Abstract
Many previous studies have aimed at spermatogenesis of male murine germ cells in vitro, but no efficient system has been established yet that covers the entire process of mammalian spermatogenesis in a culture dish permanently. In this review, we report on the requirements of spermatogenesis and the current state of different culture methods using testicular tissue fragments, single cell suspensions or three-dimensional culture environments.
Requirements of Spermatogenesis: The Challenges for In Vitro Male Germ Cell Differentiation
Spermatogenesis in a culture system provides the perspective of fertility preservation and treatment of many forms of male infertility. In vitro spermatogenesis might also provide an option to explore details of cellular differentiation under controlled experimental conditions. Nearly a century has passed after first attempts to understand and establish spermatogenesis ex situ in culture systems were performed.Citation1-Citation3 However, only little progress has been made beyond proof of principle (see ).Citation3-Citation6 We recently demonstrated that morphologically mature sperm from enzymatically dispersed testicular cells of immature mice can be generated in three-dimensional culture systems.Citation3,Citation7 For the first time offspring was generated from in vitro generated sperm when organ cultures were applied to immature mouse seminiferous tubules in culture.Citation6,Citation8 Nevertheless, effective in vitro spermatogenesis still remains a vision. Here we review reasons why it is so difficult to setup efficient in vitro spermatogenic approaches and present a novel approach using scaffolds.
Table 1. Overview of studies addressing in vitro spermatogenesis.
Mammalian spermatogenesis is a highly complex process and its understanding requires knowledge of genetics, molecular biology, cell biology and physiology. Spermatogenesis is a process starting from stem cells and ending with one of the most striking cellular transformations. Involved are many paracrine and endocrine regulatory steps influencing highly structured and compartmentalized microenvironments in the seminiferous epithelium (for a review, see ref. Citation9).The seminiferous tubules in mammals consist of a basal, intraepithelial and adluminal compartment. During differentiation from spermatogonial stem cells to mature spermatids the developing germ cells pass through all three compartments (). Spermatogonia are located at the basal membrane of the seminiferous compartmentCitation4,Citation10. The subfraction of spermatogonial stem cells (SSCs) plays a unique role in the organism undergoing balanced self-renewal and differentiation in order to generate sufficient progenitors with intact DNA integrity albeit extremely high mitotic rates. This population of stem cells is responsible for transfer of the genetic information from one generation to the next.Citation11-Citation13 The epithelial microenvironment at the basement membrane of the seminiferous tubule defines stem cell niches. The number of niches determines the size of the stem cell population.Citation11 Extracellular matrix components deposited by peritubular and Sertoli cells such as laminin and collagen play important roles and may act in concert with soluble factors provided by somatic cell types and premeiotic germ cells.Citation14 Several growth factors are released into the basal compartment. One of the most prominent is the growth factor glia cell line-derived neurotrophic factor (GDNF), secreted by Sertoli cells. The binding of GDNF triggers the activation of the multicomponent signaling GFRα-1/Ret-receptor complex which modulates the self-renewal of SSCs and the development of undifferentiated spermatogonial cells via multiple signaling pathways (Scr signaling pathway/ Ras signaling pathway)Citation12,Citation14,Citation15. In addition to spermatogonial stem cells type A and B spermatogonia are the differentiating germ cells populating the basal compartment. The blood testis barrier presents the intraepithelial border of this microenvironment and consists of tight junctions between Sertoli cells. Proteins in the basement membrane affect Sertoli cell tight junction dynamics and thus regulate the movement of germ cells across the blood testis barrier.Citation16-Citation18.Citation19
Figure 1. (A) Cytology of the mouse testis showing the relationship and the function of the interstitial, basal, intraepithelial, and adluminal compartment. (B) Schematic diagram of different approaches for male germ cell differentiation in vitro and their outcomes so far.
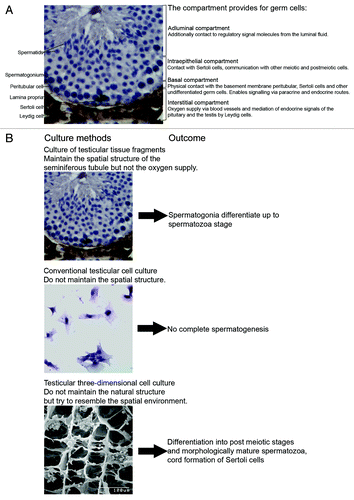
Differentiating germ cells in the basal compartment undergo incomplete mitotic divisions and form small interconnected cohorts and clones of highly synchronized cells. These clones remain stable for several weeks until they reach the elongating spermatid stage. When these clones finish meiotic S-phase and enter meiotic prophase they detach as preleptotene spermatocytes from the basement membrane and migrate through the blood testis barrier into the intraepithelial compartment.Citation17,Citation18,Citation19 Here the only other contacts are with other clones and Sertoli cells. After meiosis, during the elongation phase of spermiogenesis, for the first time the differentiating germ cells encounter the microenvironment of the third compartment (i.e., the adluminal region) of the seminiferous tubules. During the process of spermiation contact with the Sertoli cells is lost, as the newly differentiated testicular spermatozoa are released from the epithelium and hence must adapt to the conditions in the luminal fluid. Changes that were instigated previously by the Sertoli cells ().
In addition factors from the interstitial compartment of the testis may affect the maturation of germ cells. In this compartment, blood vessels, Leydig, peritubular, immune and endothelial cells are located. In response to luteinizing hormone (LH) from the pituitary, Leydig cells produce androgens which regulate biological activities, sexual functions and fertility.Citation19 Germ cells do not express androgen receptors.20The effects of androgens on germ cells may be indirect through peritubular cells and Sertoli cells which express high levels of androgen receptors. The importance of androgens in germ cell cultures may therefore be low.
These complex regulatory and spatial cellular relationships make it obvious that complete and efficient spermatogenesis is not easy to be rebuilt in artificial systems. This helps to understand why—although experimentally addressed over several decades—spermatogenesis in a culture dish has not been established yet. An effective system would have to resemble the sequential conditions provided by the three tubular compartments, ensure the survival of a combined mixture of somatic and germ cells, endocrine and paracrine signaling, the spatial arrangement, and it would have to enable the maintenance of SSC potential to self-renew and differentiate in the in vitro approach. So far attempts performed in organ or in testicular cell culture appear limited in their potential to build and maintain these requirements (). In this regard it is useful to have a closer look on a different method also dealing with the maturation of the germ line outside of the donor, but inside of an organism, the transplantation of a donor male germ line into recipient animals by xenografting or intratesticular germ cell transplantation. These approaches, which are clearly not in vitro, are nevertheless interesting in particular because of the fact that findings from these approaches gave worthy hints for the recent novel approaches for a transfer of spermatogenesis into in vitro systems by pointing out essential requirements which are here provided by the host environment.
Culture of Testicular Tissue Fragments
Historically in vitro differentiation and culture of male germ cell was first attempted using organ culture approaches aiming at maintenance of complete spermatogenesis in testicular tissue fragments. One of the first tissue culture experiments was conducted by Goldschmidt in 1915.Citation1 Culture of “sperm follicles” (which today would be designated as testicular cysts) of a moth (Samia cecropia L.) provided insights in the spermatogenic process. In this experimental setting, a hanging drop of hemolymph containing cysts with spermatogonia and early spermatocytes, provided the conditions for the maturation of spermatocytes into spermatozoa within three weeks of culture. It was shown that the first divisions of spermatogonia require weeks but meiosis and maturation of spermatids occurred in only few days. Interestingly, almost a century later, in a very similar study utilizing dissected testis from Drosophila spec., autonomous in vitro differentiation of 16-cell spermatogonial clones into mature spermatids was described.Citation20 In the second half of the last century testicular organ culture came into focus of reproductive research now aiming at mammalian spermatogenesis. In 1959 TrowellCitation21 developed a method employing a gas-liquid interphase organ culture system. Testicular tissue obtained from adult rats or mice was maintained in the interphase of culture medium and oxygen enriched air. Thus, already in these very early experiments one of the most serious problems for culture of tissues—hypoxia—had been identified. In 1964 Steinberger et al.Citation2 adapted this gas-liquid interphase method of Trowell and performed organ culture experiments with testicular fragments derived from immature 4-d-old rats. Fragments were placed on the surface of an agar strip arranged on a steel grid ensuring the simultaneous contact with the gas atmosphere and the culture medium. In these experiments, another issue of great importance for successful spermatogenesis, the endocrine regulation of the gonad, was addressed. The influence of hormonal support was examined, and it was shown that gonadotropins [FSH and human chorionic gonadotropin (hCG)] are not essential for the initiation of spermatogenic differentiation. Gonocytes developed to the stage of primary type A spermatogonia when the medium was supplemented with serum containing Vitamin A, E and C.Citation2 One year later it was demonstrated employing prepubertal rats (12 dpp) as organ donors that spermatogenesis passed to the pachytene stage after 2–3 weeks of culture. However germ cells arrested here because the culture conditions were insufficient to support the completion of meiotic division and formation of spermatids.Citation23 Interestingly, using the method developed by Steinberger on testicular tissue fragments of immature rats, it was demonstrated that FSH is indeed required in germ line differentiation for the conversion of type A spermatogonia into meiotic pachytene spermatocytes.Citation22 Afterwards, completion of meiosis of late pachytene spermatocytes and the induction of early spermiogenic events in cultured tubular segments occurred without additional testosterone or FSH.Citation23 Again, one of the first pioneering in vitro culture experiments was conducted by Steinberger and SteinbergerCitation24 with immature and adult rat testis cells defining a first set of culture conditions that differ from those of other culture aproaches (e.g., a lower temperature of 31°C and a pH between 6.8 and 7.0 having an adjuvant effect on testicular cells). Consequently, further efforts were made to translate the method into studies on human spermatogenesis. It was again Steinberger et al.Citation2 demonstrating in the organ culture setting that human testis cells can be maintained in vitro for several weeks and that preleptotene spermatocytes developed up to the pachytene stage but then got arrested. Testis tissue from other species was examined and culture approaches performed. Bovine SSCs survived and proliferated in tissue culture for two weeks and germ cell numbers increased in the seminiferous tubules from prepubertal bull calves (aged 1–2 mo).Citation25
A breaktrough was reported very recently when Sato and coworkers were able to produce spermatids and sperm from undifferentiated spermatogonia obtained from immature mouse donors (7.5−10.5 dpp) in an organ culture system. They were able to induce and maintain spermatogenesis for more than two months with serum-free culture media. The gametes obtained were capable of fertilization and produced offspring following microinsemination, thereby providing the functional proof of principle that male gametes derived from a culture dish are fertile and spermatogenesis can be maintained over certain periods in vitro. Furthermore this system can be used to transplant manipulated mouse SSCs into testicular tissue which is then organ cultured. Very recently it was demonstrated that those SSCs coming from a SSC cell line can be differentiated in this system up to donor derived sperm.Citation6,Citation8
However when summarizing the experiences reported in these studies, organ cultures are capable in maintaining the three compartments of the seminiferous epithelium as well as the interstitial environment (at least in part). Organ culture approaches result in initiation and partial maintenance of the spermatogenic progress and provide the advantage that the spatial arrangements and cell-cell relationship in the testicular microenvironment as well as the large clones of germ cells remain intact. However, organ culture has the disadvantage of requiring relative large amounts of tissue. Furthermore the disruption of blood supply hampers nutrient and oxygen supplies and eliminates endocrine signaling.
In Vitro Spermatogenesis Using Cell Suspensions
Cell culture using enzymatically dispersed cell suspensions provide good support of the cells in vitro when modern cell culture media and incubators are used. However single cell suspensions do not resemble the cellular interactions and structural conditions of the seminiferous epithelium. An ideal culture approach would contain a mixture of somatic and germ cells resembling the spatial arrangements inside the seminiferous epithelium. Such a system would have to ensure the maintenance of the spermatogonial stem cell and its stem cell niche providing appropriate balance of self-renewal and differentiation in the premeiotic phase of spermatogenesis. It should also provide the support of meiotic and postmeiotic germ cells which is mainly provided by Sertoli cells in vivo.
Isolated human testicular germ cells in conventional two dimensional dishes differentiated to some degree but full spermatogenic progression from spermatogonia into mature gametes was never achieved.Citation26 In co-culture with Vero cell (human fibroblasts) as feeder layers, human round spermatids underwent spermiogenesisCitation27 and human spermatogonia and spermatocytes co-cultured with Sertoli cells in Vero cell conditioned medium supplemented with FSH and testosterone completed meiosis and developed into late spermatids. Both FSH and testosterone support the differentiation of germ cells most likely via Sertoli cells, pointing out the importance of both, the presence of somatic cells supporting germ cells in culture and the need for endocrine signaling. This demonstrated for the first time that early meiotic mammalian germ cells can complete meiosis into haploid cells in vitro.Citation26,Citation28-Citation30 In rats, FSH and testosterone enhance the number of round spermatids in the culture of middle and late pachytene spermatocytes when cocultured with Sertoli cells. Culture conditions had a positive effect on meiotic divisions and reduced the percentage of apoptotic germ cells.Citation31 Co-culture of Sertoli cells and spermatogenic cells of pubertal rats in the alternating presence of high/low FSH concentrations induce the attachment of spermatogenic cells to Sertoli cells, the proliferation of premeiotic cells and the differentiation of cells in the meiotic prophase.Citation32 Iwanami et al. showed that type A spermatogonia of immature rats differentiate into round spermatid-like cells when they are co-cultured with Sertoli cells although the in vitro generated spermatids had abnormal gene expression patterns and microinsemination did not result in offspring.Citation33
In the presence of laminin, a component of the extracellular matrix of the seminiferous tubule, Sertoli cells re-aggregate and form organized monolayer structures. In contrast peritubular cells are not affected by FSH or laminin.Citation34 Sertoli cells can recapitulate morphogenesis of seminiferous cords in vitro and may therefore be capable to form functional environment in vitro.Citation14 Co-cultivation of Sertoli cells with germ cells might therefore provide options for restoring spermatogenesis. In the zebra fish (Danio rerio) the complete process of spermatogenesis was demonstrated when germ cells were co-cultured with Sertoli cells.Citation35
Xeno-transplantation of reconstructed seminiferous cords in vitro provides another option to generate functional testicular tissue. Xenografting of newborn testis fragments enabled the generation of spermatozoa from various species.Citation14,Citation36-Citation39 In an experiment performed by Honaramooz et al., testicular cells isolated from piglet testes were centrifuged and the pellets obtained were grafted into nude mice hosts where they formed functional testicular tissues after few months.Citation40 Similar results were achieved in rodents opening up novel strategies for testicular morphogenesis (for review, see ref. Citation41).
Three-Dimensional Culture Systems
The increasing knowledge gained from organ culture and conventional cell culture resulted in the hypothesis, that apart from growth factors and the presence of somatic testicular cells the spatial condition in testicular cell cultures might play a role, allowing the cells to rearrange and thereby also to reassemble the sequence of processes provided by the compartmentalized seminiferous epithelium. A three-dimensional environment of extracellular matrix components might reflect the testicular environment closer than a two-dimensional plastic surface in a culture dishCitation42 or might provide options for reaggregation of more complex tissue. Many extracellular matrix components (e.g., calcium alginate, matrigel, soft agar, methylcellulose or collagen) can be used. One approach using a single cell suspension obtained from neonatal bulls aimed at providing support in three dimensions by encapsulation of Sertoli and germ cells with a polysaccharide calcium alginate forming a hydrogel capsule around the cells. The aggregation of the cells in those capsules resembled the cell density and a spatial arrangement similar to that found in the seminiferous tubules.Citation43 Collagen gel provides a structure that mimics the natural protein structure of extracellular matrix found in living tissues. Using collagen gel matrix, germ cells of 18 d old rats co-cultured with all testicular somatic cell types underwent meiosis and differentiated into a post-meiotic stage after 22 d of culture. The viability of cells was better when seeded during shaking, very likely because of the increased oxygen supply.Citation44
Very few attempts have even been conducted to culture human male germ cells. In a study with male germ cells from non-obstructive azoospermic men, cells expressed postmeiotic genes after cultivation in calcium alginate capsules but did not develop into mature gametes. These cultures could not be maintained long-term.Citation43,Citation44 The cultivation of spermatogenic cells and somatic cells of non-obstructive azoospermic men in a collagen gel matrix revealed progression of meiotic cells into haploid spermatids after 12 d of culture.Citation42
The development of testicular cell culture approaches in three-dimensional matrices had always benefited from similar approaches that had been developed for different cell types. Thus, the three-dimensional Soft-Agar-Culture-System (SACS) was originally set up to examine proliferation and differentiation of bone marrow and hematopoietic cells in vitro.Citation45,Citation46 The semi-solid soft agar medium could be successfully used to propagate progenitor cells of the myeloid lineage that formed colonies and developed into granulocytes and macrophages in vitro.Citation45 We and others adapted the SACS and experimentally addressed its properties for the establishment of murine germ line differentiation in vitro.Citation3,Citation4,Citation7 SACS can be used as bi- or monophasic system and allows several options of manipulation, e.g., the separated analysis of somatic testicular cells or endocrine support. As the SACS does not enable flow cytometric analysis, we additionally used the methylcellulose matrix system (MCS), which allowed retrieval of cells post culture and therefore analysis of additional endpoints i.e., ploidy of the cultured cells. In proof of principle approaches on mouse cell cultures we demonstrated that spermatogonia underwent meiosis and differentiated into morphologically mature spermatozoa. Germ cell differentiation was assessed using histology, immunohistochemistry and gene expression profiling. Ploidy analysis additionally indicated that the conversion from B spermatogonia into spermatocytes was maintained in culture for several weeks when gonadotropins were added. From our experiments we concluded that the matrix as such is less important as long as spatial support and somatic cell contact is provided for the germ cells. However, as we obtained contradictory results concerning the need for endocrine support, this has to be addressed carefully in future studies.Citation3,Citation4,Citation7
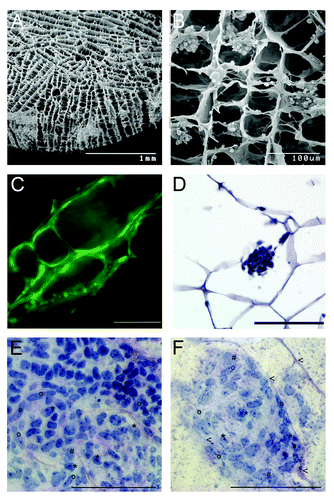
New concepts are created to generate an artificial testis by using scaffolds. For this purpose various scaffold types have been adapted. For example, carbon nanotubes in which the spermatogonial cells survived, maintained their shape and stayed adhered to the scaffold for three weeks of culture.Citation47 The most advanced technique in tissue engineering has been set up for the reconstruction of bone tissue using biodegradable sponges generated from gelatin or collagen or mixtures of those ECM components by incorporating varying amounts of β-tricalcium phosphate. These scaffolds allow migration, proliferation and differentiation of bone cells in the interconnected porous structure of the scaffolds. Agitated seeding methods resulted in homogeneous dispersion and an increased proliferation rate because the circulating medium seems to support an increased oxygen and nutrient supply.Citation48 Consequently, we conducted experiments combining those scaffold materials never tested for male germ cells so far with the experience made with three dimensional cultures of male germ cells in various matrices. We employ commercially available collagen sponges to be seeded with isolated cells of all testicular cell types obtained from immature mouse (7 dpp) or rat (10 dpp) donors. These sponges provide a three-dimensional porous structure, collagen as a component of ECM and oxygen and nutrient supply as they are placed in the culture medium. During cultivation the sponges reside in the culture medium DMEM-Glutamax (Gibco-Invitrogen) (35°C, 5% CO2). Testicular cells are supplemented with gonadotropins (hCG and FSH 5 IU/l) in some experimental settings. These scaffolds resemble spatial and physiological conditions for the cells to settle and possibly re-assemble in the sponge structures (). So far, we observed that the three-dimensional artificial collagen scaffolds (Matricel, size 5x 1.5 mm) enable the testicular cells to colonize the sponge structures, to form colonies and to survive up to several weeks. Using scanning electron microscopy we found the cells attached to the collagenous surface and histological and immunohistochemical analysis indicated a regular distribution of the various cell types over the entire sponge. Cell migration and formation of cell clusters could be observed by Live Cell imaging System (Pecon, Zeiss) making use of eGFP positive rat and mouse models. The fluorescent cells allowed following the colonization progress in the scaffold along the structure of the fibers up to 63 d, After one week of culture of mouse testicular cells, clusters consist of a mixture of somatic testicular cells (i.e peritubular cells and Sertoli cells) surrounding some undifferentiated germ cells (). After having set up this culture system ongoing systematic experimental settings aim at optimization of seeding concentration and method, endocrine support and nutritive conditions to achieve the goal of regeneration of tubular structures and complete spermatogenesis in vitro.
Figure 2. Artificial collagen sponges seeded with isolated testicular cells of mice (7 dpp). (A) Scanning electron microscope image of a collagen sponge prior to culture. (B) Scanning electron microscope image of a colonized collagen sponge. Isolated testicular cells (2 million) established aggregates in the structure of the sponge after one day of culture. (C) Testicular cells of eGFP mice on a collagen sponge after three days of culture. The cells have colonized the collagen scaffold along the given structure. Scale bar represents 100 µm. (D) Micrographs of a section of a paraffin embedded collagen sponge (hematoxylin staining) Scale bar represents 100 µm. (E, F) Histological micrographs for morphological identification of mouse testicular cell types after seven days of culture. Samples were embedded in resin (Technovit, Heraeaus Kulzer, GmbH, Wehrheim, Germany); stained with perjodic acid Schiff reagent; and cut to 3µm sections (protocol according to ref. Citation49). Scale bar represents 100 µm. (E) Cell clusters consist of a mixture of testicular cells. i.e peritubular cells (o), Sertoli cells (*) and some undifferentiated germ cells (#). (F) During colonization of the scaffolds, first signs of tubulogenic reassembly occur. The cells utilize the scaffold for structural reorganization when attaching to the collagen fibers (arrowheads).
Concluding Remarks
To date, in vitro spermatogenesis is still restricted to a limited experimental level. However, the first goals have been achieved in a proof of principle manner providing the perspective that the main requirements of a functional system might be solved: First, to maintain SSCs in self renewing and conversion potential; second, to reconstruct cell-cell relationships between somatic and germ line cells in vitro and finally to produce efficient number of sperm for assisted reproductive techniques.
Organ culture has been shown to at least maintain spermatogenesis for a certain period and has allowed the use of gametes for offspring production. Three dimensional testicular cell culture could provide morphologically mature sperm and has shown that the tendency to self-assemble in testicular cells is maintained under culture condition to some extent (see ).
The fiction of effective in vitro spermatogenesis resembling the sequential and compartmented processes in vitro could possibly become reality in the future by combining the advantages of organ culture and single cell culture approaches. The challenge to a successful culture system is to replicate the in vivo situation in the testis i.e., to achieve the spatial environment that allows the reassembling of testicular cells as well as the nutritive and endocrine support beyond the living organism. A promising novel option for spermatogenesis in vitro is the application of artificial scaffolds which replace the spatial environment in the seminiferous tubule and allow the reassembling of testicular cells in the three-dimensional structure.
Acknowledgments
K.R. and J.W. received support for the reported work by a grant from the medical faculty of the University of Münster (IMF Project No. WI 120907).
References
- Goldschmidt R. Some experiments on spermatogenesis in vitro. Proc Natl Acad Sci USA 1915; 1:220 - 2; http://dx.doi.org/10.1073/pnas.1.4.220; PMID: 16575981
- Steinberger E, Steinberger A, Perloff WH. Initiation of spermatogenesis in vitro. Endocrinology 1964; 74:788 - 92; http://dx.doi.org/10.1210/endo-74-5-788; PMID: 14168569
- Stukenborg JB, Schlatt S, Simoni M, Yeung CH, Elhija MA, Luetjens CM, et al. New horizons for in vitro spermatogenesis? An update on novel three-dimensional culture systems as tools for meiotic and post-meiotic differentiation of testicular germ cells. Mol Hum Reprod 2009; 15:521 - 9; http://dx.doi.org/10.1093/molehr/gap052; PMID: 19561342
- Stukenborg JB, Wistuba J, Luetjens CM, Elhija MA, Huleihel M, Lunenfeld E, et al. Coculture of spermatogonia with somatic cells in a novel three-dimensional soft-agar-culture-system. J Androl 2008; 29:312 - 29; http://dx.doi.org/10.2164/jandrol.107.002857; PMID: 18046051
- Gassei K, Ehmcke J, Schlatt S. Initiation of testicular tubulogenesis is controlled by neurotrophic tyrosine receptor kinases in a three-dimensional Sertoli cell aggregation assay. Reproduction 2008; 136:459 - 69; http://dx.doi.org/10.1530/REP-08-0241; PMID: 18660385
- Sato T, Katagiri K, Gohbara A, Inoue K, Ogonuki N, Ogura A, et al. In vitro production of functional sperm in cultured neonatal mouse testes. Nature 2011; 471:504 - 7; http://dx.doi.org/10.1038/nature09850; PMID: 21430778
- Abu Elhija M, Lunenfeld E, Schlatt S, Huleihel M. Differentiation of murine male germ cells to spermatozoa in a soft agar culture system. Asian J Androl 2012; 14:285 - 93; http://dx.doi.org/10.1038/aja.2011.112; PMID: 22057383
- Sato T, Katagiri K, Yokonishi T, Kubota Y, Inoue K, Ogonuki N, et al. In vitro production of fertile sperm from murine spermatogonial stem cell lines. Nat Commun 2011; 2:472; http://dx.doi.org/10.1038/ncomms1478; PMID: 21915114
- Wistuba J, Stukenborg JB, Luetjens C. Mammalian spermatogenesis. Funct Dev Embryol 2007; 1:99 - 117
- de Rooij DG. The spermatogonial stem cell niche. Microsc Res Tech 2009; 72:580 - 5; http://dx.doi.org/10.1002/jemt.20699; PMID: 19263493
- Ehmcke J, Wistuba J, Schlatt S. Spermatogonial stem cells: questions, models and perspectives. Hum Reprod Update 2006; 12:275 - 82; http://dx.doi.org/10.1093/humupd/dmk001; PMID: 16446319
- Hofmann MC. Gdnf signaling pathways within the mammalian spermatogonial stem cell niche. Mol Cell Endocrinol 2008; 288:95 - 103; http://dx.doi.org/10.1016/j.mce.2008.04.012; PMID: 18485583
- Araki Y, Sato T, Katagiri K, Kubota Y, Araki Y, Ogawa T. Proliferation of mouse spermatogonial stem cells in microdrop culture. Biol Reprod 2010; 83:951 - 7; http://dx.doi.org/10.1095/biolreprod.109.082800; PMID: 20702852
- Gassei K, Schlatt S, Ehmcke J. De novo morphogenesis of seminiferous tubules from dissociated immature rat testicular cells in xenografts. J Androl 2006; 27:611 - 8; http://dx.doi.org/10.2164/jandrol.05207; PMID: 16582408
- Meng X, Lindahl M, Hyvönen ME, Parvinen M, de Rooij DG, Hess MW, et al. Regulation of cell fate decision of undifferentiated spermatogonia by GDNF. Science 2000; 287:1489 - 93; http://dx.doi.org/10.1126/science.287.5457.1489; PMID: 10688798
- Siu MK, Cheng CY. Extracellular matrix: recent advances on its role in junction dynamics in the seminiferous epithelium during spermatogenesis. Biol Reprod 2004; 71:375 - 91; http://dx.doi.org/10.1095/biolreprod.104.028225; PMID: 15115723
- Huckins C. The spermatogonial stem cell population in adult rats. I. Their morphology, proliferation and maturation. Anat Rec 1971; 169:533 - 57; http://dx.doi.org/10.1002/ar.1091690306; PMID: 5550532
- de Rooij DG, Russell LD. All you wanted to know about spermatogonia but were afraid to ask. J Androl 2000; 21:776 - 98; PMID: 11105904
- Russell LD, Steinberger A. Sertoli cells in culture: views from the perspectives of an in vivoist and an in vitroist. Biol Reprod 1989; 41:571 - 7; http://dx.doi.org/10.1095/biolreprod41.4.571; PMID: 2695173
- Kawamoto T, Kawai K, Kodama T, Yokokura T, Niki Y. Autonomous differentiation of Drosophila spermatogonia in vitro. Dev Growth Differ 2008; 50:623 - 32; http://dx.doi.org/10.1111/j.1440-169X.2008.01060.x; PMID: 18657168
- Trowell OA. The culture of mature organs in a synthetic medium. Exp Cell Res 1959; 16:118 - 47; http://dx.doi.org/10.1016/0014-4827(59)90201-0; PMID: 13639945
- Boitani C, Politi MG, Menna T. Spermatogonial cell proliferation in organ culture of immature rat testis. Biol Reprod 1993; 48:761 - 7; http://dx.doi.org/10.1095/biolreprod48.4.761; PMID: 8485240
- Parvinen M, Wright WW, Phillips DM, Mather JP, Musto NA, Bardin CW. Spermatogenesis in vitro: completion of meiosis and early spermiogenesis. Endocrinology 1983; 112:1150 - 2; http://dx.doi.org/10.1210/endo-112-3-1150; PMID: 6822207
- Steinberger A, Steinberger E. In vitro culture of rat testicular cells. Exp Cell Res 1966; 44:443 - 52; http://dx.doi.org/10.1016/0014-4827(66)90451-4; PMID: 5337205
- Oatley JM, de Avila DM, Reeves JJ, McLean DJ. Testis tissue explant culture supports survival and proliferation of bovine spermatogonial stem cells. Biol Reprod 2004; 70:625 - 31; http://dx.doi.org/10.1095/biolreprod.103.022483; PMID: 14585812
- Tesarik J, Greco E, Rienzi L, Ubaldi F, Guido M, Cohen-Bacrie P, et al. Differentiation of spermatogenic cells during in-vitro culture of testicular biopsy samples from patients with obstructive azoospermia: effect of recombinant follicle stimulating hormone. Hum Reprod 1998; 13:10 2772 - 81; http://dx.doi.org/10.1093/humrep/13.10.2772; PMID: 9804229
- Cremades N, Bernabeu R, Barros A, Sousa M. In-vitro maturation of round spermatids using co-culture on Vero cells. Hum Reprod 1999; 14:1287 - 93; http://dx.doi.org/10.1093/humrep/14.5.1287; PMID: 10325279
- Sousa M, Cremades N, Alves C, Silva J, Barros A. Developmental potential of human spermatogenic cells co-cultured with Sertoli cells. Hum Reprod 2002; 17:161 - 72; http://dx.doi.org/10.1093/humrep/17.1.161; PMID: 11756382
- Tanaka A, Nagayoshi M, Awata S, Mawatari Y, Tanaka I, Kusunoki H. Completion of meiosis in human primary spermatocytes through in vitro coculture with Vero cells. Fertil Steril 2003; 79:Suppl 1 795 - 801; http://dx.doi.org/10.1016/S0015-0282(02)04833-1; PMID: 12620493
- Xie B, Qin Z, Huang B, Xie T, Yao H, Wei Y, et al. In vitro culture and differentiation of buffalo (Bubalus bubalis) spermatogonia. Reprod Domest Anim 2010; 45:275 - 82; http://dx.doi.org/10.1111/j.1439-0531.2008.01281.x; PMID: 19090820
- Vigier M, Weiss M, Perrard MH, Godet M, Durand P. The effects of FSH and of testosterone on the completion of meiosis and the very early steps of spermiogenesis of the rat: an in vitro study. J Mol Endocrinol 2004; 33:729 - 42; http://dx.doi.org/10.1677/jme.1.01493; PMID: 15591031
- Tres LL, Kierszenbaum AL. Viability of rat spermatogenic cells in vitro is facilitated by their coculture with Sertoli cells in serum-free hormone-supplemented medium. Proc Natl Acad Sci USA 1983; 80:3377 - 81; http://dx.doi.org/10.1073/pnas.80.11.3377; PMID: 6407012
- Iwanami Y, Kobayashi T, Kato M, Hirabayashi M, Hochi S. Characteristics of rat round spermatids differentiated from spermatogonial cells during co-culture with Sertoli cells, assessed by flow cytometry, microinsemination and RT-PCR. Theriogenology 2006; 65:288 - 98; http://dx.doi.org/10.1016/j.theriogenology.2005.04.025; PMID: 15964619
- Schlatt S, de Kretser DM, Loveland KL. Discriminative analysis of rat Sertoli and peritubular cells and their proliferation in vitro: evidence for follicle-stimulating hormone-mediated contact inhibition of Sertoli cell mitosis. Biol Reprod 1996; 55:227 - 35; http://dx.doi.org/10.1095/biolreprod55.2.227; PMID: 8828824
- Sakai N. [In vitro spermatogenesis in zebrafish]. Tanpakushitsu Kakusan Koso 2007; 52:Suppl 2124 - 9; PMID: 21089282
- Mota PC, Ramalho-Santos J, Schlatt S. Xenografting as a tool to preserve endangered species: Outcomes and challenges in model systems. Vet Med Int 2010; 2011:629409
- Abbasi S, Honaramooz A. Xenografting of testis tissue from bison calf donors into recipient mice as a strategy for salvaging genetic material. Theriogenology 2011; 76:607 - 14; http://dx.doi.org/10.1016/j.theriogenology.2011.03.011; PMID: 21529912
- Rodriguez-Sosa JR, Rathi R, Wang Z, Dobrinski I. Development of bovine fetal testis tissue after ectopic xenografting in mice. J Androl 2011; 32:271 - 81; http://dx.doi.org/10.2164/jandrol.110.010322; PMID: 20930193
- Brinster RL. Germline stem cell transplantation and transgenesis. Science 2002; 296:2174 - 6; http://dx.doi.org/10.1126/science.1071607; PMID: 12077400
- Honaramooz A, Megee SO, Rathi R, Dobrinski I. Building a testis: formation of functional testis tissue after transplantation of isolated porcine (Sus scrofa) testis cells. Biol Reprod 2007; 76:43 - 7; http://dx.doi.org/10.1095/biolreprod.106.054999; PMID: 17005941
- Gassei K, Schlatt S. Testicular morphogenesis: comparison of in vivo and in vitro models to study male gonadal development. Ann N Y Acad Sci 2007; 1120:152 - 67; http://dx.doi.org/10.1196/annals.1411.015; PMID: 18184913
- Lee JH, Gye MC, Choi KW, Hong JY, Lee YB, Park DW, et al. In vitro differentiation of germ cells from nonobstructive azoospermic patients using three-dimensional culture in a collagen gel matrix. Fertil Steril 2007; 87:824 - 33; http://dx.doi.org/10.1016/j.fertnstert.2006.09.015; PMID: 17239867
- Lee DR, Kaproth MT, Parks JE. In vitro production of haploid germ cells from fresh or frozen-thawed testicular cells of neonatal bulls. Biol Reprod 2001; 65:873 - 8; http://dx.doi.org/10.1095/biolreprod65.3.873; PMID: 11514353
- Lee JH, Kim HJ, Kim H, Lee SJ, Gye MC. In vitro spermatogenesis by three-dimensional culture of rat testicular cells in collagen gel matrix. Biomaterials 2006; 27:2845 - 53; http://dx.doi.org/10.1016/j.biomaterials.2005.12.028; PMID: 16430959
- Horowitz D, King AG. Colorimetric determination of inhibition of hematopoietic progenitor cells in soft agar. J Immunol Methods 2000; 244:49 - 58; http://dx.doi.org/10.1016/S0022-1759(00)00253-2; PMID: 11033018
- Lin HS, Kuhn C, Kuo T. Clonal growth of hamster free alveolar cells in soft agar. J Exp Med 1975; 142:877 - 86; http://dx.doi.org/10.1084/jem.142.4.877; PMID: 1080792
- Rafeeqi T, Kaul G. Carbon nanotubes as a scaffold for spermatogonial cell maintenance. J Biomed Nanotechnol 2010; 6:710 - 7; http://dx.doi.org/10.1166/jbn.2010.1167; PMID: 21361137
- Takahashi Y, Yamamoto M, Tabata Y. Osteogenic differentiation of mesenchymal stem cells in biodegradable sponges composed of gelatin and beta-tricalcium phosphate. Biomaterials 2005; 26:3587 - 96; http://dx.doi.org/10.1016/j.biomaterials.2004.09.046; PMID: 15621249
- Wistuba J, Brinkworth MH, Schlatt S, Chahoud I, Nieschlag E. Intrauterine bisphenol A exposure leads to stimulatory effects on Sertoli cell number in rats. Environ Res 2003; 91:95 - 103; http://dx.doi.org/10.1016/S0013-9351(02)00019-1; PMID: 12584010
- Mitchell RT, Saunders PT, Sharpe RM, Kelnar CJ, Wallace WH. Male fertility and strategies for fertility preservation following childhood cancer treatment. Endocr Dev 2009; 15:101 - 34; http://dx.doi.org/10.1159/000207612; PMID: 19293606
- Steinberger A, Steinberger E. Differentiation of rat seminiferous epithelium in organ culture. J Reprod Fertil 1965; 9:243 - 8; http://dx.doi.org/10.1530/jrf.0.0090243; PMID: 14273758