Abstract
The purpose of this review is to describe how the immune cells present in the testis interact with the germinal epithelium contributing to survival or apoptosis of germ cells (GCs). Physiologically, the immunosuppressor testicular microenvironment protects GCs from immune attack, whereas in inflammatory conditions, tolerance is disrupted and immune cells and their mediators respond to GC self antigens, inducing damage of the germinal epithelium. Considering that experimental models of autoimmune orchitis have clarified the local immune mechanisms by which protection of the testis is compromised, we described the following topics in the testis of normal and orchitic rats: (1) cell adhesion molecule expression of seminiferous tubule specialized junctions and modulation of blood-testis barrier permeability by cytokines (2) phenotypic and functional characteristics of testicular dendritic cells, macrophages, effector and regulatory T cells and mast cells and (3) effects of pro-inflammatory cytokines (TNF-α, IL-6 and FasL) and the nitric oxide-nitric oxide synthase system on GC apoptosis.
Introduction
It is known that genital male tract inflammations, mainly orchitis and orchi-epididymitis, are relevant co-factors in human subfertility and infertility. Lymphocyte infiltration is frequently found in testicular biopsies of patients with chronic inflammation of known or unknown etiology associated with infertility.Citation1,Citation2 Inflammation is induced mainly by viral or bacterial infections that access the testis by a hematogenous route or via excurrent ducts. Granulomatous orchitis is frequently associated with chronic inflammatory diseases. Also, lymphocyte infiltrations mimicking autoimmune orchitis may be found in testicular biopsies from patients with other pathologies involving tissue damage and spermatic antigen release such as testis trauma, cryptorchidia and testicular cancer in situ.Citation3,Citation4
The major functions of the testis are spermatogenesis and steroidogenesis. The latter is accomplished by Leydig cells localized in the interstitium as compact cell clusters closely associated with blood vessels. Loose connective tissue with thin collagen fibers and few fibroblasts and mesenchymal cells constitute the interstitial tissue, which also contains cells of the immune system involved in innate and adaptive immune responses: macrophages and scarce dendritic cells (DCs), T cells, B cells (rarely seen), mast cells and natural killer (NK) cells. Spermatogenesis occurs within the seminiferous tubules (STs), where Sertoli cells, targets of both testosterone and FSH, play a crucial role in germ cell (GC) proliferation and differentiation. These somatic cells, via specialized cell junctions, create the blood-testis barrier (BTB), between 16 and 19 d of age in the rat,Citation5 which divides the ST into a basal and an adluminal compartment.
Adherens junctions, basal gap junctions and tight junctions intermingle at the site of the BTB.Citation6 Adherens junctions are characterized by transmembrane, calcium-dependent cadherins that mediate homotypic interactions between adjacent cells. The cytoplasmic domain of cadherins interacts with γ- or β-catenin, which then link to the actin cytoskeleton through α-catenin.Citation7 The catenin p120 binds to the cytoplasmic domain of cadherin, modulating the adhesive strength of adherens junctions.Citation8 These intercellular proteins also function as signal transducers.Citation9 Gap junctions are composed of transmembrane proteins called connexins, which allow molecules smaller than 1 kDa to pass between the cytoplasmic compartments of two adjacent cells.Citation10,Citation11 Tight junctions are regions where the outer leaflets of opposing Sertoli cell membranes come into contact, completely occluding intercellular space.Citation12 These specialized junctions consist of transmembrane proteins, namely occludin, claudins and junctional adhesion molecules, linked to peripheral membrane proteins, such as ZO-1, -2 and -3,Citation13 which interact anatomically and functionally with F-actin of the cytoskeleton.Citation14
For completion of spermatogenesis, preleptotene and leptotene spermatocytes must migrate from the basal to the adluminal compartment of the seminiferous epithelium, crossing the BTB. This process requires that Sertoli-Sertoli and Sertoli-GC junctions be disassembled and reassembled.Citation15,Citation16 Many reports have implicated hormones such as testosterone, estrogens and FSH,Citation17-Citation19 cytokines such as interleukin 1-α (IL-1α), transforming growth factor-β3 (TGF-β3) and tumor necrosis factor-α (TNF-α),Citation20,Citation21 growth factors such as hepatocyte growth factorCitation22 and nitric oxide (NO)Citation23 as regulators of cell junction dynamics in the testis.
Cells of the immune system
Macrophages are by far the most prevalent cell type in the testicular interstitium, in close morphological association and functional interaction with Leydig cells. Macrophages and DCs belong to the heterogeneous group of cells collectively called “antigen-presenting cells.” Antigen presentation plays a central role in initiating and maintaining appropriate immune response to antigens. Complex molecular interactions between T cells and antigen-presenting cells ensure that T cells recognize antigenic peptides in a highly specific way. Another control necessary for T cells to respond efficiently is exerted by co-stimulatory molecules, without which tolerance may result. Finally, activation of T cells results in upregulation of cytokines and their receptors, which boost activatory signals leading to cell proliferation and differentiation into effector cells. Based on their cytokine profile and specific transcription factor expression, T cells can be further divided into multiple subsets. Among them, CD4+ T helper (Th)-1 cells are induced by IL-12 and interferon (IFN)-γ and by upregulated expression of the T-bet transcriptional factor.Citation24 CD4+ Th17 cells induced by a combination of TGF-β and IL-6 or IL-21 regulate the expression of the specific transcription factor, the orphan nuclear receptor ROR-γt.Citation25 Th1 and Th17 cells are associated with the development of many organ-specific autoimmune diseases and inflammatory tissue damage.
The Treg cell family is composed of the thymus-derived natural CD4+CD25+ Treg cells and the adaptive Treg cells generated from CD4+CD25- precursors in peripheral lymphoid organs.Citation26 The development and function of Treg cells is critically dependent on the transcriptional repressor Foxp3, currently the most reliable marker to identify this T cell subset.Citation27,Citation28 Treg cells are a relevant subset that ensures the maintenance of tolerance inhibiting potentially deleterious activities of effector T cells preventing pathogenic autoimmune responses.Citation27
Apoptotic pathways
In the testis, as in many tissues throughout the body, the number of cells in the STs is determined by a dynamic balance between cell proliferation and apoptotic cell death.Citation29 Seventy-five percent of all GCs produced in adult mammal testis are discarded through the process of apoptosis.Citation30
Programmed cell death involves many biochemical changes starting with sequential activation of proteases called caspases (cytosolic cystein aspartate proteases) that specifically cleave proteins after aspartic acid residues, which results in morphological alterations of the cellular membrane, cytoplasm and nucleus. Biochemical features of apoptosis include phosphatidylserine exposure to the external leaflet of the plasma membrane, activation of caspase cascades and DNA cleavage. Morphological events include membrane blebbing, cell volume shrinkage, chromatin condensation, cytoplasmic vacuolization and disassembly of the cell into membrane-bound remnants termed apoptotic bodies that are eventually removed by phagocytic cells.Citation31 In the testis, Sertoli cells engulf apoptotic bodies through binding of phosphatidylserine.Citation32
Apoptosis may be started by external or internal signals. External signals comprise death ligands belonging to the TNF-α family and include TNF-α and the ligand associated with the TNF (TRAIL) family and Fas ligand (FasL). Death ligands bind to their respective death receptors (R): TNF-α bind to TNFR1, TRAIL binds to TRAILR and FasL to Fas.Citation33 These interactions cause highly specific protein-protein associations that generate the oligomeric caspase-activating complex inside the cell. The death effector domain present in Fas and TNFR1 is responsible for the recruitment of caspases 8 and 10.Citation34-Citation36
Internal signals initiate the mitochondrial or internal pathway of apoptosis in which caspase 9 is the initiator caspase. Signals such as oxidative stress, DNA damage, survival factor withdrawal, hypoxia and UV irradiation activate this pathway by inducing release of cytochrome c from the internal mitochondria membrane. Together with cytocrome c, other apoptogenic proteins are released after mitochondria outer membrane permeabilization (MOMP). MOMP is modulated by the Bcl-2 family protein to which pro- (Bad, Bax, Bcl-xS, Bid, Bak) and anti-apoptotic (Bcl-2, Bcl-w, Bcl-xL, Mcl-1) proteins belong. It has been proposed that anti-apoptotic proteins are found on the outer mitochondrial membrane where they act to inhibit apoptosis through interaction and inhibition of pro-apoptotic proteins Bax and Bak. The pro-apoptotic proteins Bax and Bak are thought to promote mitochondria membrane permeabilization by oligomerizing to form pores within the outer mitochondrial membrane.
A third apoptotic pathway might be initiated by endoplasmic reticulum. This occurs when unfolded or misfolded proteins accumulate in the endoplasmic reticulum lumen and the controlled regulatory program to remove stressed cells fails.Citation37 This pathway involves many of the same Bcl-2 family proteins that regulate the mitochondrial pathway. Bax and Bak are located at the endoplasmic reticulum membrane where they regulate apoptosis and cause calcium release from it, sensitizing mitochondria to extrinsic and intrinsic death stimuli or directly activating death effectors.Citation38
Programmed cell death type 2 or autophagy is an evolutionarily conserved process involved in the degradation of intracellular proteins and organelles.Citation39 It remains unclear whether autophagy plays a role in GC death.Citation40
Interactions Between Immune Cells and Germ Cells of the Testis Under Physiological Conditions
Highly immunogenic autoantigens are expressed by haploid GCs that appear at puberty during spermatogenesis long after the establishment of immune tolerance mechanisms. The ability of the testis to tolerate these autoantigens as well as the long survival in the testicular interstitium of transplanted foreign tissue has led to the consideration that the testis is an immunoprivileged organ. This condition is restricted to sites that limit the spread of inflammation because it may threaten organ function: spermatogenesis in the case of the testis.
Immunoprivilege denotes the extended survival of cells expressing antigens that, under normal circumstances, should provoke an immune response.Citation41 It becomes evident around the pubertal period, when the BTB is formed and new GC antigens appear with the initiation of spermatogenesis. We will not explain in detail the mechanisms involved in testicular immunoprivilege since reviews on this topic have been published recently.Citation41-Citation43 We only mention the main mechanisms involved.
Immunoprivileged sites protect organ function by restricting the passage of potentially harmful molecules or cells through physical barriers. In the testis, the BTB forms an anatomical, physiological and immunological barrier.Citation44 The immunological barrier limits the access of antibodies to the adluminal compartment and the passage of most but not all GC autoantigens to the interstitium, reducing its interaction with immune cells. At tubuli recti and rete testis, modified Sertoli cells form a weak BTB relevant for immune tolerance mechanisms since continuous antigen leakage occurs. These sites are also vulnerable to autoimmune attack.Citation45,Citation46 Other mechanisms involved in immunoprivilege include local secretion by immune and non-immune cells (peritubular cells, Leydig cells and Sertoli cells) of numerous immunosuppressor molecules, including anti-inflammatory cytokines (e.g., TGF-β, IL-10) and growth factors (e.g., insulin growth factor-1, granulocyte monocyte-colony stimulating factor). Among testicular non immune cells, Sertoli cells, also called immune privileged cells,Citation47 seem to be those most responsible for maintaining a tolerogenic microenvironment. As we describe below, a relevant role in the induction of testicular immunosuppression is also played by a local and systemic network of tolerogenic DCs and T regs. The role of Tregs in the prevention of autoimmune diseases has been reported.Citation48,Citation49
As we analyzed immune and GC interactions mainly in the rat testis, most of the following data will focus on this species. Testicular leukocytes of humans are similar to that of rats and mice, and that despite some differences in the intratesticular lymphatics, these rodent species provide an appropriate model for the study of human testicular immune cells.Citation50 Although functional and/or anatomical interactions between immune cells and somatic cells (peritubular cells, Leydig cells and Sertoli cells) occur within the testis, we will address particularly the role of immune cells and their mediators in the maintenance of an immunosuppressive testis microenvironment suitable for normal spermatogenesis. Further on, we will analyze how the process of inflammation has the potential to induce GC apoptosis, thereby disrupting spermatogenesis.
Blood-testis barrier
In the normal rat testis, N-cadherin and catenins (α-, β- and p120) are highly expressed at the basal compartment of STs, following the cell borders of spermatogonia. At this region, N-cadherin co-localizes with both β-and p120 catenins. Catenins are also expressed by endothelial cells at the site of endothelial cell junctions of blood vessels. Connexin 43, the connexin predominant in the testis, is present at Sertoli-Sertoli and basal Sertoli-GC junctions, and also in the interstitial compartment between Leydig cells.Citation51-Citation53 Occludin, claudin-11 and ZO-1 localize at the basal compartment of STs where tight junctions between Sertoli cells are located. Endothelial cells of microvessels also express occludin. Tight junctions prevent the entry of molecules into the adluminal compartment of STs. In fact, when we injected biotin or lanthanum into the testis of untreated rats, both tracers were restricted to the interstitial area and basal compartment of STs, around spermatogonia and between the basolateral membrane region of Sertoli cells.Citation54 The peritubular myoid cells partially exclude the entry of small molecules, since lanthanum penetrates the myoid cell layer only in 10–15% of STs.Citation12
Immune cells
Dendritic cells
DCs in normal rat testis were identified by the monoclonal antibodies anti-OX-62 and anti-CD11c. The anti-OX-62 antibody recognizes the E2 integrin α chain expressed by rat DCs and gamma delta T cells, whereas the anti-CD11c antibody is specific for the rat integrin α x chain synthesized by DCs and a small population of myeloid cells. OX-62+ and CD11c+ cells are located only in the testicular interstitial space and the number of DCs obtained per testis with both markers is similar. However, the DC number per testis in normal Sprague-Dawley rats is about three times higher than Wistar rats.Citation55
DC maturation is the critical link between innate and adaptive T cell-dependent immunity. It is characterized by upregulation of co-stimulatory molecules, such as CD80, CD86 and CD70, production of cytokines such as IL-12 and expression of homing receptors such as CCR7 that direct DC migration into the T-cell areas of secondary lymphoid organs. These changes allow DCs to efficiently activate naive T cells.Citation56 Unexpectedly, we observed that most (95–100%) testicular DCs from normal testis express MHC class II and co-stimulatory molecules (CD80 and CD86) at levels similar to those found in DCs from inflamed rats. However, the low CCR7 mRNA level and negative IL-12p35 mRNA expression observed in DCs isolated from normal testis suggest that these cells are not mature.Citation57 In fact, testis DCs and DCs from testicular draining lymph nodes isolated from normal rats are unable to induce T-cell proliferationCitation58 confirming that in physiological conditions, DCs are tolerogenic.
Macrophages
In the rat testis, as well as in other species (e.g., human),Citation59 macrophage population is heterogeneous; two main subsets have been identified: a subpopulation of resident macrophages that express CD163, a cell-surface glycoprotein member of the scavenger receptor cysteine-rich superfamily (recognized by the monoclonal ED2 antibody in rats) and a subset of monocytes recently arrived from circulation that express the lysosomal glycoprotein CD68 (recognized by the monoclonal ED1 antibody in rats). By flow cytometry, we identified a third subset that expresses both markers (ED1+ED2+ cells)Citation60 indirectly defined by immunohistochemical techniques, previously.Citation61-Citation63 It has been proposed, but not experimentally demonstrated, that the ED1+ED2+ subpopulation is an intermediate population arising from circulating monocytes that could differentiate into resident macrophages in the testicular immunosuppressor microenvironment.Citation61,Citation62 Based on their ability to secrete pro-inflammatory cytokines such as TNF-α and IFN-γ, ED1+ testicular macrophages are identified with a pro-inflammatory profile. In contrast, ED2+ cells, the main subpopulation present in the normal rat testis, secrete immunosuppressor factors, contributing to the maintenance of immune privilege. Winnall et al.Citation64 demonstrated that the mRNA of the anti-inflammatory cytokine IL-10 was upregulated in ED2+ testicular macrophages.
When we studied expression of nitric oxide synthase (NOS), the enzyme that synthesizes NO, we observed that all macrophage subpopulations present in a normal testis expressed the inducible and the constitutive endothelial and neuronal isoforms. However, the three NOS isoforms were expressed mainly in ED1+ED2+ compared with ED1+ED2- macrophage subsets, whereas a minor percentage of ED1- ED2+ expressed NOS.Citation60 It is relevant to study ED1+ED2+, ED1+ED2- and ED1-ED2+ macrophage subsets separately, since the different phenotypes probably correlate with functional differences.
Effector T cells
CD4+ and CD8+ T cells are distributed in the testicular interstitium and CD8+ T cells are the predominant subset in normal rat testis.Citation65 These cells have the potential to produce pro-inflammatory cytokines such as TNF-α and IFN-γ following in vitro activation; however, we detected neither the presence of T cells expressing T-bet or ROR-γt in the interstitium nor IL-17 or IL-23 in interstitial fluid.Citation66
Regulatory T cells
In the normal rat testis, CD4+ and CD8+ T cells expressing Foxp3 are distributed in the testicular interstitium, mainly in the subalbuginea and peritubular areas.Citation67 It has been proposed that peripheral tolerance for internal organs depends on the control of autoreactive effector T cells by strategic enrichment of antigen-specific Tregs in the regional lymph nodes.Citation49 Concordantly, we observed that CD4+CD25+Foxp3+ Treg cells isolated from testicular draining lymph nodes, but not those derived from other regions, specifically proliferate in response to testicular antigens and suppress in vitro T cell proliferation. The fact that these cells express TGF-β1 suggests that this cytokine could be involved in the suppressive function.Citation68
Natural killer T cells
Tompkins et al.Citation69 demonstrated that NK cells are a major lymphocyte subpopulation within the rat testis. The apparent bias of the testicular cell population toward cells involved in cell killing and phagocytosis (macrophages, cytotoxic CD8+ T cells and NK cells), particularly in rats, suggests that the testis may possess enhanced innate immunoprotection.Citation50
Mast cells
Mast cells are distributed in different areas of the testis depending on the species; in the rat, they are restricted to the subalbuginea area near blood vessels, in contrast with human testis where they are localized in the interstitium and peritubular areas. Aside from their known role in inflammation and autoimmunity, it has been suggested that mast cells are essential intermediaries in regulatory T-cell tolerance.Citation70
Germ cell apoptosis
Proliferation and apoptosis of GCs occur during embryonic and postnatal periods and are sustained during the adulthood. Programmed GC death starts earlier during fetal life to eliminate primordial GCs that did not migrate properly, to remove excess cells and discard unfit cells. Excess cells generated during this period die by apoptosis that is largely dependent on Bcl-xL and Bax.Citation71 Gonadal apoptosis occurs not only in GCs but also in Sertoli cells and Leydig cells at all gestational stages.Citation72 During postnatal life in rodents, initial apoptosis of GCs occurs concurrently with maturation of Sertoli cells (10–15 d of age) and with the first wave of spermatogenesis, whereas it declines to occasional findings in adult normal testis. The physiological early apoptotic wave in the testis coincides in timing and localization with a temporary high expression of the pro-apoptotic protein Bax, which disappears at sexual maturity and seems to be necessary for the development of normal mature spermatogenesis.Citation73 The temporary and massive burst of GC apoptosis restricted to pubertal activation of spermatogenesis has been suggested to adjust the number of maturing GCs to the supportive capacity of Sertoli cells. This wave of apoptosis occurs 20–30 d post-natal, together with increased expression of Fas on apoptotic GCs and caspase 8 activation, indicating the important role of the extrinsic pathway of apoptosis in this event.Citation74 Numerous factors such as NO and cytokines are known to be involved in regulation of testicular homeostasis/function.
The free radical NO is a highly reactive molecule that acts as an intra and intercellular messenger that modulates cell death and proliferation, among other biological processes. It has been generally stated that low NO concentrations tend to favor pro-growth and anti-apoptotic responses whereas high NO levels favor cell cycle arrest, senescence or apoptosis pathways. NO is synthesized by enzymatic conversion of l-arginine to L-citrulline catalyzed by NOS. There are three isoforms of NOS: constitutively active and Ca2+-dependent endothelial (eNOS), neuronal (nNOS) and an inducible isoform (iNOS).Citation75 We demonstrated that iNOS, constitutive eNOS and nNOS are expressed in the normal rat testis by interstitial and tubular cells. These enzymes are functional since we demonstrated NO production and NOS activity.Citation60 Cells from the interstitial compartment release a higher amount of NO than cells from the STs. Macrophage subsets as well as T cells express the inducible and constitutive isoforms of NOS. It has been suggested that the NO-NOS system is involved in the determination of germ and Sertoli cell numbers and testis size, since iNOS-null mutant mice show a reduced rate of spermatocyte apoptosis and increased sperm count and Sertoli cell number.Citation76,Citation77
We demonstrated that cytokines known to be involved in GC apoptosis such as TNF-α, IL-6 and the soluble form of FasL (sFasL) are produced in the normal rat testis.Citation58,Citation78 We detected membrane FasL expressed by T cells but not by macrophages. Membrane-bound FasL may be converted to sFasL by the action of some matrix metalloproteases and by some members of the “a disintegrin and transmembrane metalloprotease” (ADAM) family.Citation79,Citation80 In the testis, ADAM10 and 17 are expressed by pre-pubertal GCs and ADAM17 has been proposed as one of the molecules triggering GC apoptosis during the first wave of spermatogenesis.Citation34,Citation81 T cells and also Leydig cells might be sources of sFasL present in the interstitial fluid of normal testis.Citation78 Intratesticular injection of FasL conjugated in its extracellular domain to Strep-Tag molecule (FasL-Strep, BioTAGnology) showed that sFasL was able to enter the STs. This short form of FasL also detected by Richburg et al.Citation82 may participate in the control of GC apoptosis by binding to Fas expressed by these cells. We demonstrated that FasL-Strep induces GC apoptosis in vitro.Citation78
Other authors reported that the Fas-FasL system is involved in the control of physiological GC apoptosis. In fact, gld/gld (generalized lymphoproliferative disease) homozygous mice in which FasL is unable to bind to Fas, have a small but significant increase in testis weight and spermatid head number per testis compared with wild-type mice. Also, gld mice are more sensitive to apoptosis induced by Sertoli cell injury.Citation83,Citation84 Moreover, an increase in GC survival after disruption of FasL expression by antisense oligonucleotide treatment and by intraperitoneal injection of anti-Fas antibody has been reported.Citation85,Citation86
Interactions Between Immune Cells and Germ Cells in Inflammatory Conditions
Although the testis is an immunoprivileged organ, immune cells present in the interstitium retain the ability to mount inflammatory and innate immune responses. They constitute, with other testicular cells bearing Toll-like receptors, the first line of defense against pathogens from the blood stream. In inflammatory conditions, tolerance may be disrupted: autoreactive T cells and B cells respond to GC self antigens, inducing immunopathologic damage of STs.
Studies using experimental models of autoimmune orchitis (EAO) clarified local immune mechanisms by which protection of the testis is compromised leading to autoimmune tissue injury.Citation58,Citation87 In this section, we describe immune-GC interactions in the testis of rats with autoimmune orchitis induced by active immunization with sperm antigens and adjuvants.
Testicular histopathology of rats with autoimmune orchitis is characterized by the presence of interstitial, subcapsular and peritubular lymphomononuclear cell infiltrates and damaged STs in which GCs undergo apoptosis and sloughing. At the onset of disease, lesions are distributed in several foci of few STs, after which they extend to the whole organ in the chronic severe stage in which formation of granulomas is frequent. Finally, STs become atrophic and peritubular fibrosis and infertility occur.Citation88 Even though cellular immune mechanisms are mainly involved in autoimmune orchitis, the presence of autoantibodies to GC antigens enhances the severity of the disease.
Blood-testis barrier
In association with the apoptosis and GC sloughing that occur in the testis of rats with EAO, we detected changes in the expression of cell junction adhesion molecules. By immunofluorescence and western blot, we observed a significant increase in N-cadherin expression in rats with focal and severe EAO, whereas an increase in α-catenin was observed only in rats with severe EAO. The loss of N-cadherin and β-catenin co-localization that we observed in the testis of EAO rats reflects impaired association between these two proteins.Citation51 Also, we detected an increase in tyrosine phosphorylation of β-catenin, which favors dissociation of the N-cadherin ⁄ β-catenin complex and leads to loss of cell adhesion function.Citation89 Moreover, the strong co-localization of N-cadherin and p120 catenin that we observed in EAO rats may function as an inhibitory regulator in the cadherin adhesion system as has been suggested by several authors.Citation90-Citation92 All these phenomena might explain the impairment of cell adhesion detected in the seminiferous epithelium of EAO rats. Expression of connexin 43 gradually decreased during EAO development. Concomitant with ST damage, the BTB function is impaired since tracers such as biotin and lanthanum were detected within the ST adluminal compartment surrounding remaining GCs. Therefore, increased BTB permeability is associated with the significant decrease of occludin expression and de-localization of claudin-11 and ZO-1.Citation51,Citation54
By in vitro experiments we demonstrated that IL-6, a pro-inflammatory cytokine that is increased in the testis of EAO rats, has the ability to modify the distribution of Sertoli cell tight junction proteins and to perturb the Sertoli cell tight junction barrier via the p38 MAPK signaling pathway, reducing transepithelial electrical resistance across the cell epithelium.Citation54 A similar effect on BTB dynamics was observed with IL-17 (unpublished results).
Immune cells
Immune cells infiltrate the testicular interstitium of rats with EAO. Chemokines and cytokines upregulating endothelial cell adhesion molecules support the initial attachment of leukocytes to endothelial cells and their extravasation into the interstitial space. Leukocyte extravasation begins with initial contact between the activated form of CD44 on lymphocytes and its major ligand, hyaluronan, on endothelial cells.Citation93 CCL2, CCL3 and CCL4 chemokines expressed by testicular cells come into play and convert leukocyte rolling into cell arrest. CD49d integrin expressed by leukocytes in conjunction with its endothelial ligand, CD106, upregulated during orchitis, mediates the firm adhesion step. Finally, migration or diapedesis occurs via interaction of CD106 and CD31.Citation58,Citation94 The expression of chemokines and tissue-homing receptors in T cells is relevant for the entrance and distribution of these cells in the testis.Citation45,Citation58
Dendritic cells
In the testis of rats with EAO, there is a significant increase in the number of DCs that undergo a process of maturation initially visualized by higher expression of the chemokine receptor CCR7 involved in cell migration to draining lymph nodes. This result is associated with increased DC percentages only in the testicular draining lymph nodes where the CCR7 ligand CCL19 is expressed. Furthermore, DCs from testicular draining lymph nodes are mature and express a higher level of MHC class II and IL-12p35 mRNA, a product of activated DCs. In fact, EAO-DCs from testicular draining lymph nodes, but not from other lymphatic sites, significantly enhance the proliferation of “naive” T cells.Citation95
Progressive amplification of the autoimmune response finally leading to chronification of EAO may result from continuous antigen presentation of DCs to lymphocytes in lymph nodes and the testis.
Macrophages
Macrophages that express MHC class II, CD80 and CD86 increase in number in the testicular interstitium of rats with EAO.Citation63 During the course of the disease, the influx of ED1+ED2- pro-inflammatory macrophages in the testicular interstitium arising from monocytes in the bloodstream drastically alters the composition of the macrophage population, inducing a shift in cytokine balance in favor of inflammatory response. The double positive (ED1+ED2+) cells, together with ED1+ED2-, are the main subpopulations contributing to the increased number of testicular macrophages in EAO. The fact that a further increase in the ED1-ED2+ resident macrophage subset does not occur in the severe phase of the disease led us to speculate that ED1+ED2+ macrophages remain in an “undifferentiated” stage, closer to the profile of their ED1+ED2- precursors, probably under the influence of pro-inflammatory factors.Citation63
In the testis of EAO rats, we observed an increase in the percentage of ED1+ED2- and ED1+ED2+ macrophages that expressed iNOS, eNOS and nNOS isoforms compared with normal rats. These results indicate that the ED1+ED2+ subset is sensitive to stimuli from an inflammatory microenvironment. Data also suggest that the high NO production by EAO interstitial macrophages is due mainly to the rise in the number of ED1+ED2+ macrophages and to NO production per macrophage.Citation60
Different cytokines present in the testicular microenvironment may modulate NOS expression and activity, generating different levels of NO production from focal and severe EAO macrophages. Similarly, in a model of autoimmune uveitis, resident retinal macrophages at the earliest phase of the disease generated little NO spontaneously and were unable to release NO in response to IFN-γ and TNF-α in vitro. In contrast, during the late phase of the disease, macrophages released higher levels of NO in response to pro-inflammatory cytokines.Citation96
The critical role of testicular macrophages and DCs in EAO development was confirmed by an in vivo experiment in which cell depletion induced by intraperitoneal injection of clodronate-containing liposomes significantly reduced EAO incidence and severity.Citation63
Effector T cells
During the course of EAO, a large increase in the number of T cells was observed in the testicular interstitium associated with initiation of testicular damage. We detected TNF-α/IFN-γ-producing CD4+ and CD8+ T cells and identified for the first time CD4+ and CD8+ T cells producing IL-17 in the inflammatory interstitial infiltrate present in the testis of EAO rats.Citation65,Citation66 Chronological changes in the composition of effector T cell subsets were detected during disease development. Expression of T-bet (Th1 transcription factor) and ROR-γt (Th17 transcription factor) increased to a similar extent in the testis of rats with focal EAO. Whereas the expression of T-bet peaked at EAO onset, ROR-γt expression remained unchanged as the disease progressed. Concordantly, content of IL-17 (the prototypical Th17 cytokine) and IL-23 (a cytokine involved in expansion and stabilization of the Th17 phenotype) also increased in the testis, reaching maximum level during the EAO chronic phase. Our results highlight the involvement of CD4+ Th1 and Th17 subsets as co-effector cells governing EAO onset as well as the central contribution of CD8+ T cells producing Th1 and Th17 cytokines in the maintenance of chronic inflammation.
Regulatory T cells
Concomitant with changes in the composition of effector T cell subsets, a large increase in the number of CD4+ and CD8+ cells expressing Foxp3 was observed in the testicular interstitium at EAO onset; then CD4+ Foxp3+ Treg cells declined during the chronic phase of the disease, probably as a consequence of severe GC sloughing occurred during this phase.Citation65
Functional analysis of the CD4+ CD25high T cell subset (which contains over 90% of Foxp3+ cells) isolated from testis draining lymph nodes of normal and EAO rats showed that these cells are able to suppress responder T cell proliferation induced by testicular antigens. However, EAO Treg cells were more efficient than those of normal rats (a 2-fold increase in % suppression). Since each regional lymph node is enriched in Treg cells specific to self-antigen derived from drained tissue,Citation49,Citation97 we speculate that Treg cells arriving to the testis during EAO development are also functionally active.
Overall results suggest the involvement of CD4+Th1 and Th17 subsets as co-effector cells driving EAO development as well as the central contribution of CD8+ T cells producing Th1 and Th17 cytokines in autoimmune pathogenesis. We propose that pro-inflammatory cytokines (mainly TNF-α, IFN-γ and IL-17) produced locally by these cells orchestrate tissue inflammation by contributing to recruit and activate other effector cells to the organ, inducing GC apoptosis and modulating Treg function. Concomitantly, the number of suppressor Tregs increases due to enhanced spermatic antigen release. Nevertheless, Tregs fail to effectively suppress inflammation, probably because these cells are outnumbered by even more vigorously expanding effector T cell subsets. Alternatively, pro-inflammatory cytokines in the inflamed testis may render effector T cells resistant to the suppressive effect of Tregs.
Natural killer T cells
NK T cells are known to modulate cell-mediated immunity in a broad spectrum of diseases including autoimmunity. However, very few data have been reported up to now on the immunoregulatory role of NK T cell population in the testis in inflammatory conditions. This is a relevant area of research for the future.
Mast cells
We observed that mast cells distributed throughout the parenchyma and mainly around STs significantly increased in rats with EAO. Iosub et al.Citation98 demonstrated that mast cell tryptase activated proteinase-activated receptor-2 (PAR-2) strongly expressed in macrophages and peritubular-like cells localized around granulomas of rats with EAO. PAR-2 activation upregulates cytokines and inflammatory mediators contributing to testicular inflammation. Moreover, mast cell tryptase inducing fibroblast proliferation is relevant for the process of testicular fibrosis as described for human testis.Citation99,Citation100
Apoptosis in the inflamed testis
In rats with EAO, we detected an increase of IL-6 in the macrophage conditioned medium. Concomitantly, activated ED1+ testicular macrophages and peritubular cells exhibited increased IL-6 reactivity, whereas Sertoli cells showed downregulation of IL-6 expression at the chronic EAO stage. A significant increase in the number of IL-6R+ GCs occurred simultaneously with increasing testicular damage and an increased number of apoptotic GCs. Also, in vitro experiments showed that IL-6 induced GC apoptosis when added to cultures of rat STs.Citation101 By in vivo experiments, we demonstrated that IL-6 induces focal inflammatory cell infiltration and GC sloughing in adjacent STs associated with alterations of tight junction protein expression and distribution.Citation54 Overall results suggest involvement of the IL-6/IL-6R system in the pathogenesis of EAO through stimulation of inflammation and apoptosis.
In relation to TNF-α, we demonstrated that in EAO, interstitial macrophages release TNF-α and lymphocytes express the membrane-bound form of this cytokine, suggesting that these cells may also be a source of TNF-α.Citation102,Citation103 In contrast, TNFR1 is expressed by spermatocytes and cells of the interstitium. The number of TNFR1+ spermatocytes increases during severe orchitis and most of these cells are apoptotic. We observed that conditioned medium of testicular macrophages from rats with severe orchitis, which contains high levels of TNF-α, induced apoptosis of GCs in vitro. Neutralization of TNF-α with Etanercept, a protein that mimics the inhibitory effects of soluble TNF receptors, reverted this apoptotic effect.Citation104 Moreover, TNF-α induces in vitro apoptosis of GCs, thereby showing that TNFR1 is functionally active.Citation103
In different experimental models of testicular cell damage, such as cryptorchidia, irradiation, toxicants, heat exposure and hormone suppression, the Fas-FasL system also appeared to be the major pathway of GC apoptosis. We analyzed the modulation of Fas-FasL in the testis under inflammatory stimulus, not studied before. In the STs of rats with EAO, the number of Fas+ and FasL+ GCs (mainly spermatocytes and spermatids) correlated with the increased number of apoptotic cells during the course of the disease. By in vitro experiments, we demonstrated that GC Fas activation induces apoptosis, suggesting that the Fas-FasL system is one of the main mediators of GC death.Citation105
In rats with severe EAO, we detected increased levels of sFasL in interstitial fluid as well as an increased number of testicular CD4+ and CD8+ T cells expressing membrane-FasL (mFasL).Citation78 The absence of detectable differences between cell surface and total expression of FasL by T cells supports the notion that in T cells mFasL, but not intracellular preformed FasL, could be the sole source of the soluble form released into the extracellular milieu. Preliminary results showed selective ADAM17 expression on cells from testicular interstitium in rats with orchitis. This metalloprotease has been implicated in the proteolysis of several substrates like TNF-α, TNFR1 and TNFR2, TGF-α and IL-6R.Citation79 We detected neither membrane nor intracellular FasL expression in testicular macrophages, in contrast with other authors who found that activated human monocytes released high levels of sFasL from preformed FasL stored within the intracellular compartment.Citation106
In addition to the increase in sFasL content, intratesticular injection experiments performed with FasL-Strep showed that it has the ability to enter STs. Moreover, FasL-Strep was able to induce GC apoptosis.Citation78 The impairment of BTB in rats undergoing autoimmune orchitis might facilitate sFasL passage into the adluminal compartment where it has the capacity to induce GC apoptosis by binding to its receptor. Testicular content of sFasL in rats with focal EAO is similar to that detected in normal rats; however, we cannot rule out sFasL participation in GC death during this phase of the disease; the higher number of GCs expressing Fas may sensitize the testis to sFasL action. Intratesticular content of sFasL reaches its highest level in the severe stage of the disease concomitantly with the increased number of Fas+ GCs. During severe orchitis, when interactions between Fas and FasL bearing GCs are maximally disturbed, apoptosis could be mediated primarily by sFasL. Our results showing that the number of GCs expressing Fas, FasL or co-expressing both molecules increased in testis of rats with EAO suggests that GC apoptosis could be mediated by an autocrine and/or paracrine mechanism.Citation107
Concomitant with high levels of pro-inflammatory cytokines in the testicular microenvironment, NO production by macrophages increases. By ex-vivo experiments, we demonstrated that NO released by testicular macrophages from rats with orchitis induced GC apoptosis. The presence of a competitive NOS inhibitor, L-NAME, reduced NO production by these cells and the percentage of STs presenting apoptotic GCs (unpublished results).
Our previous resultsCitation101,Citation102,Citation105,Citation108 showed that activation of external and mitochondrial pathways occurs in EAO, which, in turn, activates executor caspase 3 in spermatocytes of damaged STs. EAO apoptotic GCs, mostly spermatocytes and spermatids, expressed death receptors Fas, TNFR1 and IL-6R and pro-apoptotic protein Bax translocated from cytosol to mitochondria. Data suggest that apoptosis may be triggered by TNF-α, FasL and IL-6 released from immune cells activating caspase 8 and/or the mitochondrial pathway upon binding to its receptors expressed on GCs. NO may contribute to GC death by activating mitochondrial pathway or modulating the expression of death receptor Fas on GCs.
Immunopathology of Testis Sections from Subfertile or Infertile Patients
In the human testis a scarce number of lymphocytes is present in the rete testis (mainly CD8+ T cells)Citation107 and in the interstitium.Citation109 The analysis of testis biopsies showed that interstitial lymphomononuclear cells close to STs with impairment of the germinal epithelium are frequently associated with subfertility or infertility. Early studies of Suominem et al.Citation110 and el-Demiry et al.Citation107 revealed the presence of testicular leukocyte infiltrates in a low percentage of infertile patients. However, Schuppe et al.Citation1 in a recent systematic evaluation of testis biopsies from infertile patients, detected peritubular and perivascular inflammatory cell infiltrates in 50% of samples analyzed. The presence of inflammatory infiltrates in testis biopsies is also detected in other testicular pathologies in which tissue destruction facilitates the release of GC antigens. In fact, testicular infiltration of macrophages, CD4+ and CD8+ T cells, were detected in patients with untreated cryptorchidiaCitation3 and GC neoplasia.Citation4 Besides the increase of Th1 cells, Duan et al.Citation111 detected interstitial Th17 cells expressing IL-17, IL-21 and IL-22 in the testis of azoospermic patients with chronic inflammation. Overexpression of inflammatory cytokines in the testicular microenvironment is a risk factor in pathologies with impairment of spermatogenesis and steroidogenesis. Increased expression of TNFR1 and IL-6 mRNA were reported in testis from patients with arrested maturation and tumors, respectively.Citation112 The increase in the number of macrophages (CD68+)Citation59 and mast cells has also been reported in pathologies such as GC arrest and Sertoli cell-only syndrome.Citation113,Citation114 Also, the phenotype of testicular mast cells changes with respect to the ability to express COX2 and prostaglandin enzymes, these cells thus emerging as players in spermatogenic dysfunction and fibrosis.Citation99,Citation100,Citation115
Deposits of IgG, C3 components and/or immune-complex at ST basement membranes have been early reported in testis biopsies of infertile menCitation116,Citation117 as well as in experimental models of autoimmune orchitis.Citation118,Citation119 However, the pathogenic role on GCs of immune deposits present in the ST wall has only been partially explored.
Besides, anti-sperm antibodies, mainly those detected on spermatozoa and in genital tract secretions, are known to be involved in testis autoimmunity and have been linked to subfertility or infertility. Anti-sperm antibodies may interfere with sperm motility; high titers of antibodies have been detected in men after vasectomy or with vas deferens agenesia. In the female genital tract, they may interfere with sperm transport and other fertilization steps.
Concluding Remarks
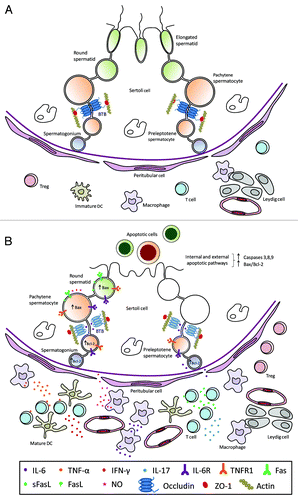
The data reviewed here show that testicular immunoregulation depends on a delicate equilibrium between immunoprivilege and inflammation and is modulated by the dual role played by tolerogenic or pathogenic cells of the immune system and by cytokines acting as immunosuppressors or pro-inflammatory mediators. Under physiological conditions, antigen-specific auto immune responses are prevented by systemic and local tolerance mechanisms. In the testis (), tolerogenic DCs and Treg subsets allow the survival of GCs expressing auto-antigens and the normal completion of spermatogenesis. Under inflammatory conditions (), mature DCs and macrophages secreting high levels of NO, IL-6 and TNF-α, infiltrate the testicular interstitium. TNF-α and IFN-γ-producing Th1 cells and IL-17-producing Th17 cells increase in number in association with testicular damage. With the action of pro-inflammatory cytokines (e.g., IL-6) on tight junction molecule expression and distribution, BTB permeability increases. Cytokines entering the ST adluminal compartment induce apoptosis of GCs expressing death receptors TNFR1, Fas and IL-6R. NO is another relevant factor contributing to GC apoptosis; however, the chronology of GC sloughing and apoptosis remains incompletely understood. Spermatic antigens released during GC apoptosis interacting with immune cells amplify the autoimmune response. Although Tregs increase in the testicular interstitium, they fail to effectively suppress inflammation. All these events contribute to GC sloughing and infertility.
Figure 1. Schematic drawing illustrating the seminiferous epithelium and intestitium under normal (A) or inflammatory (B) conditions. (A) The seminiferous epithelium is composed of Sertoli cells and germ cells at different stages of development. The blood-testis barrier (BTB) is constituted by coexisting adherens, gap and tight junctions between adjacent Sertoli cells. As representative tight junction molecules, occludin and ZO-1 are shown. Macrophages close to Leydig cells and few immature dendritic cells (DCs), T cells and regulatory T cells (Treg) are present in the interstitium. (B) Spermatocytes and spermatids undergo apoptosis and sloughing in the lumen of the seminiferous tubule. Decreased occludin expression in Sertoli cell tight junctions associated to impairment of BTB is shown. An increased number of mature DCs, pro-inflammatory and intermediate type macrophages, CD4+ and CD8+ T cells and T regs are present in the interstitium. Pro-inflammatory cytokines (IL-6, TNF-α, FasL, IFN-γ, IL-17) and nitric oxide (NO) released by macrophages and T cells are involved in the induction of inflammation and germ cell apoptosis. Cytokines and other factors secreted by Sertoli, Leydig and peritubular cells are not illustrated.
Further investigation of testicular immune regulation will continue to better understanding of Treg function, tolerance and immune-privilege mechanisms. On the other hand, knowledge of the immune cells and factors involved in alterations of BTB permeability, GC apoptosis and impairment of spermatogenesis will help to unravel the autoimmune basis of testicular inflammation associated with subfertility and infertility.
The understanding of these topics may improve diagnosis and facilitate the search for reliable peripheral markers of testis inflammation and the exploration of specific therapies in human testicular pathology associated with immune mechanisms.
| Abbreviations: | ||
| BTB | = | blood-testis barrier |
| DC | = | dendritic cell |
| EAO | = | experimental autoimmune orchitis |
| FasL | = | Fas ligand |
| GC | = | germ cell |
| IFN | = | interferon |
| IL | = | interleukin |
| NK | = | natural killer |
| NO | = | nitric oxide |
| NOS | = | nitric oxide synthase |
| ST | = | seminiferous tubule |
| TGF | = | transforming growth factor |
| Th | = | T helper |
| TNF | = | tumor necrosis factor |
| Treg | = | regulatory T cell |
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- Schuppe HC, Meinhardt A, Allam JP, Bergmann M, Weidner W, Haidl G. Chronic orchitis: a neglected cause of male infertility?. Andrologia 2008; 40:84 - 91; http://dx.doi.org/10.1111/j.1439-0272.2008.00837.x; PMID: 18336456
- Haidl G, Allam JP, Schuppe HC. Chronic epididymitis: impact on semen parameters and therapeutic options. Andrologia 2008; 40:92 - 6; http://dx.doi.org/10.1111/j.1439-0272.2007.00819.x; PMID: 18336457
- Nistal M, Riestra ML, Paniagua R. Focal orchitis in undescended testes: discussion of pathogenetic mechanisms of tubular atrophy. Arch Pathol Lab Med 2002; 126:64 - 9; PMID: 11800649
- Jahnukainen K, JørgensenN, Pöllänen P, Giwercman A, Skakkebaek NE. Incidence of testicular mononuclear cell infiltrates in normal human males and in patients with germ cell neoplasia. Int J Androl 1995; 18:313 - 20; PMID: 8719847
- Bergmann M, Dierichs R. Postnatal formation of the blood-testis barrier in the rat with special reference to the initiation of meiosis. Anat Embryol (Berl) 1983; 168:269 - 75; http://dx.doi.org/10.1007/BF00315821; PMID: 6660566
- Morrow CM, Mruk D, Cheng CY, Hess RA. Claudin and occludin expression and function in the seminiferous epithelium. Philos Trans R Soc Lond B Biol Sci 2010; 365:1679 - 96; http://dx.doi.org/10.1098/rstb.2010.0025; PMID: 20403878
- Yan HH, Mruk DD, Lee WM, Cheng CY. Ectoplasmic specialization: a friend or a foe of spermatogenesis?. Bioessays 2007; 29:36 - 48; http://dx.doi.org/10.1002/bies.20513; PMID: 17187371
- Johnson KJ, Boekelheide K. Dynamic testicular adhesion junctions are immunologically unique. I. Localization of p120 catenin in rat testis. Biol Reprod 2002; 66:983 - 91; http://dx.doi.org/10.1095/biolreprod66.4.983; PMID: 11906917
- Cheng CY, Mruk DD. Cell junction dynamics in the testis: Sertoli-germ cell interactions and male contraceptive development. Physiol Rev 2002; 82:825 - 74; PMID: 12270945
- Pointis G, Fiorini C, Defamie N, Segretain D. Gap junctional communication in the male reproductive system. Biochim Biophys Acta 2005; 1719:102 - 16; http://dx.doi.org/10.1016/j.bbamem.2005.09.017; PMID: 16259941
- Gilleron J, Carette D, Durand P, Pointis G, Segretain D. Connexin 43 a potential regulator of cell proliferation and apoptosis within the seminiferous epithelium. Int J Biochem Cell Biol 2009; 41:1381 - 90; http://dx.doi.org/10.1016/j.biocel.2008.12.008; PMID: 19136074
- Dym M, Fawcett DW. The blood-testis barrier in the rat and the physiological compartmentation of the seminiferous epithelium. Biol Reprod 1970; 3:308 - 26; PMID: 4108372
- Hermo L, Pelletier RM, Cyr DG, Smith CE. Surfing the wave, cycle, life history, and genes/proteins expressed by testicular germ cells. Part 5: intercellular junctions and contacts between germs cells and Sertoli cells and their regulatory interactions, testicular cholesterol, and genes/proteins associated with more than one germ cell generation. Microsc Res Tech 2010; 73:409 - 94; PMID: 19941291
- González-Mariscal L, Betanzos A, Avila-Flores A. MAGUK proteins: structure and role in the tight junction. Semin Cell Dev Biol 2000; 11:315 - 24; http://dx.doi.org/10.1006/scdb.2000.0178; PMID: 10966866
- Mruk DD, Cheng CY. Sertoli-Sertoli and Sertoli-germ cell interactions and their significance in germ cell movement in the seminiferous epithelium during spermatogenesis. Endocr Rev 2004; 25:747 - 806; http://dx.doi.org/10.1210/er.2003-0022; PMID: 15466940
- Smith BE, Braun RE. Germ cell migration across Sertoli cell tight junctions. Science 2012; 338:798 - 802; http://dx.doi.org/10.1126/science.1219969; PMID: 22997133
- Yan HH, Mruk DD, Lee WM, Cheng CY. Blood-testis barrier dynamics are regulated by testosterone and cytokines via their differential effects on the kinetics of protein endocytosis and recycling in Sertoli cells. FASEB J 2008; 22:1945 - 59; http://dx.doi.org/10.1096/fj.06-070342; PMID: 18192323
- MacCalman CD, Getsios S, Farookhi R, Blaschuk OW. Estrogens potentiate the stimulatory effects of follicle-stimulating hormone on N-cadherin messenger ribonucleic acid levels in cultured mouse Sertoli cells. Endocrinology 1997; 138:41 - 8; http://dx.doi.org/10.1210/en.138.1.41; PMID: 8977383
- Sluka P, O’Donnell L, Bartles JR, Stanton PG. FSH regulates the formation of adherens junctions and ectoplasmic specialisations between rat Sertoli cells in vitro and in vivo. J Endocrinol 2006; 189:381 - 95; http://dx.doi.org/10.1677/joe.1.06634; PMID: 16648304
- Sarkar O, Mathur PP, Cheng CY, Mruk DD. Interleukin 1 alpha (IL1A) is a novel regulator of the blood-testis barrier in the rat. Biol Reprod 2008; 78:445 - 54; http://dx.doi.org/10.1095/biolreprod.107.064501; PMID: 18057314
- Xia W, Wong EW, Mruk DD, Cheng CY. TGF-beta3 and TNFalpha perturb blood-testis barrier (BTB) dynamics by accelerating the clathrin-mediated endocytosis of integral membrane proteins: a new concept of BTB regulation during spermatogenesis. Dev Biol 2009; 327:48 - 61; http://dx.doi.org/10.1016/j.ydbio.2008.11.028; PMID: 19103189
- Catizone A, Ricci G, Caruso M, Ferranti F, Canipari R, Galdieri M. Hepatocyte growth factor (HGF) regulates blood-testis barrier (BTB) in adult rats. Mol Cell Endocrinol 2012; 348:135 - 46; http://dx.doi.org/10.1016/j.mce.2011.07.050; PMID: 21843593
- Lee NP, Cheng CY. Nitric oxide and cyclic nucleotides: their roles in junction dynamics and spermatogenesis. Adv Exp Med Biol 2008; 636:172 - 85; http://dx.doi.org/10.1007/978-0-387-09597-4_10; PMID: 19856168
- Szabo SJ, Kim ST, Costa GL, Zhang X, Fathman CG, Glimcher LH. A novel transcription factor, T-bet, directs Th1 lineage commitment. Cell 2000; 100:655 - 69; http://dx.doi.org/10.1016/S0092-8674(00)80702-3; PMID: 10761931
- Ivanov II, McKenzie BS, Zhou L, Tadokoro CE, Lepelley A, Lafaille JJ, et al. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell 2006; 126:1121 - 33; http://dx.doi.org/10.1016/j.cell.2006.07.035; PMID: 16990136
- Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol 2003; 4:330 - 6; http://dx.doi.org/10.1038/ni904; PMID: 12612578
- Corthay A. How do regulatory T cells work?. Scand J Immunol 2009; 70:326 - 36; http://dx.doi.org/10.1111/j.1365-3083.2009.02308.x; PMID: 19751267
- Heiber JF, Geiger TL. Context and location dependence of adaptive Foxp3(+) regulatory T cell formation during immunopathological conditions. Cell Immunol 2012; 279:60 - 5; http://dx.doi.org/10.1016/j.cellimm.2012.09.009; PMID: 23089195
- Russell LD, Chiarini-Garcia H, Korsmeyer SJ, Knudson CM. Bax-dependent spermatogonia apoptosis is required for testicular development and spermatogenesis. Biol Reprod 2002; 66:950 - 8; http://dx.doi.org/10.1095/biolreprod66.4.950; PMID: 11906913
- Blanco-Rodríguez J. A matter of death and life: the significance of germ cell death during spermatogenesis. Int J Androl 1998; 21:236 - 48; http://dx.doi.org/10.1046/j.1365-2605.1998.00133.x; PMID: 9805237
- Huppertz B, Frank HG, Kaufmann P. The apoptosis cascade--morphological and immunohistochemical methods for its visualization. Anat Embryol (Berl) 1999; 200:1 - 18; http://dx.doi.org/10.1007/s004290050254; PMID: 10395001
- Shiratsuchi A, Umeda M, Ohba Y, Nakanishi Y. Recognition of phosphatidylserine on the surface of apoptotic spermatogenic cells and subsequent phagocytosis by Sertoli cells of the rat. J Biol Chem 1997; 272:2354 - 8; http://dx.doi.org/10.1074/jbc.272.4.2354; PMID: 8999945
- LeBlanc HN, Ashkenazi A. Apo2L/TRAIL and its death and decoy receptors. Cell Death Differ 2003; 10:66 - 75; http://dx.doi.org/10.1038/sj.cdd.4401187; PMID: 12655296
- Moreno RD, Urriola-Muñoz P, Lagos-Cabré R. The emerging role of matrix metalloproteases of the ADAM family in male germ cell apoptosis. Spermatogenesis 2011; 1:195 - 208; http://dx.doi.org/10.4161/spmg.1.3.17894; PMID: 22319668
- Thorburn A. Tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) pathway signaling. J Thorac Oncol 2007; 2:461 - 5; http://dx.doi.org/10.1097/JTO.0b013e31805fea64; PMID: 17545839
- Yi CH, Yuan J. The Jekyll and Hyde functions of caspases. Dev Cell 2009; 16:21 - 34; http://dx.doi.org/10.1016/j.devcel.2008.12.012; PMID: 19154716
- Jing G, Wang JJ, Zhang SX. ER stress and apoptosis: a new mechanism for retinal cell death. Exp Diabetes Res 2012; 2012:589589; http://dx.doi.org/10.1155/2012/589589; PMID: 22216020
- Breckenridge DG, Germain M, Mathai JP, Nguyen M, Shore GC. Regulation of apoptosis by endoplasmic reticulum pathways. Oncogene 2003; 22:8608 - 18; http://dx.doi.org/10.1038/sj.onc.1207108; PMID: 14634622
- Bustamante-Marín X, Quiroga C, Lavandero S, Reyes JG, Moreno RD. Apoptosis, necrosis and autophagy are influenced by metabolic energy sources in cultured rat spermatocytes. Apoptosis 2012; 17:539 - 50; http://dx.doi.org/10.1007/s10495-012-0709-2; PMID: 22484449
- Shaha C, Tripathi R, Mishra DP. Male germ cell apoptosis: regulation and biology. Philos Trans R Soc Lond B Biol Sci 2010; 365:1501 - 15; http://dx.doi.org/10.1098/rstb.2009.0124; PMID: 20403866
- Meinhardt A, Hedger MP. Immunological, paracrine and endocrine aspects of testicular immune privilege. Mol Cell Endocrinol 2011; 335:60 - 8; http://dx.doi.org/10.1016/j.mce.2010.03.022; PMID: 20363290
- Hedger MP. Immunophysiology and pathology of inflammation in the testis and epididymis. J Androl 2011; 32:625 - 40; http://dx.doi.org/10.2164/jandrol.111.012989; PMID: 21764900
- Li N, Wang T, Han D. Structural, cellular and molecular aspects of immune privilege in the testis. Front Immunol 2012; 3:152; http://dx.doi.org/10.3389/fimmu.2012.00152; PMID: 22701457
- Mital P, Hinton BT, Dufour JM. The blood-testis and blood-epididymis barriers are more than just their tight junctions. Biol Reprod 2011; 84:851 - 8; http://dx.doi.org/10.1095/biolreprod.110.087452; PMID: 21209417
- Tung KS, Yule TD, Mahi-Brown CA, Listrom MB. Distribution of histopathology and Ia positive cells in actively induced and passively transferred experimental autoimmune orchitis. J Immunol 1987; 138:752 - 9; PMID: 3492532
- Takahashi K, Naito M, Terayama H, Qu N, Cheng L, Tainosho S, et al. Immunomorphological aspects of the tubuli recti and the surrounding interstitium in normal mice. Int J Androl 2007; 30:21 - 7; http://dx.doi.org/10.1111/j.1365-2605.2006.00704.x; PMID: 17328721
- Kaur G, Long CR, Dufour JM. Genetically engineered immune privileged Sertoli cells: A new road to cell based gene therapy. Spermatogenesis 2012; 2:23 - 31; http://dx.doi.org/10.4161/spmg.19119; PMID: 22553487
- Tung KS, Agersborg SS, Alard P, Garza KM, Lou YH. Regulatory T-cell, endogenous antigen and neonatal environment in the prevention and induction of autoimmune disease. Immunol Rev 2001; 182:135 - 48; http://dx.doi.org/10.1034/j.1600-065X.2001.1820111.x; PMID: 11722630
- Wheeler KM, Samy ET, Tung KS. Cutting edge: normal regional lymph node enrichment of antigen-specific regulatory T cells with autoimmune disease-suppressive capacity. J Immunol 2009; 183:7635 - 8; http://dx.doi.org/10.4049/jimmunol.0804251; PMID: 19923458
- Hedger MP. Testicular leukocytes: what are they doing?. Rev Reprod 1997; 2:38 - 47; http://dx.doi.org/10.1530/ror.0.0020038; PMID: 9414464
- Pérez C, Sobarzo C, Jacobo P, Jarazo Dietrich S, Theas M, Denduchis B, et al. Impaired expression and distribution of adherens and gap junction proteins in the seminiferous tubules of rats undergoing autoimmune orchitis. Int J Androl 2011; 34:e566 - 77; http://dx.doi.org/10.1111/j.1365-2605.2011.01165.x; PMID: 21615420
- Risley MS, Tan IP, Roy C, Sáez JC. Cell-, age- and stage-dependent distribution of connexin43 gap junctions in testes. J Cell Sci 1992; 103:81 - 96; PMID: 1331136
- Johnson KJ, Boekelheide K. Dynamic testicular adhesion junctions are immunologically unique. II. Localization of classic cadherins in rat testis. Biol Reprod 2002; 66:992 - 1000; http://dx.doi.org/10.1095/biolreprod66.4.992; PMID: 11906918
- Pérez CV, Sobarzo CM, Jacobo PV, Pellizzari EH, Cigorraga SB, Denduchis B, et al. Loss of occludin expression and impairment of blood-testis barrier permeability in rats with autoimmune orchitis: effect of interleukin 6 on sertoli cell tight junctions. Biol Reprod 2012; 87:122; http://dx.doi.org/10.1095/biolreprod.112.101709; PMID: 23018187
- Rival C, Lustig L, Iosub R, Guazzone VA, Schneider E, Meinhardt A, et al. Identification of a dendritic cell population in normal testis and in chronically inflamed testis of rats with autoimmune orchitis. Cell Tissue Res 2006; 324:311 - 8; http://dx.doi.org/10.1007/s00441-005-0129-5; PMID: 16432710
- Muth S, Schütze K, Schild H, Probst HC. Release of dendritic cells from cognate CD4+ T-cell recognition results in impaired peripheral tolerance and fatal cytotoxic T-cell mediated autoimmunity. Proc Natl Acad Sci USA 2012; 109:9059 - 64; http://dx.doi.org/10.1073/pnas.1110620109; PMID: 22615402
- Rival C, Guazzone VA, von Wulffen W, Hackstein H, Schneider E, Lustig L, et al. Expression of co-stimulatory molecules, chemokine receptors and proinflammatory cytokines in dendritic cells from normal and chronically inflamed rat testis. Mol Hum Reprod 2007; 13:853 - 61; http://dx.doi.org/10.1093/molehr/gam067; PMID: 17884838
- Guazzone VA, Jacobo P, Theas MS, Lustig L. Cytokines and chemokines in testicular inflammation: A brief review. Microsc Res Tech 2009; 72:620 - 8; http://dx.doi.org/10.1002/jemt.20704; PMID: 19263422
- Frungieri MB, Calandra RS, Lustig L, Meineke V, Köhn FM, Vogt HJ, et al. Number, distribution pattern, and identification of macrophages in the testes of infertile men. Fertil Steril 2002; 78:298 - 306; http://dx.doi.org/10.1016/S0015-0282(02)03206-5; PMID: 12137866
- Jarazo-Dietrich S, Jacobo P, Pérez CV, Guazzone VA, Lustig L, Theas MS. Up regulation of nitric oxide synthase-nitric oxide system in the testis of rats undergoing autoimmune orchitis. Immunobiology 2012; 217:778 - 87; http://dx.doi.org/10.1016/j.imbio.2012.04.007; PMID: 22672990
- Dijkstra CD, Döpp EA, Joling P, Kraal G. The heterogeneity of mononuclear phagocytes in lymphoid organs: distinct macrophage subpopulations in the rat recognized by monoclonal antibodies ED1, ED2 and ED3. Immunology 1985; 54:589 - 99; PMID: 3882559
- Hedger MP. Macrophages and the immune responsiveness of the testis. J Reprod Immunol 2002; 57:19 - 34; http://dx.doi.org/10.1016/S0165-0378(02)00016-5; PMID: 12385831
- Rival C, Theas MS, Suescun MO, Jacobo P, Guazzone V, van Rooijen N, et al. Functional and phenotypic characteristics of testicular macrophages in experimental autoimmune orchitis. J Pathol 2008; 215:108 - 17; http://dx.doi.org/10.1002/path.2328; PMID: 18381617
- Winnall WR, Muir JA, Hedger MP. Rat resident testicular macrophages have an alternatively activated phenotype and constitutively produce interleukin-10 in vitro. J Leukoc Biol 2011; 90:133 - 43; http://dx.doi.org/10.1189/jlb.1010557; PMID: 21498587
- Jacobo P, Guazzone VA, Jarazo-Dietrich S, Theas MS, Lustig L. Differential changes in CD4+ and CD8+ effector and regulatory T lymphocyte subsets in the testis of rats undergoing autoimmune orchitis. J Reprod Immunol 2009; 81:44 - 54; http://dx.doi.org/10.1016/j.jri.2009.04.005; PMID: 19520436
- Jacobo P, Pérez CV, Theas MS, Guazzone VA, Lustig L. CD4+ and CD8+ T cells producing Th1 and Th17 cytokines are involved in the pathogenesis of autoimmune orchitis. Reproduction 2011; 141:249 - 58; http://dx.doi.org/10.1530/REP-10-0362; PMID: 21109610
- Jacobo P, Guazzone VA, Theas MS, Lustig L. Testicular autoimmunity. Autoimmun Rev 2011; 10:201 - 4; http://dx.doi.org/10.1016/j.autrev.2010.09.026; PMID: 20932942
- Jacobo P, Guazzone V, Pérez C, Jarazo-Dietrich S, Theas M, Lustig L. Characterization of Foxp3+ regulatory T cells in experimental autoimmune orchitis. [Abstract] Transl Biomed 2010; 1:72
- Tompkins AB, Hutchinson P, de Kretser DM, Hedger MP. Characterization of lymphocytes in the adult rat testis by flow cytometry: effects of activin and transforming growth factor beta on lymphocyte subsets in vitro. Biol Reprod 1998; 58:943 - 51; http://dx.doi.org/10.1095/biolreprod58.4.943; PMID: 9546724
- Lu LF, Lind EF, Gondek DC, Bennett KA, Gleeson MW, Pino-Lagos K, et al. Mast cells are essential intermediaries in regulatory T-cell tolerance. Nature 2006; 442:997 - 1002; http://dx.doi.org/10.1038/nature05010; PMID: 16921386
- Rucker EB 3rd, Dierisseau P, Wagner KU, Garrett L, Wynshaw-Boris A, Flaws JA, et al. Bcl-x and Bax regulate mouse primordial germ cell survival and apoptosis during embryogenesis. Mol Endocrinol 2000; 14:1038 - 52; http://dx.doi.org/10.1210/me.14.7.1038; PMID: 10894153
- Helal MA, Mehmet H, Thomas NS, Cox PM, Ralph DJ, Bajoria R, et al. Ontogeny of human fetal testicular apoptosis during first, second, and third trimesters of pregnancy. J Clin Endocrinol Metab 2002; 87:1189 - 93; http://dx.doi.org/10.1210/jc.87.3.1189; PMID: 11889186
- Rodriguez I, Ody C, Araki K, Garcia I, Vassalli P. An early and massive wave of germinal cell apoptosis is required for the development of functional spermatogenesis. EMBO J 1997; 16:2262 - 70; http://dx.doi.org/10.1093/emboj/16.9.2262; PMID: 9171341
- Lizama C, Alfaro I, Reyes JG, Moreno RD. Up-regulation of CD95 (Apo-1/Fas) is associated with spermatocyte apoptosis during the first round of spermatogenesis in the rat. Apoptosis 2007; 12:499 - 512; http://dx.doi.org/10.1007/s10495-006-0012-1; PMID: 17195944
- Thomas DD, Ridnour LA, Isenberg JS, Flores-Santana W, Switzer CH, Donzelli S, et al. The chemical biology of nitric oxide: implications in cellular signaling. Free Radic Biol Med 2008; 45:18 - 31; http://dx.doi.org/10.1016/j.freeradbiomed.2008.03.020; PMID: 18439435
- Lue Y, Sinha Hikim AP, Wang C, Leung A, Swerdloff RS. Functional role of inducible nitric oxide synthase in the induction of male germ cell apoptosis, regulation of sperm number, and determination of testes size: evidence from null mutant mice. Endocrinology 2003; 144:3092 - 100; http://dx.doi.org/10.1210/en.2002-0142; PMID: 12810565
- Auharek SA, Lara NL, Avelar GF, Sharpe RM, França LR. Effects of inducible nitric oxide synthase (iNOS) deficiency in mice on Sertoli cell proliferation and perinatal testis development. Int J Androl 2012; 35:741 - 51; http://dx.doi.org/10.1111/j.1365-2605.2012.01264.x; PMID: 22420564
- Jacobo PV, Fass M, Pérez CV, Jarazo-Dietrich S, Lustig L, Theas MS. Involvement of soluble Fas Ligand in germ cell apoptosis in testis of rats undergoing autoimmune orchitis. Cytokine 2012; 60:385 - 92; http://dx.doi.org/10.1016/j.cyto.2012.07.020; PMID: 22892327
- Schulte M, Reiss K, Lettau M, Maretzky T, Ludwig A, Hartmann D, et al. ADAM10 regulates FasL cell surface expression and modulates FasL-induced cytotoxicity and activation-induced cell death. Cell Death Differ 2007; 14:1040 - 9; PMID: 17290285
- Kayagaki N, Kawasaki A, Ebata T, Ohmoto H, Ikeda S, Inoue S, et al. Metalloproteinase-mediated release of human Fas ligand. J Exp Med 1995; 182:1777 - 83; http://dx.doi.org/10.1084/jem.182.6.1777; PMID: 7500022
- Lizama C, Rojas-Benítez D, Antonelli M, Ludwig A, Bustamante-Marín X, Brouwer-Visser J, et al. TACE/ADAM17 is involved in germ cell apoptosis during rat spermatogenesis. Reproduction 2010; 140:305 - 17; http://dx.doi.org/10.1530/REP-10-0104; PMID: 20501791
- Richburg JH, Nañez A, Gao H. Participation of the Fas-signaling system in the initiation of germ cell apoptosis in young rat testes after exposure to mono-(2-ethylhexyl) phthalate. Toxicol Appl Pharmacol 1999; 160:271 - 8; http://dx.doi.org/10.1006/taap.1999.8786; PMID: 10544061
- Richburg JH, Nañez A. Fas- or FasL-deficient mice display an increased sensitivity to nitrobenzene-induced testicular germ cell apoptosis. Toxicol Lett 2003; 139:1 - 10; http://dx.doi.org/10.1016/S0378-4274(02)00419-8; PMID: 12595153
- Richburg JH, Nañez A, Williams LR, Embree ME, Boekelheide K. Sensitivity of testicular germ cells to toxicant-induced apoptosis in gld mice that express a nonfunctional form of Fas ligand. Endocrinology 2000; 141:787 - 93; http://dx.doi.org/10.1210/en.141.2.787; PMID: 10650961
- Lee J, Richburg JH, Younkin SC, Boekelheide K. The Fas system is a key regulator of germ cell apoptosis in the testis. Endocrinology 1997; 138:2081 - 8; http://dx.doi.org/10.1210/en.138.5.2081; PMID: 9112408
- Koji T, Hishikawa Y, Ando H, Nakanishi Y, Kobayashi N. Expression of Fas and Fas ligand in normal and ischemia-reperfusion testes: involvement of the Fas system in the induction of germ cell apoptosis in the damaged mouse testis. Biol Reprod 2001; 64:946 - 54; http://dx.doi.org/10.1095/biolreprod64.3.946; PMID: 11207212
- Tung KSK, Fusi F, Teuscher C. Autoimmune disease of spermatozoa, ovary and testis. In: Theofilopoulos AN and Boma CA, eds. The molecular pathology of autoimmune diseases. 2nd ed. New York, NY: CRC Press, 2002: 1031–1045.
- Doncel GF, Di Paola JA, Lustig L. Sequential study of the histopathology and cellular and humoral immune response during the development of an autoimmune orchitis in Wistar rats. Am J Reprod Immunol 1989; 20:44 - 51; PMID: 2803528
- Zhang J, Wong CH, Xia W, Mruk DD, Lee NP, Lee WM, et al. Regulation of Sertoli-germ cell adherens junction dynamics via changes in protein-protein interactions of the N-cadherin-beta-catenin protein complex which are possibly mediated by c-Src and myotubularin-related protein 2: an in vivo study using an androgen suppression model. Endocrinology 2005; 146:1268 - 84; http://dx.doi.org/10.1210/en.2004-1194; PMID: 15591133
- Skoudy A, Llosas MD, García de Herreros A. Intestinal HT-29 cells with dysfunction of E-cadherin show increased pp60src activity and tyrosine phosphorylation of p120-catenin. Biochem J 1996; 317:279 - 84; PMID: 8694775
- Aono S, Nakagawa S, Reynolds AB, Takeichi M. p120(ctn) acts as an inhibitory regulator of cadherin function in colon carcinoma cells. J Cell Biol 1999; 145:551 - 62; http://dx.doi.org/10.1083/jcb.145.3.551; PMID: 10225956
- Ozawa M, Ohkubo T. Tyrosine phosphorylation of p120(ctn) in v-Src transfected L cells depends on its association with E-cadherin and reduces adhesion activity. J Cell Sci 2001; 114:503 - 12; PMID: 11171320
- Guazzone VA, Denduchis B, Lustig L. Involvement of CD44 in leukocyte recruitment to the rat testis in experimental autoimmune orchitis. Reproduction 2005; 129:603 - 9; http://dx.doi.org/10.1530/rep.1.00329; PMID: 15855623
- Guazzone VA, Jacobo P, Denduchis B, Lustig L. Expression of cell adhesion molecules, chemokines and chemokine receptors involved in leukocyte traffic in rats undergoing autoimmune orchitis. Reproduction 2012; 143:651 - 62; http://dx.doi.org/10.1530/REP-11-0079; PMID: 22351899
- Guazzone VA, Hollwegs S, Mardirosian M, Jacobo P, Hackstein H, Wygrecka M, et al. Characterization of dendritic cells in testicular draining lymph nodes in a rat model of experimental autoimmune orchitis. Int J Androl 2011; 34:276 - 89; http://dx.doi.org/10.1111/j.1365-2605.2010.01082.x; PMID: 20584093
- Robertson MJ, Erwig LP, Liversidge J, Forrester JV, Rees AJ, Dick AD. Retinal microenvironment controls resident and infiltrating macrophage function during uveoretinitis. Invest Ophthalmol Vis Sci 2002; 43:2250 - 7; PMID: 12091424
- Fisson S, Darrasse-Jèze G, Litvinova E, Septier F, Klatzmann D, Liblau R, et al. Continuous activation of autoreactive CD4+ CD25+ regulatory T cells in the steady state. J Exp Med 2003; 198:737 - 46; http://dx.doi.org/10.1084/jem.20030686; PMID: 12939344
- Iosub R, Klug J, Fijak M, Schneider E, Fröhlich S, Blumbach K, et al. Development of testicular inflammation in the rat involves activation of proteinase-activated receptor-2. J Pathol 2006; 208:686 - 98; http://dx.doi.org/10.1002/path.1938; PMID: 16450334
- Meineke V, Frungieri MB, Jessberger B, Vogt H, Mayerhofer A. Human testicular mast cells contain tryptase: increased mast cell number and altered distribution in the testes of infertile men. Fertil Steril 2000; 74:239 - 44; http://dx.doi.org/10.1016/S0015-0282(00)00626-9; PMID: 10927038
- Frungieri MB, Weidinger S, Meineke V, Köhn FM, Mayerhofer A. Proliferative action of mast-cell tryptase is mediated by PAR2, COX2, prostaglandins, and PPARgamma : Possible relevance to human fibrotic disorders. Proc Natl Acad Sci USA 2002; 99:15072 - 7; http://dx.doi.org/10.1073/pnas.232422999; PMID: 12397176
- Rival C, Theas MS, Guazzone VA, Lustig L. Interleukin-6 and IL-6 receptor cell expression in testis of rats with autoimmune orchitis. J Reprod Immunol 2006; 70:43 - 58; http://dx.doi.org/10.1016/j.jri.2005.10.006; PMID: 16458979
- Suescun MO, Rival C, Theas MS, Calandra RS, Lustig L. Involvement of tumor necrosis factor-alpha in the pathogenesis of autoimmune orchitis in rats. Biol Reprod 2003; 68:2114 - 21; http://dx.doi.org/10.1095/biolreprod.102.011189; PMID: 12606341
- Theas MS, Rival C, Jarazo-Dietrich S, Jacobo P, Guazzone VA, Lustig L. Tumour necrosis factor-alpha released by testicular macrophages induces apoptosis of germ cells in autoimmune orchitis. Hum Reprod 2008; 23:1865 - 72; http://dx.doi.org/10.1093/humrep/den240; PMID: 18579514
- Scallon B, Cai A, Solowski N, Rosenberg A, Song XY, Shealy D, et al. Binding and functional comparisons of two types of tumor necrosis factor antagonists. J Pharmacol Exp Ther 2002; 301:418 - 26; http://dx.doi.org/10.1124/jpet.301.2.418; PMID: 11961039
- Theas S, Rival C, Lustig L. Germ cell apoptosis in autoimmune orchitis: involvement of the Fas-FasL system. Am J Reprod Immunol 2003; 50:166 - 76; http://dx.doi.org/10.1034/j.1600-0897.2003.00074.x; PMID: 12846681
- Kiener PA, Davis PM, Rankin BM, Klebanoff SJ, Ledbetter JA, Starling GC, et al. Human monocytic cells contain high levels of intracellular Fas ligand: rapid release following cellular activation. J Immunol 1997; 159:1594 - 8; PMID: 9257817
- el-Demiry MI, Hargreave TB, Busuttil A, Elton R, James K, Chisholm GD. Immunocompetent cells in human testis in health and disease. Fertil Steril 1987; 48:470 - 9; PMID: 2957238
- Theas MS, Rival C, Dietrich SJ, Guazzone VA, Lustig L. Death receptor and mitochondrial pathways are involved in germ cell apoptosis in an experimental model of autoimmune orchitis. Hum Reprod 2006; 21:1734 - 42; http://dx.doi.org/10.1093/humrep/del066; PMID: 16585127
- Pöllänen P, Niemi M. Immunohistochemical identification of macrophages, lymphoid cells and HLA antigens in the human testis. Int J Androl 1987; 10:37 - 42; http://dx.doi.org/10.1111/j.1365-2605.1987.tb00163.x; PMID: 3294605
- Suominen J, Söderström KO. Lymphocyte infiltration in human testicular biopsies. Int J Androl 1982; 5:461 - 6; http://dx.doi.org/10.1111/j.1365-2605.1982.tb00277.x; PMID: 7174127
- Duan YG, Yu CF, Novak N, Bieber T, Zhu CH, Schuppe HC, et al. Immunodeviation towards a Th17 immune response associated with testicular damage in azoospermic men. Int J Androl 2011; 34:e536 - 45; http://dx.doi.org/10.1111/j.1365-2605.2010.01137.x; PMID: 21332504
- Białas M, Fiszer D, Rozwadowska N, Kosicki W, Jedrzejczak P, Kurpisz M. The role of IL-6, IL-10, TNF-alpha and its receptors TNFR1 and TNFR2 in the local regulatory system of normal and impaired human spermatogenesis. Am J Reprod Immunol 2009; 62:51 - 9; http://dx.doi.org/10.1111/j.1600-0897.2009.00711.x; PMID: 19527232
- Hussein MR, Abou-Deif ES, Bedaiwy MA, Said TM, Mustafa MG, Nada E, et al. Phenotypic characterization of the immune and mast cell infiltrates in the human testis shows normal and abnormal spermatogenesis. Fertil Steril 2005; 83:1447 - 53; http://dx.doi.org/10.1016/j.fertnstert.2004.11.062; PMID: 15866583
- Haidl G, Duan YG, Chen SJ, Kohn FM, Schuppe HC, Allam JP. The role of mast cells in male infertility. Expert Rev Clin Immunol 2011; 7:627 - 34; http://dx.doi.org/10.1586/eci.11.57; PMID: 21895475
- Welter H, Köhn FM, Mayerhofer A. Mast cells in human testicular biopsies from patients with mixed atrophy: increased numbers, heterogeneity, and expression of cyclooxygenase 2 and prostaglandin D2 synthase. Fertil Steril 2011; 96:309 - 13; http://dx.doi.org/10.1016/j.fertnstert.2011.05.035; PMID: 21683347
- Salomon F, Saremaslani PP, Jakob M, Hedinger CE. Immune complex orchitis in infertile men. Immunoelectron microscopy of abnormal basement membrane structures. Lab Invest 1982; 47:555 - 67; PMID: 6755064
- Lehmann D, Temminck B, Da Rugna D, Leibundgut B, Sulmoni A, Müller HJ. Role of immunological factors in male infertility. Immunohistochemical and serological evidence. Lab Invest 1987; 57:21 - 8; PMID: 3298848
- Lustig L, Doncel GF, Berensztein E, Denduchis B. Testis lesions and cellular and humoral immune responses induced in rats by immunization with laminin. Am J Reprod Immunol Microbiol 1987; 14:123 - 8; PMID: 3324774
- Lustig L, Denduchis B, Ponzio R, Lauzon M, Pelletier RM. Passive immunization with anti-laminin immunoglobulin G modifies the integrity of the seminiferous epithelium and induces arrest of spermatogenesis in the guinea pig. Biol Reprod 2000; 62:1505 - 14; http://dx.doi.org/10.1095/biolreprod62.6.1505; PMID: 10819750