjakov A, et al. Flies without centrioles. Cell 2006; 125:1375 - 86; http://dx.doi.org/10.1016/j.cell.2006.05.025; PMID: 16814722
- Glater EE, Megeath LJ, Stowers RS, Schwarz TL. Axonal transport of mitochondria requires milton to recruit kinesin heavy chain and is light chain independent. J Cell Biol 2006; 173:545 - 57; http://dx.doi.org/10.1083/jcb.200601067; PMID: 16717129
- Metzendorf C, Lind MI. Drosophila mitoferrin is essential for male fertility: evidence for a role of mitochondrial iron metabolism during spermatogenesis. BMC Dev Biol 2010; 10:68; http://dx.doi.org/10.1186/1471-213X-10-68; PMID: 20565922
- Hales KG. Iron testes: sperm mitochondria as a context for dissecting iron metabolism. BMC Biol 2010; 8:79; http://dx.doi.org/10.1186/1741-7007-8-79; PMID: 20598113
- Bazinet C, Rollins JE. Rickettsia-like mitochondrial motility in Drosophila spermiogenesis. Evol Dev 2003; 5:379 - 85; http://dx.doi.org/10.1046/j.1525-142X.2003.03045.x; PMID: 12823454
- DeLuca SZ, O’Farrell PH. Barriers to male transmission of mitochondrial DNA in sperm development. Dev Cell 2012; 22:660 - 8; http://dx.doi.org/10.1016/j.devcel.2011.12.021; PMID: 22421049
- Li MG, Serr M, Newman EA, Hays TS. The Drosophila tctex-1 light chain is dispensable for essential cytoplasmic dynein functions but is required during spermatid differentiation. Mol Biol Cell 2004; 15:3005 - 14; http://dx.doi.org/10.1091/mbc.E04-01-0013; PMID: 15090621
- Schatten G. The centrosome and its mode of inheritance: the reduction of the centrosome during gametogenesis and its restoration during fertilization. Dev Biol 1994; 165:299 - 335; http://dx.doi.org/10.1006/dbio.1994.1256; PMID: 7958403
- Bettencourt-Dias M, Rodrigues-Martins A, Carpenter L, Riparbelli M, Lehmann L, Gatt MK, et al. SAK/PLK4 is required for centriole duplication and flagella development. Curr Biol 2005; 15:2199 - 207; http://dx.doi.org/10.1016/j.cub.2005.11.042; PMID: 16326102
- Rodrigues-Martins A, Bettencourt-Dias M, Riparbelli M, Ferreira C, Ferreira I, Callaini G, et al. DSAS-6 organizes a tube-like centriole precursor, and its absence suggests modularity in centriole assembly. Curr Biol 2007; 17:1465 - 72; http://dx.doi.org/10.1016/j.cub.2007.07.034; PMID: 17689959
- Blachon S, Gopalakrishnan J, Omori Y, Polyanovsky A, Church A, Nicastro D, et al. Drosophila asterless and vertebrate Cep152 Are orthologs essential for centriole duplication. Genetics 2008; 180:2081 - 94; http://dx.doi.org/10.1534/genetics.108.095141; PMID: 18854586
- Blachon S, Cai X, Roberts KA, Yang K, Polyanovsky A, Church A, et al. A proximal centriole-like structure is present in Drosophila spermatids and can serve as a model to study centriole duplication. Genetics 2009; 182:133 - 44; http://dx.doi.org/10.1534/genetics.109.101709; PMID: 19293139
- Gopalakrishnan J, Mennella V, Blachon S, Zhai B, Smith AH, Megraw TL, et al. Sas-4 provides a scaffold for cytoplasmic complexes and tethers them in a centrosome. Nat Commun 2011; 2:359; http://dx.doi.org/10.1038/ncomms1367; PMID: 21694707
- Gogendeau D, Basto R. Centrioles in flies: the exception to the rule?. Semin Cell Dev Biol 2010; 21:163 - 73; http://dx.doi.org/10.1016/j.semcdb.2009.07.001; PMID: 19596460
- Martinez-Campos M, Basto R, Baker J, Kernan M, Raff JW. The Drosophila pericentrin-like protein is essential for cilia/flagella function, but appears to be dispensable for mitosis. J Cell Biol 2004; 165:673 - 83; http://dx.doi.org/10.1083/jcb.200402130; PMID: 15184400
- Kawaguchi S, Zheng Y. Characterization of a Drosophila centrosome protein CP309 that shares homology with Kendrin and CG-NAP. Mol Biol Cell 2004; 15:37 - 45; http://dx.doi.org/10.1091/mbc.E03-03-0191; PMID: 14565985
- Dix CI, Raff JW. Drosophila Spd-2 recruits PCM to the sperm centriole, but is dispensable for centriole duplication. Curr Biol 2007; 17:1759 - 64; http://dx.doi.org/10.1016/j.cub.2007.08.065; PMID: 17919907
- Giansanti MG, Bucciarelli E, Bonaccorsi S, Gatti M. Drosophila SPD-2 is an essential centriole component required for PCM recruitment and astral-microtubule nucleation. Curr Biol 2008; 18:303 - 9; http://dx.doi.org/10.1016/j.cub.2008.01.058; PMID: 18291647
- Li K, Xu EY, Cecil JK, Turner FR, Megraw TL, Kaufman TC. Drosophila centrosomin protein is required for male meiosis and assembly of the flagellar axoneme. J Cell Biol 1998; 141:455 - 67; http://dx.doi.org/10.1083/jcb.141.2.455; PMID: 9548723
- Baker JD, Adhikarakunnathu S, Kernan MJ. Mechanosensory-defective, male-sterile unc mutants identify a novel basal body protein required for ciliogenesis in Drosophila. Development 2004; 131:3411 - 22; http://dx.doi.org/10.1242/dev.01229; PMID: 15226257
- Wei HC, Rollins J, Fabian L, Hayes M, Polevoy G, Bazinet C, et al. Depletion of plasma membrane PtdIns(4,5)P2 reveals essential roles for phosphoinositides in flagellar biogenesis. J Cell Sci 2008; 121:1076 - 84; http://dx.doi.org/10.1242/jcs.024927; PMID: 18334551
- Enjolras C, Thomas J, Chhin B, Cortier E, Duteyrat JL, Soulavie F, et al. Drosophila chibby is required for basal body formation and ciliogenesis but not for Wg signaling. J Cell Biol 2012; 197:313 - 25; http://dx.doi.org/10.1083/jcb.201109148; PMID: 22508513
- Mottier-Pavie V, Megraw TL. Drosophila bld10 is a centriolar protein that regulates centriole, basal body, and motile cilium assembly. Mol Biol Cell 2009; 20:2605 - 14; http://dx.doi.org/10.1091/mbc.E08-11-1115; PMID: 19321663
- Carvalho-Santos Z, Machado P, Branco P, Tavares-Cadete F, Rodrigues-Martins A, Pereira-Leal JB, et al. Stepwise evolution of the centriole-assembly pathway. J Cell Sci 2010; 123:1414 - 26; http://dx.doi.org/10.1242/jcs.064931; PMID: 20392737
- Vogt N, Koch I, Schwarz H, Schnorrer F, Nüsslein-Volhard C. The gammaTuRC components Grip75 and Grip128 have an essential microtubule-anchoring function in the Drosophila germline. Development 2006; 133:3963 - 72; http://dx.doi.org/10.1242/dev.02570; PMID: 16971473
- Ma L, Jarman AP. Dilatory is a Drosophila protein related to AZI1 (CEP131) that is located at the ciliary base and required for cilium formation. J Cell Sci 2011; 124:2622 - 30; http://dx.doi.org/10.1242/jcs.084798; PMID: 21750193
- Anderson MA, Jodoin JN, Lee E, Hales KG, Hays TS, Lee LA. Asunder is a critical regulator of dynein-dynactin localization during Drosophila spermatogenesis. Mol Biol Cell 2009; 20:2709 - 21; http://dx.doi.org/10.1091/mbc.E08-12-1165; PMID: 19357193
- Kracklauer MP, Wiora HM, Deery WJ, Chen X, Bolival B Jr., Romanowicz D, et al. The Drosophila SUN protein Spag4 cooperates with the coiled-coil protein Yuri Gagarin to maintain association of the basal body and spermatid nucleus. J Cell Sci 2010; 123:2763 - 72; http://dx.doi.org/10.1242/jcs.066589; PMID: 20647369
- Texada MJ, Simonette RA, Johnson CB, Deery WJ, Beckingham KM. Yuri gagarin is required for actin, tubulin and basal body functions in Drosophila spermatogenesis. J Cell Sci 2008; 121:1926 - 36; http://dx.doi.org/10.1242/jcs.026559; PMID: 18477609
- Méjat A, Misteli T. LINC complexes in health and disease. Nucleus 2010; 1:40 - 52; PMID: 21327104
- Sartain CV, Cui J, Meisel RP, Wolfner MF. The poly(A) polymerase GLD2 is required for spermatogenesis in Drosophila melanogaster.. Development 2011; 138:1619 - 29; http://dx.doi.org/10.1242/dev.059618; PMID: 21427144
- Gepner J, Hays TS. A fertility region on the Y chromosome of Drosophila melanogaster encodes a dynein microtubule motor. Proc Natl Acad Sci U S A 1993; 90:11132 - 6; http://dx.doi.org/10.1073/pnas.90.23.11132; PMID: 8248219
- Carvalho AB, Lazzaro BP, Clark AG. Y chromosomal fertility factors kl-2 and kl-3 of Drosophila melanogaster encode dynein heavy chain polypeptides. Proc Natl Acad Sci U S A 2000; 97:13239 - 44; http://dx.doi.org/10.1073/pnas.230438397; PMID: 11069293
- Bridges CB. Non-Disjunction as Proof of the Chromosome Theory of Heredity. Genetics 1916; 1:1 - 52, 107-63; PMID: 17245850
- Mencarelli C, Lupetti P, Dallai R. New insights into the cell biology of insect axonemes. Int Rev Cell Mol Biol 2008; 268:95 - 145; http://dx.doi.org/10.1016/S1937-6448(08)00804-6; PMID: 18703405
- Mencarelli C, Bré MH, Levilliers N, Dallai R. Accessory tubules and axonemal microtubules of Apis mellifera sperm flagellum differ in their tubulin isoform content. Cell Motil Cytoskeleton 2000; 47:1 - 12; http://dx.doi.org/10.1002/1097-0169(200009)47:1<1::AID-CM1>3.0.CO;2-U; PMID: 11002306
- Raff EC. Genetics of microtubule systems. J Cell Biol 1984; 99:1 - 10; http://dx.doi.org/10.1083/jcb.99.1.1; PMID: 6429152
- Matthews KA, Miller DF, Kaufman TC. Developmental distribution of RNA and protein products of the Drosophila α-tubulin gene family. Dev Biol 1989; 132:45 - 61; http://dx.doi.org/10.1016/0012-1606(89)90203-0; PMID: 2492961
- Hoyle HD, Hutchens JA, Turner FR, Raff EC. Regulation of β-tubulin function and expression in Drosophila spermatogenesis. Dev Genet 1995; 16:148 - 70; http://dx.doi.org/10.1002/dvg.1020160208; PMID: 7736665
- Hutchens JA, Hoyle HD, Turner FR, Raff EC. Structurally similar Drosophila α-tubulins are functionally distinct in vivo. Mol Biol Cell 1997; 8:481 - 500; PMID: 9188100
- Raff EC, Hutchens JA, Hoyle HD, Nielsen MG, Turner FR. Conserved axoneme symmetry altered by a component β-tubulin. Curr Biol 2000; 10:1391 - 4; http://dx.doi.org/10.1016/S0960-9822(00)00784-3; PMID: 11084342
- Fackenthal JD, Turner FR, Raff EC. Tissue-specific microtubule functions in Drosophila spermatogenesis require the β 2-tubulin isotype-specific carboxy terminus. Dev Biol 1993; 158:213 - 27; http://dx.doi.org/10.1006/dbio.1993.1180; PMID: 8330671
- Nielsen MG, Turner FR, Hutchens JA, Raff EC. Axoneme-specific β-tubulin specialization: a conserved C-terminal motif specifies the central pair. Curr Biol 2001; 11:529 - 33; http://dx.doi.org/10.1016/S0960-9822(01)00150-6; PMID: 11413005
- Sorokin SP. Reconstructions of centriole formation and ciliogenesis in mammalian lungs. J Cell Sci 1968; 3:207 - 30; PMID: 5661997
- Ishikawa H, Marshall WF. Ciliogenesis: building the cell’s antenna. Nat Rev Mol Cell Biol 2011; 12:222 - 34; http://dx.doi.org/10.1038/nrm3085; PMID: 21427764
- Han YG, Kwok BH, Kernan MJ. Intraflagellar transport is required in Drosophila to differentiate sensory cilia but not sperm. Curr Biol 2003; 13:1679 - 86; http://dx.doi.org/10.1016/j.cub.2003.08.034; PMID: 14521833
- Sarpal R, Todi SV, Sivan-Loukianova E, Shirolikar S, Subramanian N, Raff EC, et al. Drosophila KAP interacts with the kinesin II motor subunit KLP64D to assemble chordotonal sensory cilia, but not sperm tails. Curr Biol 2003; 13:1687 - 96; http://dx.doi.org/10.1016/j.cub.2003.09.025; PMID: 14521834
- Zhang P, Stankiewicz RL. Y-Linked male sterile mutations induced by P element in Drosophila melanogaster.. Genetics 1998; 150:735 - 44; PMID: 9755204
- Fatima R. Drosophila Dynein intermediate chain gene, Dic61B, is required for spermatogenesis. PLoS One 2011; 6:e27822; http://dx.doi.org/10.1371/journal.pone.0027822; PMID: 22145020
- Eberl DF, Hardy RW, Kernan MJ. Genetically similar transduction mechanisms for touch and hearing in Drosophila. J Neurosci 2000; 20:5981 - 8; PMID: 10934246
- Janke C, Bulinski JC. Post-translational regulation of the microtubule cytoskeleton: mechanisms and functions. Nat Rev Mol Cell Biol 2011; 12:773 - 86; http://dx.doi.org/10.1038/nrm3227; PMID: 22086369
- Nogales E. Structural insights into microtubule function. Annu Rev Biochem 2000; 69:277 - 302; http://dx.doi.org/10.1146/annurev.biochem.69.1.277; PMID: 10966460
- Piperno G, Fuller MT. Monoclonal antibodies specific for an acetylated form of α-tubulin recognize the antigen in cilia and flagella from a variety of organisms. J Cell Biol 1985; 101:2085 - 94; http://dx.doi.org/10.1083/jcb.101.6.2085; PMID: 2415535
- Bressac C, Bré MH, Darmanaden-Delorme J, Laurent M, Levilliers N, Fleury A. A massive new posttranslational modification occurs on axonemal tubulin at the final step of spermatogenesis in Drosophila. Eur J Cell Biol 1995; 67:346 - 55; PMID: 8521874
- Bobinnec Y, Marcaillou C, Debec A. Microtubule polyglutamylation in Drosophila melanogaster brain and testis. Eur J Cell Biol 1999; 78:671 - 4; http://dx.doi.org/10.1016/S0171-9335(99)80053-3; PMID: 10535310
- Popodi EM, Hoyle HD, Turner FR, Raff EC. The proximal region of the β-tubulin C-terminal tail is sufficient for axoneme assembly. Cell Motil Cytoskeleton 2005; 62:48 - 64; http://dx.doi.org/10.1002/cm.20085; PMID: 16080206
- Hoyle HD, Turner FR, Raff EC. Axoneme-dependent tubulin modifications in singlet microtubules of the Drosophila sperm tail. Cell Motil Cytoskeleton 2008; 65:295 - 313; http://dx.doi.org/10.1002/cm.20261; PMID: 18205200
- Bré MH, Redeker V, Quibell M, Darmanaden-Delorme J, Bressac C, Cosson J, et al. Axonemal tubulin polyglycylation probed with two monoclonal antibodies: widespread evolutionary distribution, appearance during spermatozoan maturation and possible function in motility. J Cell Sci 1996; 109:727 - 38; PMID: 8718664
- Raff EC, Hoyle HD, Popodi EM, Turner FR. Axoneme β-tubulin sequence determines attachment of outer dynein arms. Curr Biol 2008; 18:911 - 4; http://dx.doi.org/10.1016/j.cub.2008.05.031; PMID: 18571413
- Rogowski K, Juge F, van Dijk J, Wloga D, Strub JM, Levilliers N, et al. Evolutionary divergence of enzymatic mechanisms for posttranslational polyglycylation. Cell 2009; 137:1076 - 87; http://dx.doi.org/10.1016/j.cell.2009.05.020; PMID: 19524510
- Chatterjee N, Rollins J, Mahowald AP, Bazinet C. Neurotransmitter Transporter-Like: a male germline-specific SLC6 transporter required for Drosophila spermiogenesis. PLoS One 2011; 6:e16275; http://dx.doi.org/10.1371/journal.pone.0016275; PMID: 21298005
- Rattner JB, Brinkley BR. Ultrastructure of mammalian spermiogenesis. 3. The organization and morphogenesis of the manchette during rodent spermiogenesis. J Ultrastruct Res 1972; 41:209 - 18; http://dx.doi.org/10.1016/S0022-5320(72)90065-2; PMID: 4636018
- Rathke C, Barckmann B, Burkhard S, Jayaramaiah-Raja S, Roote J, Renkawitz-Pohl R. Distinct functions of Mst77F and protamines in nuclear shaping and chromatin condensation during Drosophila spermiogenesis. Eur J Cell Biol 2010; 89:326 - 38; http://dx.doi.org/10.1016/j.ejcb.2009.09.001; PMID: 20138392
- Fuller MT, Regan CL, Green LL, Robertson B, Deuring R, Hays TS. Interacting genes identify interacting proteins involved in microtubule function in Drosophila. Cell Motil Cytoskeleton 1989; 14:128 - 35; http://dx.doi.org/10.1002/cm.970140122; PMID: 2684419
- Jayaramaiah Raja S, Renkawitz-Pohl R. Replacement by Drosophila melanogaster protamines and Mst77F of histones during chromatin condensation in late spermatids and role of sesame in the removal of these proteins from the male pronucleus. Mol Cell Biol 2005; 25:6165 - 77; http://dx.doi.org/10.1128/MCB.25.14.6165-6177.2005; PMID: 15988027
- Rathke C, Baarends WM, Jayaramaiah-Raja S, Bartkuhn M, Renkawitz R, Renkawitz-Pohl R. Transition from a nucleosome-based to a protamine-based chromatin configuration during spermiogenesis in Drosophila. J Cell Sci 2007; 120:1689 - 700; http://dx.doi.org/10.1242/jcs.004663; PMID: 17452629
- Zhong L, Belote JM. The testis-specific proteasome subunit Prosalpha6T of D. melanogaster is required for individualization and nuclear maturation during spermatogenesis. Development 2007; 134:3517 - 25; http://dx.doi.org/10.1242/dev.004770; PMID: 17728345
- Awe S, Renkawitz-Pohl R. Histone H4 acetylation is essential to proceed from a histone- to a protamine-based chromatin structure in spermatid nuclei of Drosophila melanogaster.. Syst Biol Reprod Med 2010; 56:44 - 61; http://dx.doi.org/10.3109/19396360903490790; PMID: 20170286
- Vigodner M, Morris PL. Testicular expression of small ubiquitin-related modifier-1 (SUMO-1) supports multiple roles in spermatogenesis: silencing of sex chromosomes in spermatocytes, spermatid microtubule nucleation, and nuclear reshaping. Dev Biol 2005; 282:480 - 92; http://dx.doi.org/10.1016/j.ydbio.2005.03.034; PMID: 15950612
- Roest HP, van Klaveren J, de Wit J, van Gurp CG, Koken MH, Vermey M, et al. Inactivation of the HR6B ubiquitin-conjugating DNA repair enzyme in mice causes male sterility associated with chromatin modification. Cell 1996; 86:799 - 810; http://dx.doi.org/10.1016/S0092-8674(00)80154-3; PMID: 8797826
- Baarends WM, Hoogerbrugge JW, Roest HP, Ooms M, Vreeburg J, Hoeijmakers JH, et al. Histone ubiquitination and chromatin remodeling in mouse spermatogenesis. Dev Biol 1999; 207:322 - 33; http://dx.doi.org/10.1006/dbio.1998.9155; PMID: 10068466
- Cho C, Willis WD, Goulding EH, Jung-Ha H, Choi YC, Hecht NB, et al. Haploinsufficiency of protamine-1 or -2 causes infertility in mice. Nat Genet 2001; 28:82 - 6; http://dx.doi.org/10.1038/ng0501-82; PMID: 11326282
- Barreau C, Benson E, Gudmannsdottir E, Newton F, White-Cooper H. Post-meiotic transcription in Drosophila testes. Development 2008; 135:1897 - 902; http://dx.doi.org/10.1242/dev.021949; PMID: 18434411
- Vibranovski MD, Chalopin DS, Lopes HF, Long M, Karr TL. Direct evidence for postmeiotic transcription during Drosophila melanogaster spermatogenesis. Genetics 2010; 186:431 - 3; http://dx.doi.org/10.1534/genetics.110.118919; PMID: 20610406
- Dubruille R, Orsi GA, Delabaere L, Cortier E, Couble P, Marais GA, et al. Specialization of a Drosophila capping protein essential for the protection of sperm telomeres. Curr Biol 2010; 20:2090 - 9; http://dx.doi.org/10.1016/j.cub.2010.11.013; PMID: 21093267
- Gao G, Cheng Y, Wesolowska N, Rong YS. Paternal imprint essential for the inheritance of telomere identity in Drosophila. Proc Natl Acad Sci U S A 2011; 108:4932 - 7; http://dx.doi.org/10.1073/pnas.1016792108; PMID: 21383184
- Dubruille R, Loppin B. Epigenetic maintenance of telomere identity in Drosophila: buckle up for the sperm ride. Cell Cycle 2011; 10:1037 - 42; http://dx.doi.org/10.4161/cc.10.7.15071; PMID: 21386659
- Farkas RM, Giansanti MG, Gatti M, Fuller MT. The Drosophila Cog5 homologue is required for cytokinesis, cell elongation, and assembly of specialized Golgi architecture during spermatogenesis. Mol Biol Cell 2003; 14:190 - 200; http://dx.doi.org/10.1091/mbc.E02-06-0343; PMID: 12529436
- Giansanti MG, Bonaccorsi S, Kurek R, Farkas RM, Dimitri P, Fuller MT, et al. The class I PITP giotto is required for Drosophila cytokinesis. Curr Biol 2006; 16:195 - 201; http://dx.doi.org/10.1016/j.cub.2005.12.011; PMID: 16431372
- Giansanti MG, Belloni G, Gatti M. Rab11 is required for membrane trafficking and actomyosin ring constriction in meiotic cytokinesis of Drosophila males. Mol Biol Cell 2007; 18:5034 - 47; http://dx.doi.org/10.1091/mbc.E07-05-0415; PMID: 17914057
- Robinett CC, Giansanti MG, Gatti M, Fuller MT. TRAPPII is required for cleavage furrow ingression and localization of Rab11 in dividing male meiotic cells of Drosophila. J Cell Sci 2009; 122:4526 - 34; http://dx.doi.org/10.1242/jcs.054536; PMID: 19934220
- Yao R, Ito C, Natsume Y, Sugitani Y, Yamanaka H, Kuretake S, et al. Lack of acrosome formation in mice lacking a Golgi protein, GOPC. Proc Natl Acad Sci U S A 2002; 99:11211 - 6; http://dx.doi.org/10.1073/pnas.162027899; PMID: 12149515
- Kondylis V, Rabouille C. The Golgi apparatus: lessons from Drosophila. FEBS Lett 2009; 583:3827 - 38; http://dx.doi.org/10.1016/j.febslet.2009.09.048; PMID: 19800333
- Perotti ME, Pasini ME. Glycoconjugates of the surface of the spermatozoa of Drosophila melanogaster: a qualitative and quantitative study. J Exp Zool 1995; 272:311 - 8; http://dx.doi.org/10.1002/jez.1402720409; PMID: 7650518
- Hirst J, Carmichael J. A potential role for the clathrin adaptor GGA in Drosophila spermatogenesis. BMC Cell Biol 2011; 12:22; http://dx.doi.org/10.1186/1471-2121-12-22; PMID: 21599933
- Kitazawa D, Yamaguchi M, Mori H, Inoue YH. COPI-mediated membrane trafficking is required for cytokinesis in Drosophila male meiotic divisions. J Cell Sci 2012; http://dx.doi.org/10.1242/jcs.103317; PMID: 22553212
- Wilson KL, Fitch KR, Bafus BT, Wakimoto BT. Sperm plasma membrane breakdown during Drosophila fertilization requires sneaky, an acrosomal membrane protein. Development 2006; 133:4871 - 9; http://dx.doi.org/10.1242/dev.02671; PMID: 17092953
- Ohsako T, Hirai K, Yamamoto MT. The Drosophila misfire gene has an essential role in sperm activation during fertilization. Genes Genet Syst 2003; 78:253 - 66; http://dx.doi.org/10.1266/ggs.78.253; PMID: 12893967
- Smith MK, Wakimoto BT. Complex regulation and multiple developmental functions of misfire, the Drosophila melanogaster ferlin gene. BMC Dev Biol 2007; 7:21; http://dx.doi.org/10.1186/1471-213X-7-21; PMID: 17386097
- Dorogova NV, Akhmametyeva EM, Kopyl SA, Gubanova NV, Yudina OS, Omelyanchuk LV, et al. The role of Drosophila Merlin in spermatogenesis. BMC Cell Biol 2008; 9:1; http://dx.doi.org/10.1186/1471-2121-9-1; PMID: 18186933
- Xu H, Brill JA, Hsien J, McBride R, Boulianne GL, Trimble WS. Syntaxin 5 is required for cytokinesis and spermatid differentiation in Drosophila. Dev Biol 2002; 251:294 - 306; http://dx.doi.org/10.1006/dbio.2002.0830; PMID: 12435359
- Giansanti MG, Farkas RM, Bonaccorsi S, Lindsley DL, Wakimoto BT, Fuller MT, et al. Genetic dissection of meiotic cytokinesis in Drosophila males. Mol Biol Cell 2004; 15:2509 - 22; http://dx.doi.org/10.1091/mbc.E03-08-0603; PMID: 15004238
- Chen H, Liu Z, Huang X. Drosophila models of peroxisomal biogenesis disorder: peroxins are required for spermatogenesis and very-long-chain fatty acid metabolism. Hum Mol Genet 2010; 19:494 - 505; http://dx.doi.org/10.1093/hmg/ddp518; PMID: 19933170
- Brill JA, Hime GR, Scharer-Schuksz M, Fuller MT. A phospholipid kinase regulates actin organization and intercellular bridge formation during germline cytokinesis. Development 2000; 127:3855 - 64; PMID: 10934029
- Zhou X, Fabian L, Bayraktar JL, Ding HM, Brill JA, Chang HC. Auxilin is required for formation of Golgi-derived clathrin-coated vesicles during Drosophila spermatogenesis. Development 2011; 138:1111 - 20; http://dx.doi.org/10.1242/dev.057422; PMID: 21343365
- Bergeret E, Pignot-Paintrand I, Guichard A, Raymond K, Fauvarque MO, Cazemajor M, et al. RotundRacGAP functions with Ras during spermatogenesis and retinal differentiation in Drosophila melanogaster.. Mol Cell Biol 2001; 21:6280 - 91; http://dx.doi.org/10.1128/MCB.21.18.6280-6291.2001; PMID: 11509670
- Raymond K, Bergeret E, Avet-Rochex A, Griffin-Shea R, Fauvarque MO. A screen for modifiers of RacGAP(84C) gain-of-function in the Drosophila eye revealed the LIM kinase Cdi/TESK1 as a downstream effector of Rac1 during spermatogenesis. J Cell Sci 2004; 117:2777 - 89; http://dx.doi.org/10.1242/jcs.01123; PMID: 15169836
- Ghosh-Roy A, Kulkarni M, Kumar V, Shirolikar S, Ray K. Cytoplasmic dynein-dynactin complex is required for spermatid growth but not axoneme assembly in Drosophila. Mol Biol Cell 2004; 15:2470 - 83; http://dx.doi.org/10.1091/mbc.E03-11-0848; PMID: 15020714
- Perotti ME, Riva A. Concanavalin A binding sites on the surface of Drosophila melanogaster sperm: a fluorescence and ultrastructural study. J Ultrastruct Mol Struct Res 1988; 100:173 - 82; http://dx.doi.org/10.1016/0889-1605(88)90024-9; PMID: 3147297
- Cattaneo F, Pasini ME, Perotti ME. Glycosidases are present on the surface of Drosophila melanogaster spermatozoa. Mol Reprod Dev 1997; 48:276 - 81; http://dx.doi.org/10.1002/(SICI)1098-2795(199710)48:2<276::AID-MRD16>3.0.CO;2-W; PMID: 9291478
- Cattaneo F, Pasini ME, Intra J, Matsumoto M, Briani F, Hoshi M, et al. Identification and expression analysis of Drosophila melanogaster genes encoding β-hexosaminidases of the sperm plasma membrane. Glycobiology 2006; 16:786 - 800; http://dx.doi.org/10.1093/glycob/cwl007; PMID: 16733265
- Intra J, Cenni F, Perotti ME. An α-L-fucosidase potentially involved in fertilization is present on Drosophila spermatozoa surface. Mol Reprod Dev 2006; 73:1149 - 58; http://dx.doi.org/10.1002/mrd.20425; PMID: 16736526
- Intra J, Cenni F, Pavesi G, Pasini M, Perotti ME. Interspecific analysis of the glycosidases of the sperm plasma membrane in Drosophila. Mol Reprod Dev 2009; 76:85 - 100; http://dx.doi.org/10.1002/mrd.20932; PMID: 18484570
- Perotti ME, Cattaneo F, Pasini ME, Vernì F, Hackstein JH. Male sterile mutant casanova gives clues to mechanisms of sperm-egg interactions in Drosophila melanogaster.. Mol Reprod Dev 2001; 60:248 - 59; http://dx.doi.org/10.1002/mrd.1085; PMID: 11553926
- Macek MB, Shur BD. Protein-carbohydrate complementarity in mammalian gamete recognition. Gamete Res 1988; 20:93 - 109; http://dx.doi.org/10.1002/mrd.1120200109; PMID: 2853128
- Shur BD, Rodeheffer C, Ensslin MA, Lyng R, Raymond A. Identification of novel gamete receptors that mediate sperm adhesion to the egg coat. Mol Cell Endocrinol 2006; 250:137 - 48; http://dx.doi.org/10.1016/j.mce.2005.12.037; PMID: 16417965
- Gao Z, Ruden DM, Lu X. PKD2 cation channel is required for directional sperm movement and male fertility. Curr Biol 2003; 13:2175 - 8; http://dx.doi.org/10.1016/j.cub.2003.11.053; PMID: 14680633
- Watnick TJ, Jin Y, Matunis E, Kernan MJ, Montell C. A flagellar polycystin-2 homolog required for male fertility in Drosophila. Curr Biol 2003; 13:2179 - 84; http://dx.doi.org/10.1016/j.cub.2003.12.002; PMID: 14680634
- Köttgen M, Hofherr A, Li W, Chu K, Cook S, Montell C, et al. Drosophila sperm swim backwards in the female reproductive tract and are activated via TRPP2 ion channels. PLoS One 2011; 6:e20031; http://dx.doi.org/10.1371/journal.pone.0020031; PMID: 21625494
- Fabrizio JJ, Hime G, Lemmon SK, Bazinet C. Genetic dissection of sperm individualization in Drosophila melanogaster.. Development 1998; 125:1833 - 43; PMID: 9550716
- Mermall V, Bonafé N, Jones L, Sellers JR, Cooley L, Mooseker MS. Drosophila myosin V is required for larval development and spermatid individualization. Dev Biol 2005; 286:238 - 55; http://dx.doi.org/10.1016/j.ydbio.2005.07.028; PMID: 16126191
- Ghosh-Roy A, Desai BS, Ray K. Dynein light chain 1 regulates dynamin-mediated F-actin assembly during sperm individualization in Drosophila. Mol Biol Cell 2005; 16:3107 - 16; http://dx.doi.org/10.1091/mbc.E05-02-0103; PMID: 15829565
- Hicks JL, Deng WM, Rogat AD, Miller KG, Bownes M. Class VI unconventional myosin is required for spermatogenesis in Drosophila. Mol Biol Cell 1999; 10:4341 - 53; PMID: 10588662
- Noguchi T, Lenartowska M, Miller KG. Myosin VI stabilizes an actin network during Drosophila spermatid individualization. Mol Biol Cell 2006; 17:2559 - 71; http://dx.doi.org/10.1091/mbc.E06-01-0031; PMID: 16571671
- Noguchi T, Frank DJ, Isaji M, Miller KG. Coiled-coil-mediated dimerization is not required for myosin VI to stabilize actin during spermatid individualization in Drosophila melanogaster.. Mol Biol Cell 2009; 20:358 - 67; http://dx.doi.org/10.1091/mbc.E08-07-0776; PMID: 19005209
- Noguchi T, Lenartowska M, Rogat AD, Frank DJ, Miller KG. Proper cellular reorganization during Drosophila spermatid individualization depends on actin structures composed of two domains, bundles and meshwork, that are differentially regulated and have different functions. Mol Biol Cell 2008; 19:2363 - 72; http://dx.doi.org/10.1091/mbc.E07-08-0840; PMID: 18353976
- Rogat AD, Miller KG. A role for myosin VI in actin dynamics at sites of membrane remodeling during Drosophila spermatogenesis. J Cell Sci 2002; 115:4855 - 65; http://dx.doi.org/10.1242/jcs.00149; PMID: 12432073
- Sevrioukov EA, Moghrabi N, Kuhn M, Krämer H. A mutation in dVps28 reveals a link between a subunit of the endosomal sorting complex required for transport-I complex and the actin cytoskeleton in Drosophila. Mol Biol Cell 2005; 16:2301 - 12; http://dx.doi.org/10.1091/mbc.E04-11-1013; PMID: 15728719
- Lee S, Zhou L, Kim J, Kalbfleisch S, Schöck F. Lasp anchors the Drosophila male stem cell niche and mediates spermatid individualization. Mech Dev 2008; 125:768 - 76; http://dx.doi.org/10.1016/j.mod.2008.06.012; PMID: 18655828
- Baehrecke EH. Caspase activation finds fertile ground. Dev Cell 2003; 4:608 - 9; http://dx.doi.org/10.1016/S1534-5807(03)00134-5; PMID: 12737795
- Cagan RL. Spermatogenesis: borrowing the apoptotic machinery. Curr Biol 2003; 13:R600 - 2; http://dx.doi.org/10.1016/S0960-9822(03)00525-6; PMID: 12906812
- Feinstein-Rotkopf Y, Arama E. Can’t live without them, can live with them: roles of caspases during vital cellular processes. Apoptosis 2009; 14:980 - 95; http://dx.doi.org/10.1007/s10495-009-0346-6; PMID: 19373560
- Arama E, Agapite J, Steller H. Caspase activity and a specific cytochrome C are required for sperm differentiation in Drosophila. Dev Cell 2003; 4:687 - 97; http://dx.doi.org/10.1016/S1534-5807(03)00120-5; PMID: 12737804
- Huh JR, Vernooy SY, Yu H, Yan N, Shi Y, Guo M, et al. Multiple apoptotic caspase cascades are required in nonapoptotic roles for Drosophila spermatid individualization. PLoS Biol 2004; 2:E15; http://dx.doi.org/10.1371/journal.pbio.0020015; PMID: 14737191
- Muro I, Berry DL, Huh JR, Chen CH, Huang H, Yoo SJ, et al. The Drosophila caspase Ice is important for many apoptotic cell deaths and for spermatid individualization, a nonapoptotic process. Development 2006; 133:3305 - 15; http://dx.doi.org/10.1242/dev.02495; PMID: 16887831
- Kaplan Y, Gibbs-Bar L, Kalifa Y, Feinstein-Rotkopf Y, Arama E. Gradients of a ubiquitin E3 ligase inhibitor and a caspase inhibitor determine differentiation or death in spermatids. Dev Cell 2010; 19:160 - 73; http://dx.doi.org/10.1016/j.devcel.2010.06.009; PMID: 20643358
- Arama E, Bader M, Rieckhof GE, Steller H. A ubiquitin ligase complex regulates caspase activation during sperm differentiation in Drosophila. PLoS Biol 2007; 5:e251; http://dx.doi.org/10.1371/journal.pbio.0050251; PMID: 17880263
- Bader M, Arama E, Steller H. A novel F-box protein is required for caspase activation during cellular remodeling in Drosophila. Development 2010; 137:1679 - 88; http://dx.doi.org/10.1242/dev.050088; PMID: 20392747
- Arama E, Bader M, Srivastava M, Bergmann A, Steller H. The two Drosophila cytochrome C proteins can function in both respiration and caspase activation. EMBO J 2006; 25:232 - 43; http://dx.doi.org/10.1038/sj.emboj.7600920; PMID: 16362035
- Handel MA. Genetic control of spermatogenesis in mice. In: Hennig W, ed. Results and Problems in Cell Differentiation, Spermatogenesis: Genetic Aspects. Berlin Heidelberg: Springer-Verlag, 1987:1-62.
- Kierszenbaum AL. Sperm axoneme: a tale of tubulin posttranslation diversity. Mol Reprod Dev 2002; 62:1 - 3; http://dx.doi.org/10.1002/mrd.10139; PMID: 11933155
- Kierszenbaum AL, Tres LL. The acrosome-acroplaxome-manchette complex and the shaping of the spermatid head. Arch Histol Cytol 2004; 67:271 - 84; http://dx.doi.org/10.1679/aohc.67.271; PMID: 15700535
- Moreno RD, Alvarado CP. The mammalian acrosome as a secretory lysosome: new and old evidence. Mol Reprod Dev 2006; 73:1430 - 4; http://dx.doi.org/10.1002/mrd.20581; PMID: 16894549
- Escalier D. Knockout mouse models of sperm flagellum anomalies. Hum Reprod Update 2006; 12:449 - 61; http://dx.doi.org/10.1093/humupd/dml013; PMID: 16565154
- Toure A, Rode B, Hunnicutt GR, Escalier D, Gacon G. Septins at the annulus of mammalian sperm. Biol Chem 2011; 392:799 - 803; http://dx.doi.org/10.1515/BC.2011.074; PMID: 21740329
- Kierszenbaum AL, Rivkin E, Tres LL. Molecular biology of sperm head shaping. Soc Reprod Fertil Suppl 2007; 65:33 - 43; PMID: 17644953
- Ward WS. Function of sperm chromatin structural elements in fertilization and development. Mol Hum Reprod 2010; 16:30 - 6; http://dx.doi.org/10.1093/molehr/gap080; PMID: 19748904
- Blanco-Rodríguez J, Martínez-García C. Apoptosis is physiologically restricted to a specialized cytoplasmic compartment in rat spermatids. Biol Reprod 1999; 61:1541 - 7; http://dx.doi.org/10.1095/biolreprod61.6.1541; PMID: 10570001
- Buffone MG, Ijiri TW, Cao W, Merdiushev T, Aghajanian HK, Gerton GL. Heads or tails? Structural events and molecular mechanisms that promote mammalian sperm acrosomal exocytosis and motility. Mol Reprod Dev 2012; 79:4 - 18; http://dx.doi.org/10.1002/mrd.21393; PMID: 22031228