Abstract
Primary cell culture is an established and widely used technique to study Sertoli cell function in vitro. However, the relative difficulty of stably overexpressing or knocking down genes in Sertoli cell culture has limited progress in the field. In this technical report, we present a method to transduce 20 dpp rat Sertoli cell cultures with VSV-G pseudotyped lentiviral based vectors at a high rate (~80%), with stable reporter gene expression. Although high transgene expression is desirable, it was noted that at transduction rates > 60% inter-Sertoli cell tight junction integrity and, hence, Sertoli cell function, were transiently compromised. We envisage that this optimized procedure has the potential to stimulate Sertoli cell research, and motivate the use of Sertoli cells in various cell therapy applications.
Introduction
The somatic Sertoli cell of the testis provides structural and nutritional support to germ cells in the process of spermatogenesis. The specialized “nurse” functions of the Sertoli cell has been studied extensively in vitro since the first isolation and culture of primary cells in the 1970s.Citation1-Citation3 Various techniques to manipulate Sertoli cell gene expression, including transfection have been used,Citation4,Citation5 but the efficiency nor longevity of these methods is rarely reported as they do not lead to stable genomic integration.
Recently, adenovirus vectors have been used to transduce Sertoli cells in vivo,Citation6,Citation7 although low rates of transduction and cellular toxicity were noted. An alternative virus of the retroviridae family, lentivirus, possess the inherent capacity to transduce and stably integrate into non-dividing cells.Citation8,Citation9 Recently, retroviral transduction was used to overexpress a panel of neuronal transcription factors in proliferative phase Sertoli cells, directing the cells toward an induced neural stem/progenitor lineage.Citation10 This study demonstrated the viability and potential use of retroviral vector transgene expression in primary juvenile Sertoli cells. Lentiviral based vectors have also been used to transduce Sertoli cells in an elegant in vivo study, in which the overexpression kit ligand in c-kit lignad null mice was able to restore fertility.Citation6
Our laboratory has extensive experience in primary rat Sertoli cell culture as a model to study the regulation of cell junctions,Citation11,Citation12 growth factor signalingCitation13 and gene expression,Citation14 and we are currently utilizing a lentiviral based vector approach to manipulate Sertoli cell gene function in vitro. In early experiments, we noted that rat Sertoli cell monolayers were relatively inert to retroviral transduction. Herein, we describe a modified inoculation and centrifugation protocol (“spinoculation”),Citation15,Citation16 which considerably improves Sertoli cell transduction in vitro.
Methods
Lentiviral vector production
To produce VSV-G-pseudotyped lentiviral based vectors capable of expressing the green fluorescent protein (GFP) reporter gene, HEK293T cells were transfected with the minimal lentivirus genomic requirements, divided across four DNA plasmids, to produce lentiviral based vectors capable of cell transduction.Citation17 The transfer plasmid encoding reporter GFP protein used in these experiments was pSIH-H1-copGFP, with GFP expression under the control of a CMV promoter (System Biosciences). The remainder of the viral genome was encoded by an HIV gag/pol packaging plasmid, a rev expression plasmid, and the VSV-G expression plasmid.Citation17 Transfection of HEK293T cells at 60% confluence proceeded for 24 h, with subsequent medium replacement and virus collection 48 h later. Supernatant (50 ml) was filtered through 0.2 µm filters, and virus concentrated by ultracentrifugal pelleting at 20,000 g for 16 h at 4°C. The lentiviral based vector was resuspended in 100 μl PBS and stored at -80°C. The functional titer of viral aliquots was assayed by serial limiting dilution on HEK293T cells and measurement of reporter gene expression (GFP) by flow cytometry to determine the number of transducing units (TU/ml) as previously described.Citation18 This viral production procedure regularly gave yields of ≥ 1 × 109 TU/ml when assayed on 293T cells. The pSIH transfer vector used in these experiments generates lentiviral based vectors with an insert size of 4,267 bp.
Primary cell culture and transduction
Sertoli cells were isolated as previously described.Citation13 Briefly, Sertoli cells from 20 dpp Sprague Dawley rats were plated at 6.13 × 105 cells/cm2 at 37°C in 24-well culture plates (Nalge Nunc) or into Millicell PCF bicameral chambers (12mm diameter, 0.4 µm pore size, 0.6 cm2 surface area; Millipore) pre-coated with Matrigel (BD Biosciences), for measurement of transepithelial electrical resistance (TER). Cell cultures proceeded in DMEM/Hams F12 (Gibco) supplemented with non-essential amino acids (10 mM, Gibco), dialysed bovine serum albumin (1% w/v, Sigma), HEPES (10 mM, Gibco), insulin (5 μg/ml, Novo-Nordisk), transferrin (5 μg/ml, Sigma), sodium selenite (50 ng/ml, Sigma), penicillin (200 U/ml)-streptomycin (200 μg/ml)-fungizone (0.5 μg/ml) (CSL), testosterone (28 ng/ml, Sigma) and FSH (150 mIU/ml, Puregon, N.V. Organon). After 72 h, Sertoli cell cultures were hypotonically shocked with 10% DMEM/F12 in distilled H2O for 45 sec to remove contaminating germ cells, and then replaced with culture medium. Sertoli cell cultures using this method are typically 92% pure after 5 d, with the contaminant cells predominantly being peritubular myoid cells, or residual germ cells.Citation19 In initial experiments, 5 × 106 TU/ml lentiviral based vectors were applied directly to established Sertoli cell monolayers immediately following hypotonic shock (72 h after commencement of culture). The virus containing media was subsequently replaced 24 h later with fresh medium. Cells were then cultured for at least a further 48 h to allow for reporter gene expression. Transgene expression was confirmed by live imaging of GFP fluorescence. Cells were removed from cell culture for DNA extraction and flow cytometry (see below) using a combination of trypsin, collagenase and hyaluronidase (2 mg/ml each, Sigma) for 20 min at 37°C, in an orbital shaker at 180 r.p.m. The enzymatic activity was then quenched using 10% FCS, and the cells washed and pelleted by centrifugation at 500 g at 4°C for 5 min.
Centrifugation and inoculation (“spinoculation”) of primary Sertoli cell cultures with lentivirus-based vectors was performed using an Eppendorf plate centrifuge at 1,000 g at room temperature for 30 min.
Assessment of inter-Sertoli cell tight junction integrity
Tight junction function was assessed by measurement of transepithelial electrical resistance (TER) of cells plated in bicameral chambers (EMD Millipore) on a daily basis at 37°C using a Millicell-electrical resistance system (EMD Millipore), as previously described.Citation13
Genomic DNA extraction, RNA extraction and qPCR
DNA was extracted from Sertoli cell pellets using an isopropanol precipitation method.Citation20,Citation21 RNA was extracted using Trizol following the manufacturer’s instructions (Life Technologies). Contaminating DNA was removed using a DNase free kit (Life Technologies), RNA concentration quantified using a nanodrop spectrophotometer (Thermo Scientific) and reverse transcribed using SuperScript-VILO (Life Technologies). Quantitative real-time PCR analysis was performed using the Roche Lightcycler 380 (Roche) with the FastStart DNA Master SYBR-Green 1 polymerase (Roche). Oligonucleotide primers specific to the GFP transfer vector (pSIH-cop-GFP, F: 5′-AATGTCTTTGGATTTGGGAATCTTAT-3′, R: 5′-TGGTCTAACCAGAGAGACCCAGTA-3′) were used to determine viral DNA incorporation, or RNA expression, by comparing expression against reference DNA (β-actin, F: 5′-CCGTAAAGACCTCTATGCCAACA-3′, R: 5′-GATTACTGCCCTGGCTCCTAGC-3′), using previously published conditions.Citation22
Flow cytometry
Following cell harvesting, cells were washed once in 0.5% BSA/PBS (%w/v), and fixed in 4% paraformaldehyde for 10 min at room temperature. Cells were subsequently washed and resuspended in 0.5% BSA/PBS. Cell population and reporter GFP expression were analyzed using a LSRII flow cytometer (BD Biosciences) and summary data of at least 30,000 cells per data point collated using FlowJo software (Tree Star Inc.).
Immunocytochemistry and microscopy
Sertoli cell monolayers cultured on Millicell bicameral chambers (EMD Millipore) were washed once in PBS, and then fixed in 4% paraformaldehyde in PBS for 30 min at room temperature. Cells were then washed in PBS, and permeabilised with 0.05% (v/v) Triton X-100 in PBS for 5 min on ice. The chamber was then washed in PBS and stored at 4°C until use. Immunocytochemistry was performed by first blocking non-specific binding sites with CAS block (Zymed) containing 10% normal goat serum (Vector Laboratories) for 20 min. Primary antiserum (rat anti-Gata4 1:500, C#14–9980, eBioscience; rabbit anti-Cldn11 1:200 C#36–4500 and rabbit-Tjp1 1:200 #61–7300, Zymed Laboratories) was applied and incubated for 16 h at 4°C. Cell monolayers were then washed, and secondary antisera (goat anti-rat AlexaFluor-568, goat anti-rabbit AlexaFluor-647, Molecular Probes) applied. Confocal analysis was performed using an Olympus Fluoview FV300 confocal system attached to an Olympus IX 81 inverted microscope.
Statistics
All measurements were conducted with replicates from which the mean and SD were calculated. Statistics were performed using SigmaStat version 3.5 (Systat Software, Inc.). Before analysis, homogeneity of variance was confirmed, and assessed using a one-way ANOVA, followed by the Student-Newman-Keuls post hoc multiple group comparisons test for significance. A p-value of ≤ 0.05 was used as a measure of statistical significance for all experiments.
Results
Optimization of Sertoli cell transduction procedure
To confirm that the lentiviral preparations could express transgene-GFP in a model cell line, HEK293T cells were transduced with limiting dilutions of a GFP-virus. Using a titer of 5 x 106 TU/ml, robust GFP fluorescence was observed by live cell imaging () and quantitative flow cytometry () after 48 h. When a similar protocol was employed in primary Sertoli cells 72 h after isolation, using an identical viral titer of GFP-virus, we determined that transduction efficiency of primary Sertoli cells was very low by live cell imaging of reporter GFP fluorescence (), which equated to 3% GFP+ cells by flow cytometry (). To improve transduction efficiency in primary Sertoli cell cultures, we performed viral transduction in combination with centrifugation of the culture plate at 1,000 g for 30 min at room temperature in a procedure known as ‘spinoculation’.Citation15 This procedure initially improved transduction efficiency by 9-fold, as measured by genomic incorporation of the lentiviral GFP transfer vector by real-time PCR (, p < 0.01). We next performed this viral transduction in combination with the poly-cationic reagent, polybrene (4 μg/ml, Sigma), at the time of viral addition. The addition of polybrene resulted in a further improvement of transduction and genomic incorporation by 1.6-fold, to 14-fold above the initial level (, p = ns). During primary Sertoli cell culture, tight junctions become established within 72 h of culture,Citation12,Citation13 and we reasoned that tight junction formation may limit transduction efficiency. We subsequently repeated viral transduction 24 h after commencement of cell culture, rather than after 72 h of culture, and noted a further improvement in genomic incorporation by 12.4-fold, with a total improvement in transduction efficiency of 222-fold above basal conditions ( < 0.01). Using alternative combinations of the optimization steps outlined above, we consistently observed that the combination of centrifugation, polybrene and addition of lentiviral vectors 24 h after the initial culture produced the greatest enhancement in viral transduction (data not shown). Live cell imaging () and flow cytometry () confirmed that the increased genomic transduction under optimized conditions also resulted in a significant improvement in the proportion of reporter GFP-fluorescent cells (from 3 to 64%, ).
Figure 1. Transduction of primary Sertoli cells using lentvirus vector. (A) Live reporter GFP fluorescence of HEK293T cells transduced at 5 × 106 TU/ml (B) Flow cytometric analysis of control (no virus, black) and lentivirus transduced HEK293T cells (red). (C) Live GFP fluorescence of Sertoli cells transduced with 5 × 106 TU/ml prior to and (D) after optimization. (E) Flow cytometric analysis of Sertoli cells prior to (red) and after (blue) optimization. (F) Expression of pSIH transfer vector normalized to β-actin in primary Sertoli cells 4 d (white) and 14 d (black) after transduction. (G) Experimental outline of optimized lentiviral vector transduction procedure in primary Sertoli cell culture. Scale bar = 50 μm.
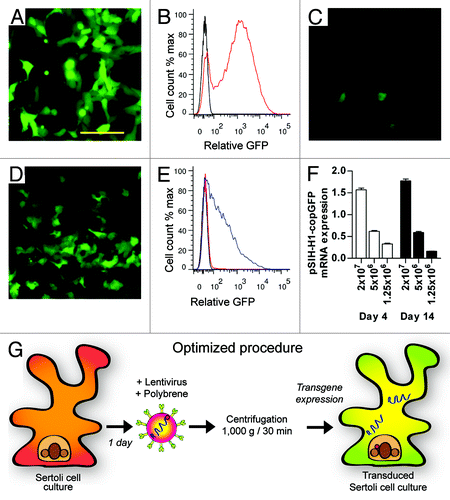
Table 1. Fold increase in genomic incorporation of lentiviral transfer vector by qPCR.
Stability of DNA incorporation was determined by examining the expression of the lentiviral transfer vector in transduced primary Sertoli cells at 3 and 14 d (). Using a range of viral titers, expression was dependent upon lentiviral titer administration, but remained consistent across this time course. An outline of the optimized transduction procedure is presented in .
Effect of high transduction rate upon tight junction integrity
Flow cytometric analysis of reporter GFP fluorescence indicated that our optimized procedure resulted in Sertoli cell transduction rates ~80% at high titer (2 × 107 TU/ml, ). We noted that increased lentiviral vector titer allowed for a parallel increase in reporter gene expression (). As a surrogate measure of Sertoli cell function, we assessed the integrity of tight junctions following lentiviral vector transduction by real-time measurement of transepithelial electrical resistance (TER).Citation13 Interestingly, monolayers with a high titer of virus and reporter GFP fluorescence exhibited reduced tight junction integrity (). When tight junction function was assessed over an eight-day period, TER function only recovered to control levels with titers ≤ 5 × 106 TU/ml after one week ().
Figure 2. Effect of lentivirus administration on inter-Sertoli cell tight junction. (A) Transepithelial electrical resistance (%TER of control monolayers, white boxes) taken 48 h after Sertoli cell transduction with between 1.25 × 106 and 2 × 107 TU/ml. GFP reporter fluorescence was determined by flow cytometry (% GFP+ cells, flow cytometry, black circles). Increased lentivirus addition (and increased %GFP+ cells) is associated with loss of TER. TER data are mean, ± SD, n = 3, letters denote significant difference p < 0.01. (B) Daily TER of control (black boxes) and lentivirus transduced cells (5 x 106 TU/ml, white boxes) over 8 d culture period. Black arrow indicates lentivirus addition 24 h after primary cell isolation. TER data are mean, ± SD, n = 3, * p < 0.01 between control and transduced cells. (C–F) Co-localization of endogenous reporter gene expression (GFP, green), Gata4 (blue) and tight junction protein (red); (C and D) Tjp1 and (E and F) Cldn11 with 1.25 × 106 or 2 × 107 TU/ml. White arrow indicates TJ co-localization between non-transduced cells, *indicates TJ localization between transduced (GFP) cells, and white arrowhead in (F) indicates reduced Cldn11 localization between adjacent transduced cells after high viral titer addition. Scale bar = 20 μm.
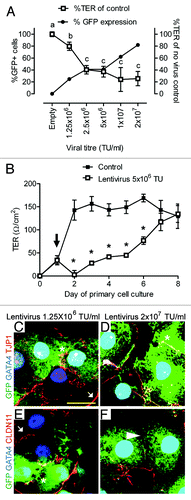
Sertoli cell tight junctions are composed of several transmembrane proteins, including Claudin-11 (Cldn11), bound to intracellular plaque proteins, such as zona occludens-1 (Tjp1). To determine the effect of lentiviral vector transduction upon Sertoli cell tight junctions, cell monolayers were then visualized by the co-localization of reporter GFP fluorescence with the somatic nuclear marker, Gata4, and the Sertoli cell tight junction-related proteins Tjp1 and Cldn11. Reporter gene intensity appeared highly variable between adjacent Sertoli cells (−F), consistent with a broad range of GFP reporter fluorescence observed by flow cytometry (). Immunocytochemical co-localization with Tjp1 indicated a normal localization, consistent with inter-Sertoli cell tight junctions (). However, Cldn11 immunostaining appeared reduced in cells treated with high titer lentiviral vector (). Of particular note, Cldn11 was reduced at inter-Sertoli cell contacts between transduced (GFP, green) cells (), suggesting that the reduced TER following high titer lentiviral vector administration () may be mediated through Cldn11.
Discussion
Use of lentiviral vector transduction to study Sertoli cell gene function
The data presented in this technical report validate lentiviral based vectors as a feasible methodology to manipulate rat Sertoli cell gene function in vitro. Using this optimized procedure, we achieve robust and stable expression of a GFP transgene in primary Sertoli cells through two weeks of culture. Our optimized procedure likely results from an enhanced interaction between lentivirus vectors and Sertoli cells,Citation16 with multiple vectors likely transducing each cell at high titer. Given that lentiviral based vectors are susceptible to gene silencing,Citation23 the increased rate of incorporation achieved with high titer likely overcomes silencing phenomena to allow for more consistent gene expression across individual cells.
The improved efficiency of viral transduction described following “spinoculation” represents a significant improvement upon basal transduction rates. Consistent with this, previous reports have described the necessity to undertake multiple rounds of infection of primary Sertoli cells to ensure high rates of transduction.Citation10 The proportion of transduced cells using this optimized methodology is much greater than previous reports which describe low rates of primary Sertoli cell transfection (25–38%) for overexpression of genes or luciferase assays.Citation5,Citation24 We envisage that this optimized method could be exploited to overexpress genes implicated in Sertoli cell development and dysfunction in primary cell culture. This method may be particularly useful as an alternative to immortalized Sertoli-like cell lines, which do not fully recapitulate Sertoli cell gene expression and function.Citation25 Previous reports indicate that Sertoli cells express the gene silencing machinery required for siRNA mediated gene knockdown,Citation4 and consequently, the protocol outlined in this report could also be utilized to express an shRNA transcript to knockdown gene function in a differentiated Sertoli cell. Furthermore, miRNAs are highly expressed and hormonally-regulated in Sertoli cells, but their functional significance is largely unknown.Citation14 The protocol described here could be easily adapted to overexpress specific miRNA hairpins, or miRNA inhibitors such as “sponges”Citation26 to define the role of these non-coding RNAs in Sertoli cell function.
Given that Sertoli cell tight junction function appears compromised at high viral titer, we aim to transduce 60% of Sertoli cells in culture to maintain optimal Sertoli cell function.
Sertoli cell therapy
Sertoli cell transplantation into diseased organs has been suggested as a cell therapy approach to chronic disease.Citation27 Analogous to their “nurse” cell function within the testis, transplanted Sertoli cells provide immunoprotective, and structural support functions within host tissues.Citation28 In a model of type I diabetes in mice, Sertoli cell therapy has been utilized to prevent, or revert the disease.Citation29,Citation30 Furthermore, Sertoli cell transplantation into the striatum of the brain has been observed to reduce neuronal hyperactivity in a rodent model of early Huntington’s disease,Citation31 and have trophic effects upon dopaminergic neurons in Parkinson disease.Citation32
The ability to engineer Sertoli cells using a retroviral approach may significantly improve the scope and feasibility of Sertoli cell therapy.Citation27,Citation33 This approach was recently demonstrated with the induction of Sertoli cells into a functional, induced neuronal stem/progenitor cell population using virally engineered cells.Citation10 Furthermore, the integration of lentiviral based vectors into post-mitotic cells (such as differentiated Sertoli cells) offers significant safety advantage when compared with proliferative cells, including reduced integration into protein coding, and potentially oncogenic, regions of the genome.Citation34
Safety considerations
Use of HIV-based vectors falls within the NIH biosafety Level 2, and as such, appropriate technical and physical safeguards should be put into practice to protect researchers. Due to the broad tropism of lentivirus, there is the potential for human transmission, and as such, it should be used under strict safety requirements specific to the host institution. Our laboratory utilizes a ‘3rd generation’ vector system, with several enhanced safety features compared with previous generations. Significantly, these vectors encode a self-inactivating (SIN) construct in the 3′ LTR, which prevents the production of wildtype viral particles by infected cells.Citation35 Novel commercially available lentiviral based vector plasmids now frequently utilize inducible promoters, which have enhanced safety and experimental benefits.
Summary
The generation of transgenic mice to selectively knock out Sertoli cell gene products is hampered by financial and time constraints, fertility defects, and by the limited access to inducible Sertoli cell transgenic mouse lines.Citation36 The technique presented in this technical report provides a novel, in-expensive, and rapid method to assess gene function in primary Sertoli cells. The ability to manipulate gene function in Sertoli cells in vitro is an important technical development, which will allow researchers to realize new advances in the field of Sertoli cell biology.
Using this transduction approach, we have stably expressed GFP in primary Sertoli cell cultures, and in un-published observations, confirmed that these cells express the translational and processing “machinery” for shRNA mediated gene knockdown. Finally, this lentiviral based approach could be used in a range of applications, including engineering Sertoli cells for potential cell-based gene therapy.
Acknowledgments
The lentiviral packaging plasmids were a kind gift of William Osborne (University of Washington). This work was supported by an Australian post-graduate award (P.K.N.), National Health and Medical Research Council (Australia) Career Development Fellowship #1013533 (C.A.H.), Project Grant #1009144 (P.G.S. and C.A.H), Program Grant #494802 (P.G.S.), a Pfizer Australia Senior Research Fellowship (P.G.), and by the Victorian Government's Operational Infrastructure Support Program. PHI data audit #12–03.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- Dorrington JH, Fritz IB. Cellular localization of 5alpha-reductase and 3alpha-hydroxysteroid dehydrogenase in the seminiferous tubule of the rat testis. Endocrinology 1975; 96:897 - 89; http://dx.doi.org/10.1210/endo-96-4-879; PMID: 1120478
- Steinberger A, Heindel JJ, Lindsey JN, Elkington JS, Sanborn BM, Steinberger E. Isolation and culture of FSH responsive Sertoli cells. Endocr Res Commun 1975; 2:261 - 72; http://dx.doi.org/10.3109/07435807509053853; PMID: 170059
- Welsh MJ, Wiebe JP. Rat sertoli cells: a rapid method for obtaining viable cells. Endocrinology 1975; 96:618 - 24; http://dx.doi.org/10.1210/endo-96-3-618; PMID: 163729
- Li MW, Mruk DD, Lee WM, Cheng CY. Connexin 43 and plakophilin-2 as a protein complex that regulates blood-testis barrier dynamics. Proc Natl Acad Sci USA 2009; 106:10213 - 8; http://dx.doi.org/10.1073/pnas.0901700106; PMID: 19509333
- González-González E, López-Casas PP, Del Mazo J. Gene silencing by RNAi in mouse Sertoli cells. Reprod Biol Endocrinol 2008; 6:29; http://dx.doi.org/10.1186/1477-7827-6-29; PMID: 18620581
- Ikawa M, Tergaonkar V, Ogura A, Ogonuki N, Inoue K, Verma IM. Restoration of spermatogenesis by lentiviral gene transfer: offspring from infertile mice. Proc Natl Acad Sci USA 2002; 99:7524 - 9; http://dx.doi.org/10.1073/pnas.072207299; PMID: 12032316
- Hooley RP, Paterson M, Brown P, Kerr K, Saunders PT. Intra-testicular injection of adenoviral constructs results in Sertoli cell-specific gene expression and disruption of the seminiferous epithelium. Reproduction 2009; 137:361 - 70; http://dx.doi.org/10.1530/REP-08-0247; PMID: 18955374
- Thomas CE, Ehrhardt A, Kay MA. Progress and problems with the use of viral vectors for gene therapy. Nat Rev Genet 2003; 4:346 - 58; http://dx.doi.org/10.1038/nrg1066; PMID: 12728277
- De Rijck J, Vandekerckhove L, Christ F, Debyser Z. Lentiviral nuclear import: a complex interplay between virus and host. Bioessays 2007; 29:441 - 51; http://dx.doi.org/10.1002/bies.20561; PMID: 17450594
- Sheng C, Zheng Q, Wu J, Xu Z, Wang L, Li W, et al. Direct reprogramming of Sertoli cells into multipotent neural stem cells by defined factors. Cell Res 2012; 22:208 - 18; http://dx.doi.org/10.1038/cr.2011.175; PMID: 22064700
- Sluka P, O’Donnell L, Bartles JR, Stanton PG. FSH regulates the formation of adherens junctions and ectoplasmic specialisations between rat Sertoli cells in vitro and in vivo. J Endocrinol 2006; 189:381 - 95; http://dx.doi.org/10.1677/joe.1.06634; PMID: 16648304
- Kaitu’u-Lino TJ, Sluka P, Foo CF, Stanton PG. Claudin-11 expression and localisation is regulated by androgens in rat Sertoli cells in vitro. Reproduction 2007; 133:1169 - 79; http://dx.doi.org/10.1530/REP-06-0385; PMID: 17636171
- Nicholls PK, Harrison CA, Gilchrist RB, Farnworth PG, Stanton PG. Growth differentiation factor 9 is a germ cell regulator of Sertoli cell function. Endocrinology 2009; 150:2481 - 90; http://dx.doi.org/10.1210/en.2008-1048; PMID: 19106224
- Nicholls PK, Harrison CA, Walton KL, McLachlan RI, O’Donnell L, Stanton PG. Hormonal regulation of sertoli cell micro-RNAs at spermiation. Endocrinology 2011; 152:1670 - 83; http://dx.doi.org/10.1210/en.2010-1341; PMID: 21325043
- Forestell SP, Dando JS, Böhnlein E, Rigg RJ. Improved detection of replication-competent retrovirus. J Virol Methods 1996; 60:171 - 8; http://dx.doi.org/10.1016/0166-0934(96)02052-6; PMID: 8844623
- O’Doherty U, Swiggard WJ, Malim MH. Human immunodeficiency virus type 1 spinoculation enhances infection through virus binding. J Virol 2000; 74:10074 - 80; http://dx.doi.org/10.1128/JVI.74.21.10074-10080.2000; PMID: 11024136
- Barry SC, Harder B, Brzezinski M, Flint LY, Seppen J, Osborne WR. Lentivirus vectors encoding both central polypurine tract and posttranscriptional regulatory element provide enhanced transduction and transgene expression. Hum Gene Ther 2001; 12:1103 - 8; http://dx.doi.org/10.1089/104303401750214311; PMID: 11399231
- Tiscornia G, Singer O, Verma IM. Production and purification of lentiviral vectors. Nat Protoc 2006; 1:241 - 5; http://dx.doi.org/10.1038/nprot.2006.37; PMID: 17406239
- Lampa J, Hoogerbrugge JW, Baarends WM, Stanton PG, Perryman KJ, Grootegoed JA, et al. Follicle-stimulating hormone and testosterone stimulation of immature and mature Sertoli cells in vitro: inhibin and N-cadherin levels and round spermatid binding. J Androl 1999; 20:399 - 406; PMID: 10386820
- John SW, Weitzner G, Rozen R, Scriver CR. A rapid procedure for extracting genomic DNA from leukocytes. Nucleic Acids Res 1991; 19:408; http://dx.doi.org/10.1093/nar/19.2.408; PMID: 2014181
- Laird PW, Zijderveld A, Linders K, Rudnicki MA, Jaenisch R, Berns A. Simplified mammalian DNA isolation procedure. Nucleic Acids Res 1991; 19:4293; http://dx.doi.org/10.1093/nar/19.15.4293; PMID: 1870982
- McCabe MJ, Tarulli GA, Meachem SJ, Robertson DM, Smooker PM, Stanton PG. Gonadotropins regulate rat testicular tight junctions in vivo. Endocrinology 2010; 151:2911 - 22; http://dx.doi.org/10.1210/en.2009-1278; PMID: 20357222
- Ellis J. Silencing and variegation of gammaretrovirus and lentivirus vectors. Hum Gene Ther 2005; 16:1241 - 6; http://dx.doi.org/10.1089/hum.2005.16.1241; PMID: 16259557
- Chaudhary J, Skinner MK. E-box and cyclic adenosine monophosphate response elements are both required for follicle-stimulating hormone-induced transferrin promoter activation in Sertoli cells. Endocrinology 1999; 140:1262 - 71; http://dx.doi.org/10.1210/en.140.3.1262; PMID: 10067852
- Kaur G, Dufour JM. Cell lines: Valuable tools or useless artifacts. Spermatogenesis 2012; 2:1 - 5; http://dx.doi.org/10.4161/spmg.19885; PMID: 22553484
- Ebert MS, Neilson JR, Sharp PA. MicroRNA sponges: competitive inhibitors of small RNAs in mammalian cells. Nat Methods 2007; 4:721 - 6; http://dx.doi.org/10.1038/nmeth1079; PMID: 17694064
- Kaur G, Long CR, Dufour JM. Genetically engineered immune privileged Sertoli cells: A new road to cell based gene therapy. Spermatogenesis 2012; 2:23 - 31; http://dx.doi.org/10.4161/spmg.19119; PMID: 22553487
- Mital P, Kaur G, Dufour JM. Immunoprotective sertoli cells: making allogeneic and xenogeneic transplantation feasible. Reproduction 2010; 139:495 - 504; http://dx.doi.org/10.1530/REP-09-0384; PMID: 19995832
- Fallarino F, Luca G, Calvitti M, Mancuso F, Nastruzzi C, Fioretti MC, et al. Therapy of experimental type 1 diabetes by isolated Sertoli cell xenografts alone. J Exp Med 2009; 206:2511 - 26; http://dx.doi.org/10.1084/jem.20090134; PMID: 19822646
- Luca G, Fallarino F, Calvitti M, Mancuso F, Nastruzzi C, Arato I, et al. Xenograft of microencapsulated sertoli cells reverses T1DM in NOD mice by inducing neogenesis of beta-cells. Transplantation 2010; 90:1352 - 7; http://dx.doi.org/10.1097/TP.0b013e3181ffb9d2; PMID: 21197711
- Rodriguez AI, Willing AE, Saporta S, Cameron DF, Sanberg PR. Effects of Sertoli cell transplants in a 3-nitropropionic acid model of early Huntington’s disease: a preliminary study. Neurotox Res 2003; 5:443 - 50; http://dx.doi.org/10.1007/BF03033174; PMID: 14715448
- Sanberg PR, Borlongan CV, Othberg AI, Saporta S, Freeman TB, Cameron DF. Testis-derived Sertoli cells have a trophic effect on dopamine neurons and alleviate hemiparkinsonism in rats. Nat Med 1997; 3:1129 - 32; http://dx.doi.org/10.1038/nm1097-1129; PMID: 9334725
- Loftis JM. Sertoli cell therapy: a novel possible treatment strategy for treatment-resistant major depressive disorder. Med Hypotheses 2011; 77:35 - 42; http://dx.doi.org/10.1016/j.mehy.2011.03.017; PMID: 21454019
- Bartholomae CC, Arens A, Balaggan KS, Yáñez-Muñoz RJ, Montini E, Howe SJ, et al. Lentiviral vector integration profiles differ in rodent postmitotic tissues. Mol Ther 2011; 19:703 - 10; http://dx.doi.org/10.1038/mt.2011.19; PMID: 21364536
- Miyoshi H, Blömer U, Takahashi M, Gage FH, Verma IM. Development of a self-inactivating lentivirus vector. J Virol 1998; 72:8150 - 7; PMID: 9733856
- Smith L. Good planning and serendipity: exploiting the Cre/Lox system in the testis. Reproduction 2011; 141:151 - 61; http://dx.doi.org/10.1530/REP-10-0404; PMID: 21084571