Abstract
During spermatogenesis, spermatogonia (2n, diploid) undergo a series of mitotic divisions as well as differentiation to become spermatocytes, which enter meiosis I to be followed by meiosis II to form round spermatids (1n, haploid), and then differentiate into spermatozoa (1n, haploid) via spermiogenesis. These events take place in the epithelium of the seminiferous tubule, involving extensive junction restructuring at the Sertoli-Sertoli and Sertoli-germ cell interface to allow the transport of developing germ cells across the epithelium. Although structural aspects of these cell-cell junctions have been studied, the underlying mechanism(s) that governs these events has yet to be explored. Earlier studies have shown that a non-receptor protein tyrosine kinase known as focal adhesion kinase (FAK) is a likely regulator of these events due to the stage-specific and spatiotemporal expression of its various phosphorylated/activated forms at the testis-specific anchoring junctions in the testis, as well as its association with actin regulatory proteins. Recent studies have shown that FAK, in particular its two activated phosphorylated forms p-FAK-Tyr407 and p-FAK-Tyr397, are crucial regulators in modulating junction restructuring at the Sertoli cell-cell interface at the blood-testis barrier (BTB) known as the basal ectoplasmic specialization (basal ES), as well as at the Sertoli-spermatid interface called apical ES during spermiogenesis via its effects on the filamentous (F)-actin organization at the ES. We herein summarize and critically evaluate the current knowledge regarding the physiological significance of FAK in regulating BTB and apical ES dynamics by governing the conversion of actin filaments at the ES from a “bundled” to a “de-bundled/branched” configuration and vice versa. We also provide a molecular model on the role of FAK in regulating these events based on the latest findings in the field.
Introduction
Spermatogenesis takes place in the seminiferous tubule—the functional unit in the testis—to produce spermatozoa (haploid, 1n) from spermatogonia (diploid, 2n) via spermatogenesis.Citation1-Citation3 This highly complex cellular process contains four distinct events, namely mitosis, meiosis, spermiogenesis, and spermiation that constitute the seminiferous epithelial cycle. The seminiferous epithelium, on the other hand, is anatomically segregated into two compartments, the basal and the apical compartment, by the blood-testis barrier (BTB) (). The BTB is constituted by multiple co-existing junctions: (1) testis-specific adherens junction (AJ) called basal ectoplasmic specialization (basal ES), (2) tight junction (TJ), and (3) gap junction (GJ), which together with (4) desmosome at the Sertoli cell-cell interface near the basement membrane of the tunica propria, create one of the tightest blood-tissue barriers in mammals ().Citation4-Citation6 Thus, in the mammalian testis, endothelial TJ barrier of the microvessels in the interstitium contributes virtually no barrier function of the BTB.Citation4,Citation7 The hallmark ultrastructural feature of the BTB, unlike all other blood-tissue barriers,Citation8 is the tightly packed actin filament bundles that line perpendicular to the Sertoli cell plasma membrane, which are sandwiched in-between cisternae of endoplasmic reticulum and the apposing Sertoli cell plasma membranes.Citation9 This unusual ultrastructural feature of the BTB was first described in the early 1970s in the testis.Citation10,Citation11 The term ectoplasmic specialization (ES) was subsequently used in the late 1970s when similar bundles of actin filaments were also found at the Sertoli-spermatid and Sertoli cell-cell interface and designated apical and basal ES, respectively.Citation12-Citation15 The only ultrastructural difference between the apical and basal ES is that the actin filament bundles are not found in the spermatid (step 8–19 spermatids) but restricted only to the Sertoli cell, such that there are only a single array of actin filament bundles at the apical ES vs. two layers of these F-actin bundles at the basal ES (). It is also these actin filament bundles that confer the unusual adhesive strength to the ES in the testis,Citation16,Citation17 making the BTB one of the tightest blood-tissue barriers.Citation8 However, the basal ES/BTB undergoes extensive restructuring at stage VIII of the epithelial cycle to facilitate the transit of preleptotene spermatocytes across the BTB to enter the adluminal compartment to prepare for meiosis.Citation18,Citation19 Furthermore, the apical ES also restructures extensively during spermiogenesis to facilitate the transport of spermatids across the seminiferous epithelium during the epithelial cycle. Once the apical ES forms at the Sertoli-step 8 spermatid interface, it is the only anchorage device, replacing desmosome and GJ, and persists until step 19 spermatids that line up at the luminal edge of the seminiferous tubule to prepare for the release of sperm at spermiation.Citation19-Citation21 Thus, it is conceivable that during spermatogenesis, extensive junction restructuring and cytoskeletal reorganization take place in the seminiferous epithelium to facilitate the transport of: (1) preleptotene spermatocytes across the BTB and (2) spermatids across the epithelium during the epithelial cycle, yet the regulatory biomolecules and/or mechanism(s) remain elusive until recently.
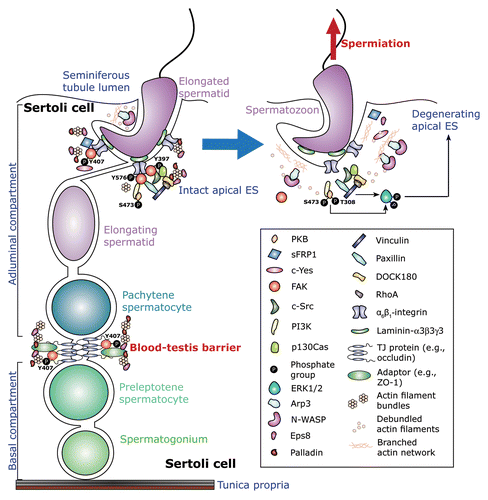
Figure 1. An schematic drawing illustrating the regulatory events mediated by the β1-integrin/FAK signaling cascades during apical ES degeneration at spermiation. This is a schematic drawing of the seminiferous epithelium in the rat testis in which the blood-testis barrier (BTB) physically divides the epithelium into the adluminal (apical) and the basal compartment with the base of the Sertoli cell lies on the basement membrane of the tunica propria. Apical ES first appears at the interface of step 8 spermatids and Sertoli cells at stage VIII of the epithelial cycle, once it forms, it is the only anchoring device in these spermatids, replacing desmosome and gap junction at the Sertoli-spermatid interface, and to confer spermatid polarity.Citation74 Also, apical ES is found in step 8–19 spermatids, and it begins to undergo degeneration at stage VII of the cycle until it is disintegrated entirely at late stage VIII to allow the release of sperm at spermiation.Citation19,Citation20,Citation54 In stage VII-early stage VIII tubules (left panel) as shown herein, spermatids attach to the Sertoli cell through an adhesion protein complex containing α6β1-integrin and laminin-α3β3γ3 (other adhesion protein complexes at the apical ES are: JAM-C-ZO-1, nectin2/3-afadin, and N-cahderin-β-cateninCitation21). This complex is known to associate with a large number of regulatory proteins,Citation23,Citation49,Citation50 which include PKB, DOCK180, PI3K, and most notably FAK, in particular p-FAK-Tyr397, p-FAK-Tyr407, and p-FAK-Tyr576, which recruit actin cross-linking and bundling proteins, such as Eps8, palladin, and filamin A, to maintain the integrity of the actin filament bundles at the ES. Collectively, these proteins to confer the integrity of the apical ES. At late stage VIII of the epithelial cycle, to prepare for spermiation, the expression of FAK, in particular p-FAK-Tyr397 and -Tyr407, at the apical ES is downregulated.Citation23,Citation32,Citation33 This loss of FAK at the apical ES fails to retain the actin filament cross-linking, barded end-capping and bundling proteins (e.g., Eps8, palladin, filamin A) at the apical ES to maintain the integrity of the actin filament bundles, instead, N-WASP activated-Arp2/3 protein complex induces branched actin polymerization, converting the actin filaments from their “unbundled” and their “de-bundled” configuration, destabilizing the apical ES, facilitating endocytic vesicle-mediated protein trafficking to further destabilize apical ES adhesion. Furthermore, apical ES disruption involves also the PI3K/PKB signaling cascades in which ERK1/2, a downstream signaling protein, is activated via phosphorylation (arrow),Citation49,Citation50,Citation85,Citation86 which further enhances apical ES disruption and, thus, facilitating the release of sperm at spermiation.
Focal adhesion kinase (FAK), a non-receptor protein tyrosine kinase, was first shown in the late 1980s to be highly expressed in the testis with an expression level significantly higher than that of other non-gonadal tissues.Citation22 Although this finding suggested its physiological significance in regulating cellular event in testes, its function has remained unexplored until almost 15 y later when FAK was first reported to be a crucial regulator of junction dynamics in spermatogenesis.Citation23 Herein, we summarize and critically evaluate the recent findings on the role of FAK in the testis.
Focal adhesion kinase (FAK)
Focal adhesion kinase (FAK, ~120 kDa), also called protein tyrosine kinase 2 (PTK2), is a non-receptor protein tyrosine kinase, which has been found to be expressed ubiquitously in mammalian tissues including brain,Citation24 lymphocytes,Citation25 and testes.Citation26,Citation27 FAK contains four linearly arranged functional domains from its N terminus: the band 4.1, Ezrin, Radixin, Moesin (FERM) domain, the catalytic kinase domain, three proline-rich regions, and the focal adhesion targeting (FAT) domain.Citation28,Citation29 FAK, as its name implies, is restricted to the focal contact (or focal adhesion complex, FAC), which is an actin-based anchoring junction limited to the cell-extracellular matrix (ECM) interface in mammalian tissues. It is mostly used by motile cells such as fibroblasts, lymphocytes, and metastatic cancer cells for their movement over basal lamina under physiological (e.g., growth, inflammation, combat bacterial/viral infection) or pathophysiological conditions (e.g., tumorigenesis). In the testis, however, FAC is absent in the seminiferous tubules at the Sertoli cell-basement membrane (BM) interface since the BM is a modified form of ECM in the testis.Citation30,Citation31 Instead, FAK is found at the Sertoli cell-cell interface at the basal ES in the BTB and also at the Sertoli-spermatid interface at the apical ES.Citation23,Citation32,Citation33 FAK, besides being a kinase that phosphorylates downstream signaling target proteins, also functions as a scaffolding and adaptor protein that mediates the assembly of signaling protein complexes via its protein-protein-interacting domains along its polypeptide sequence, in particular, to transducing the integrin-based signals.Citation34-Citation36 It plays an important role in regulating cell proliferation, apoptosis, and cell motility.Citation36,Citation37 An elevated expression of FAK also correlates with tumor cell proliferation and metastasis.Citation38-Citation40 In fact, FAK is an oncogene and a therapeutic target of cancer therapy.Citation41,Citation42 Collectively, these findings illustrate the pivotal role of FAK in cellular functions, both in health and in disease.
In order to exert its intrinsic kinase activity, FAK must first be activated. There are six putative tyrosine phosphorylation sites in FAK, including Tyr-397, -407, -576, -577, -861, and -925,Citation35,Citation43 and among them, Tyr-397 is the only autophosphorylation site. Upon phosphorylation of Tyr-397, a high-affinity binding site for Src homology 2 (SH2) domain is exposed that allows FAK to act as an adaptor protein to assemble various SH2 domain-containing regulatory proteins, such as Src family kinases,Citation34,Citation44,Citation45 to assemble a multiprotein functional complex. While Tyr-397 is autophosphorylated, other Tyr residues in FAK are phosphorylated by Src family kinases and, in turn, lead to respective downstream effects, illustrating the tight physiological relationship between FAK and Src kinases. In fact, the FAK-Src dual kinase complex is an emerging target in cancer therapy,Citation46 and a crucial functional protein complex in cellular physiological events. Apart from tyrosine phosphorylation, FAK can also be phosphorylated on several Ser residues. For instance, Ser-722 phosphorylation inhibits the intrinsic FAK kinase catalytic activity,Citation47 while Ser-732 phosphorylation leads to changes in microtubule organization, nuclear movement, and neuronal migration.Citation48
FAK is a regulator of the apical ES
Apical ES is an F-actin-rich cell-cell AJ restricted to the Sertoli-spermatid (step 8–19 and 8–16 spermatids in the rat and mouse testis, respectively) interface. FAK was first identified in the rat testis by fluorescence microscopy and shown to be a component in the basal compartment of the seminiferous epithelium as well as at the apical ES.Citation27 Further studies have shown that the activated forms of FAK, phosphorylated (p)-FAK-Tyr397 and also p-FAK-Tyr576, are restricted to the apical ES and display stage-specific and spatiotemporal expression at the apical ES at stage VI‒VIII of the epithelial cycle, whereas FAK is most predominant at the BTB in virtually all stages of the cycle.Citation23 Furthermore, p-FAK-Tyr397 forms a complex with the apical ES-associated proteins such as β1-integrin, c-Src, and vinculin complex,Citation23 indicating its role in mediating β1-integrin signaling pathway at the apical ES. Subsequent studies have confirmed that p-FAK-Tyr397 is an integrated component of the α6β1-integrin-based adhesion complex at the apical ES, which persists until spermiation.Citation32 Thus, p-FAK-Tyr397 is likely a crucial protein in conferring spermatid adhesion and also a regulator during the release of sperm at spermiation.Citation20 Furthermore, FAK that works in concert with its partner proteins can create a giant regulatory protein complex composed of p130Cas (p130 Crk-associated substrate), DOCK180 (Dedicator of cytokinese 180), RhoA and vinculin (and its associated partners such as Crk, R-ras, and Grb2), which, in turn, is associated with β1-integrin.Citation49 In studies using Sertoli-germ cell co-cultures and rats treated with adjudin (a contraceptive drug known to induce apical ES and other anchoring junction restructuring in the testis)Citation4 to investigate spermatid adhesion, the β1-integrin-p-FAK-p130Cas-DOCK180-RhoA-vinculin complex emerges as a crucial role in mediating alterations on the actin-based cytoskeleton and subsequently modulating spermatid transport and spermiation during spermatogenesis.
With the discoveries of the structural components (e.g., integral membrane protein β1-integrin, adaptor proteins vinculin, and paxillin) that are associated with FAK at the apical ES, in particular, its three activated forms p-FAK-Tyr397,Citation23,Citation50 p-FAK-Tyr576,Citation23 and p-FAK-Tyr407,Citation33 and their unique stage-specific and spatiotemporal expression in the seminiferous epithelium,Citation23,Citation33 these observations implicate their likely roles in regulating apical ES dynamics during spermatid transport and spermiation via a modulation of actin filament network in the seminiferous epithelium. For instance, while p-FAK-Tyr397 and -Tyr407 are both highly expressed at the apical ES at stage VII of the epithelial cycle, p-FAK-Tyr397 is restricted to the convex side of the spermatid head and co-localized with β1-integrin,Citation23,Citation33 whereas p-FAK-Tyr407 is expressed almost exclusively to the concave side of the spermatid head and co-localized with Arp3.Citation33 Arp3 (actin-related protein 3, which together with Arp2 forms the Arp2/3 complex, which can be by N-WASP, neuronal Wiskott-Aldrich syndrome proteinCitation51,Citation52) is known to induce barbed end nucleation of an existing actin filament, thus effectively creating an extensive branched actin network. In short, the N-WASP/Arp2/3 protein complex effectively converts actin filaments from a “bundled” to a “de-bundled/branched” configuration, thereby destabilizing the ES-based cell adhesion and to facilitate endocytic vesicle-mediated protein trafficking.Citation51,Citation53 Indeed, recent studies have shown that this site of the apical ES at the concave side of the spermatid head is where endocytic vesicle-mediated protein trafficking takes place to facilitate endocytosis, transcytosis, and recycling of apical ES proteins, such that “old” apical ES proteins can be used to assemble “new” apical ES derived from step 8 spermatids via spermiogenesis.Citation21,Citation54 On the other hand, the convex side of the spermatid head is being used to confer spermatid adhesion at stage VII of the epithelial cycle since both Eps8 (epidermal growth factor receptor pathway substrate 8, an actin barbed end capping and bundling protein) and palladin (an actin cross-linking and bundling protein) are also highly expressed at this siteCitation55,Citation56 when p-FAK-Tyr397 is upregulated.Citation33 These actin bundling proteins can thus be used to maintain the integrity of the actin filament bundles at the convex side of the spermatid head to anchor these spermatids onto the Sertoli cell in the epithelium. Interestingly, at late stage VIII of the epithelial cycle, the expression of p-FAK-Tyr397, Eps8, and palladin; as well as p-FAK-Tyr407 and Arp3 are all subsided considerably and they are virtually non-detectable at the apical ES to facilitate the release of sperm at spermiation.Citation33,Citation55-Citation57 In short, it is highly likely that these two forms of p-FAK regulate the intrinsic activity of these actin bundling and nucleation proteins to induce re-organization of the network of actin filament bundles at the apical ES during the epithelial cycle to facilitate both spermatid transport across the epithelium during spermiogenesis and the release of sperm at spermiation. This conclusion is supported by findings in a recent report, which have demonstrated that overexpression of a p-FAK-Tyr407 phosphomimetic mutant FAK Y407E in Sertoli cells with an established TJ-permeability barrier significantly enhances the kinetics of actin polymerization,Citation33 illustrating that p-FAK-Tyr407 at the apical ES can indeed modify the organization of the F-actin network. Furthermore, the knockdown of c-Yes by ~70% in the testis in vivo by RNAi also impedes the localization and downregulates the expression of p-FAK-Tyr407 at the apical ES, causing defects in spermiation in which elongated spermatids are trapped deep inside the seminiferous epithelium in stage VIII tubules, failing to undergo spermiation and these spermatids also display a loss of polarity in which their heads are no longer pointing toward the basement membrane but aligned randomly in the seminiferous epithelium.Citation58 More important, this downregulation of p-FAK-Tyr407 following the knockdown of c-Yes in the testis also associates with changes in actin polymerization.Citation58 Taken collectively, these findings have unequivocally demonstrated that the regulating roles of these phosphorylated FAK forms in F-actin reorganization at the apical ES. Furthermore, several protein kinases (e.g., PKB), lipid kinases (e.g., PI3K), and regulatory proteins (e.g., RhoA GTPase, DOCK180) that are known to be involved in regulatory actin dynamics are also binding partners of FAK and/or its phosphorylated forms (). summarizes these latest findings in the field, regarding the role of FAK in particular p-FAK-Tyr397, -Tyr407, and -Tyr576, in regulating apical ES dynamics during spermatogenesis, depicting a likely regulatory model. Obviously, much work is needed to define the role of FAK in relation to other protein kinases and actin regulatory proteins in conferring F-actin organization at the apical ES as well as other cellular events of spermatogenesis, such as apoptosis, mitosis, meiosis, and cell metabolism.
Table 1. Regulators associate with FAK and/or its phosphorylated/activated form(s) at the apical ES
FAK is a regulator of the blood-testis barrier (BTB)
In the mammalian testis, the BTB is different from other blood-tissue barriers (e.g., the blood-brain barrier, the blood-retina barrier). The blood-brain and the blood-retina barriers are constituted almost exclusively by the endothelial TJ-barrier of the microvessels in the brain and the eye, respectively.Citation59-Citation61 Instead, the BTB is morphologically marked by the presence of a testis-specific F-actin-rich adherens junction (AJ) called basal ectoplasmic specialization (basal ES), which is restricted between adjacent Sertoli cells near the basement membrane.Citation5,Citation9,Citation62,Citation63 The basal ES is ultrastructurally identical to the apical ES when examined by electron microscopy, except that bundles of actin filaments that line perpendicular to the plasma membrane are found on both sides of the Sertoli cells and are sandwiched in-between cisternae of endoplasmic reticulum and the plasma membrane, instead of limiting only to the Sertoli cell at the apical ES.Citation4,Citation9,Citation21,Citation62 Thus, two layers of actin filament bundles are found at the BTB. Furthermore, basal ES coexists with tight junction (TJ) and gap junction (GJ), which together with desmosome constitute the BTB, making it one of the tightest blood-tissue barriers.Citation7,Citation64 Previous studies have identified multiple adhesion protein complexes, such as TJ-protein complexes: occludin-zonula occludens 1 (ZO-1), JAM-A-ZO-1, JAM-B-ZO-1, and claudins-ZO-1; basal ES-protein complexes: N-cadherin-β-catenin, nectin-2-afadin; GJ-protein complexes: connexin43-plakophilin-2; and desmosome protein complexes: desmoglein-2‒desmocollin-2, which constitute the BTB.Citation21,Citation65 Interestingly, FAK is structurally associated with the occludin-ZO-1 protein complex at the BTBCitation66 instead of restricted to the Sertoli cell-basement membrane since FAC is absent in the testis.Citation4 Subsequent studies have shown that occludin is a putative substrate of FAK, since the knockdown of FAK at the Sertoli cell BTB alters the phosphorylation status of occludin, impeding occludin-ZO-1 association, thereby destabilizing the Sertoli cell TJ-permeability barrier.Citation67 These findings thus illustrate the pivotal role of FAK in conferring adhesion function at the Sertoli cell BTB via its effects on the phosphorylation status of the occludin-ZO-1 complex.
A more recent report using various mutants of p-FAK-Tyr397 and -Tyr407 for their overexpression in Sertoli cells cultured in vitro with a functional TJ-permeability barrier that mimics the BTB in vivo has shown that p-FAK-Tyr407 is promoting the Sertoli cell BTB function, tightening the TJ-barrier.Citation33 However, p-FAK-Tyr397 is promoting the BTB disruption, making the Sertoli TJ-permeability barrier “leaky.”Citation33 In short, the p-FAK-Tyr407 and -Tyr397 forms of FAK have antagonistic effects on the Sertoli cell BTB, illustrating these two non-receptor protein tyrosine kinases may serve as molecular switches to turn “on” and “off” the TJ-barrier during the transit of preleptotene spermatocytes across the BTB at stage VIII of the epithelial cycle. This concept, besides supported by the antagonistic effects of these two forms of FAK, is also strengthened by the stage-specific and spatiotemporal expression of p-FAK-Tyr407 at the BTB as well as its association with Arp3 of the Arp2/3 protein complex. For instance, p-FAK-Tyr407 is structurally associated with N-WASP,Citation33 suggesting N-WASP is also a substrate of FAK and overexpression of p-FAK-Tyr407 phosphomimetic mutant in the Sertoli cell epithelium that promotes the Sertoli TJ-barrier function also induces an increase in the association of Arp3 and N-WASP.Citation33 These findings are important because they illustrate that FAK exerts its effects via its p-FAK-Tyr407 and -Tyr397 forms to regulate F-actin organization at the BTB by modulating the conversion of actin filaments from a “bundled” to a “debundled/branched” configuration, conferring plasticity to the F-actin network at the ES. Furthermore, the two phosphorylated forms of FAK are known to interact with several regulatory proteins. For instance, SHP2 (Src homology domain-containing phosphatase-2, a ubiquitously expressed non-receptor protein tyrosine phosphatase in mammalian cells, also known as PTPN11, tyrosine-protein phosphatase non-receptor type 11, an enzyme encoded by PTPN11 gene in humans) is known to downregulate the expression of p-FAK-Tyr397 and initiates the mitogen-activated kinase (MAPK) signaling pathway, subsequently modulating actin cytoskeleton.Citation68 is a schematic drawing which depicts a hypothetical model on the role of FAK in regulating F-actin organization at the BTB during the epithelial cycle of spermatogenesis.
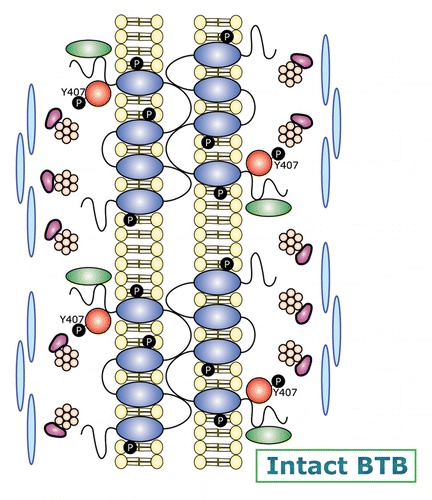
Figure 2. A schematic diagram illustrating the molecular architecture of the BTB and its restructuring events that are mediated by FAK during the seminiferous epithelial cycle. The panel on the left is a schematic drawing that illustrates the relative location of the BTB in the seminiferous epithelium. The BTB is enlarged and shown in the right panel. The upper part of the diagram on the right displays the molecular architecture of an intact BTB. The relatively high expression of p-FAK-Tyr407 at the BTB, coupled with the upregulation of Eps8 (an actin barbed end capping and bundling protein), and two actin cross-linking and bundling proteins palladin and filamin A at the BTB thus maintain the integrity of the actin filament bundles at the BTB. Occludin/ZO-1 and other TJ proteins (e.g., JAM-B/ZO-1, JAM-A-ZO-1), together with basal ES proteins (e.g., N-cadherin/β-catenin, nectin-2/afadin), gap junction proteins (e.g., connexin-43, connexin-33), and desmosomal proteins (e.g., desmoglein-2), thus confer Sertoli cell-cell adhesion to constitute the blood-testis barrier (BTB). This thus maintains the BTB integrity, such as at stage VII of the epithelial cycle. At stage VIII of the epithelial cycle, BTB undergoes modifications as shown in the lower part of the diagram on the right panel. This is likely mediated via a downregulation of p-FAK-Tyr407, which coupled with an upregulation of the Arp2/3 complex and N-WASP, thereby converting actin filament bundles from their “bundled” to their “unbundled/branched” configuration, destabilizing the BTB to facilitate endocytic vesicle-mediated protein trafficking, facilitating BTB restructuring to allow the transport of preleptotene spermatocytes across the BTB. Other signaling proteins, such as the phosphatase, SHP2 (Src homology domain-containing phosphatase-2) may also take part in this event.Citation68
FAK and the apical ES-BTB-BM (apical ectoplasmic specialization-blood-testis barrier-basement membrane) functional axis in the testis
Since the initial discovery of the seminiferous epithelial cycle of spermatogenesis in the 1950–60s in rodents and humans,Citation69-Citation72 it is known that cellular events that occur across the seminiferous epithelium are tightly regulated.Citation18,Citation19,Citation73,Citation74 However, the molecular basis that coordinates these events is virtually unknown until a report published in 2008,Citation75 demonstrating for the first time the presence of a local functional axis that coordinates these events known as the apical ES-BTB-BM axis.Citation21,Citation75 In this first report,Citation75 it was shown that overexpression of fragments of laminin chains (note: laminins, such as laminin-α3β3γ3, are components of the adhesion protein complex at the apical ESCitation76-Citation78) or inclusion of purified recombinant proteins of these fragments in Sertoli cells cultured in vitro with an established TJ-permeability barrier, they both perturbed the Sertoli cell TJ-barrier function. These observations thus suggest that MMP-2 (matrix metalloprotease-2), which is highly expressed at the apical ES at stage VIII of the epithelial cycle,Citation77 likely cleaves laminin chains at the apical ES during its degeneration at spermiation to generate the biologically active fragments to induce BTB restructuring, thereby coordinating the cellular events of spermiation and BTB restructuring that take place concurrently but at the opposite ends of the epithelium at stage VIII of the epithelial cycle. In short, there is a functional axis between the apical ES and the BTB, which is mediated by the autocrine-based laminin fragments. Since apical ES was absent in these cultures due to the lack of elongating/elongated spermatids, the knockdown of β1-integin by RNAi (note: β1-integrin is a component of the apical ES and also the hemidesmosome at the Sertoli cell-BM interface) was also found to induce BTB restructuring.Citation75 Thus, the BTB and the hemidesmosome at the BM are also functionally linked. Additionally, recent studies have shown that biologically active fragments are also released by collagen chains in the BM that regulate BTB function, confirming the presence of the BTB-BM axis.Citation79 This apical ES-BTB-BM functional axis has since been confirmed in which the biologically active domain of at least two laminin chains are identified and they have shown to be potent biologically active peptides to regulate Sertoli BTB function both in vitro and in vivo in a reversible fashion.Citation80 Furthermore, studies using the phthalate-induced Sertoli cell injury model have also confirmed the presence of this local functional axis in the testis.Citation81-Citation83
A recent report has shown that the p-FAK-Tyr397 and p-FAK-Tyr407 are the likely “on” and “off” molecular switches in this apical ES-BTB-BM functional axis that modulate the organization of actin filament bundles at the apical ES, as well as the basal ES. For instance, p-FAK-Tyr407 and p-FAK-Tyr397 promotes and disrupts the Sertoli cell TJ-permeability barrier function, respectively, which is mediated via their effects on the organization of F-actin network at the BTB.Citation33 In short, biologically active laminin fragments released from the apical ES can alter the spatiotemporal expression of these molecular “switches” in the seminiferous epithelium, which, in turn, affects re-organization of F-actin at the basal ES, promoting BTB restructuring. This hypothesis is supported by findings that following administration of the biologically active laminin F5 peptide, there is a downregulation and mis-localization of p-FAK-Tyr407 at the apical and basal ES, which is also associated with a disruption of F-actin organization at both sites, leading to spermatid loss from the epithelium and BTB disruption.Citation80 At present, the receptor(s) for the laminin fragments, such as F5 peptide, at the BTB is unknown, but β1-integrin is the likely receptor of the laminin fragments at the BM. It is likely that the p-FAK-Tyr407 and -Tyr397 serve as the downstream regulators of the laminin fragment (ligand)-integrin (receptor) complex in this functional axis that coordinates different cellular events that take place across the seminiferous epithelium during the epithelial cycle. is a schematic drawing that illustrates a hypothetical model, in particular, the early signaling cascades along the apical ES-BTB functional axis in the seminiferous epithelium.
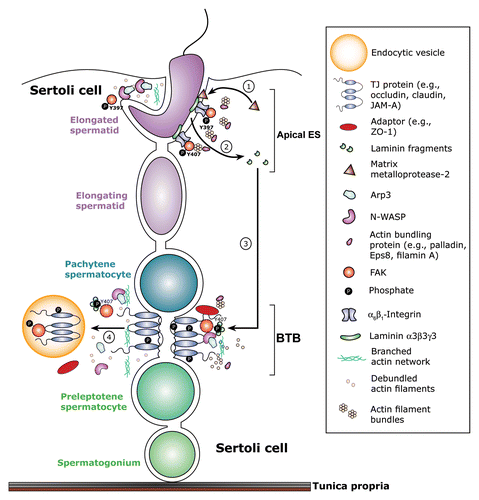
Figure 3. The role of p-FAK-Tyr397 and -Tyr407 in coordinating cellular events at the apical ES-BTB functional axis during the epithelial cycle of spermatogenesis. FAK, in particular its two activated/phosphorylated forms of p-FAK-Tyr397 and -Tyr407 is tightly involved in coordinating the events of spermiation and BTB restructuring that take place simultaneously across the epithelium at stage VIII of the seminiferous epithelial cycle. p-FAK-Tyr397 that is highly expressed at early stage VIII of the cycle may: (1) involve in the activation and/or upregulation of matrix metalloproteinase 2 (MMP-2) which cleaves laminin-β3 and -γ3 chains. (2) This thus generates biologically active laminin fragments which are released from the apical ES to activate BTB restructuring via an “inside-outside-in” signaling cascade, involving p-FAK-Tyr407.Citation80 (3) It is likely that the biologically active laminin fragments are working in concert with p-FAK-Tyr407 to recruit N-WASP-Arp2/3 complex to the site to induce F-actin re-organization, converting actin filaments from their “bundled” to their “unbundled/branched” configuration, thereby destabilizing the BTB. (4) The “unbundled/branched” F-actin network at the BTB thus favors endocytic vesicle-mediated protein trafficking, inducing protein endocytosis, such as TJ protein occludin, leading to BTB restructuring. It is obvious that this model will be rapidly updated when more functional data are available in the near future.
Concluding remarks and future perspectives
Herein, we briefly summarize the critical role of FAK in the seminiferous epithelium of the rat testis. It is likely that the stage-specific and spatiotemporal expression of p-FAK-Tyr397 and p-FAK-Tyr407 at the apical and/or basal ES serve as the downstream signal transducers of the laminin (ligand)-integrin (receptor) complex in the apical ES-BTB-BM functional axis. These signaling complexes either are working in concert with adhesion protein complexes at the ES (e.g., occludin-ZO-1 complex) or actin regulatory proteins (e.g., the N-WASP-Arp2/3 complex, palladin, drebrin E, Eps8) to modulate cell adhesion function and the organization of F-actin at the ES. A better understanding on FAK in the testis should reveal novel targets for male contraceptive development and also insightful information on toxicant-induced reproductive dysfunction since the apical ES-BTB-BT axis is an emerging target of toxicant-induced male infertility.Citation84
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding
This work was supported by grants from the National Institutes of Health, NICHD R01 HD056034 to CYC, and NICHD U54 HD029990 Project 5 to CYC.
References
- de Kretser DM, Kerr JB. The cytology of the testis. in The Physiology of Reproduction. Vol. 1 (eds. Knobil, E., et al.) 837-932 (Raven Press, New York, 1988).
- Sharpe RM. Regulation of spermatogenesis. In: The Physiology of Reproduction. Eds. Knobil, E., Neill, J.D. New York, Raven Press. pp. 1363-1434 (1994).
- Ehmcke J, Schlatt S. A revised model for spermatogonial expansion in man: lessons from non-human primates. Reproduction 2006; 132:673 - 80; http://dx.doi.org/10.1530/rep.1.01081; PMID: 17071768
- Cheng CY, Mruk DD. The blood-testis barrier and its implications for male contraception. Pharmacol Rev 2012; 64:16 - 64; http://dx.doi.org/10.1124/pr.110.002790; PMID: 22039149
- Pelletier RM. The blood-testis barrier: the junctional permeability, the proteins and the lipids. Prog Histochem Cytochem 2011; 46:49 - 127; http://dx.doi.org/10.1016/j.proghi.2011.05.001; PMID: 21705043
- França LR, Auharek SA, Hess RA, Dufour JM, Hinton BT. Blood-tissue barriers: morphofunctional and immunological aspects of the blood-testis and blood-epididymal barriers. Adv Exp Med Biol 2012; 763:237 - 59; PMID: 23397628
- Setchell BP. Blood-testis barrier, junctional and transport proteins and spermatogenesis. Adv Exp Med Biol 2008; 636:212 - 33; http://dx.doi.org/10.1007/978-0-387-09597-4_12; PMID: 19856170
- Cheng CYE. Biology and Regulation of Blood-Tissue Barriers. Austin, TX, Landes Bioscience/Springer Science+Business Media, LLC. pp. 1-361 (2012).
- Vogl AW, Vaid KS, Guttman JA. The Sertoli cell cytoskeleton. Adv Exp Med Biol 2008; 636:186 - 211; http://dx.doi.org/10.1007/978-0-387-09597-4_11; PMID: 19856169
- Fawcett DW, Leak LV, Heidger PM Jr.. Electron microscopic observations on the structural components of the blood-testis barrier. J Reprod Fertil Suppl 1970; 10:105 - 22; PMID: 4951168
- Fawcett D. Ultrastructure and function of the Sertoli cell. in Handbook of Physiology., Vol. 5 (eds. Hamilton, D. & Greep, R.) 21-25 (American Physiological Society, Washington, DC, 1975).
- Russell LD. Observations on rat Sertoli ectoplasmic (‘junctional’) specializations in their association with germ cells of the rat testis. Tissue Cell 1977; 9:475 - 98; http://dx.doi.org/10.1016/0040-8166(77)90007-6; PMID: 929577
- Russell LD. The blood-testis barrier and its formation relative to spermatocyte maturation in the adult rat: a lanthanum tracer study. Anat Rec 1978; 190:99 - 111; http://dx.doi.org/10.1002/ar.1091900109; PMID: 626419
- Russell LD. Observations on the inter-relationships of Sertoli cells at the level of the blood- testis barrier: evidence for formation and resorption of Sertoli-Sertoli tubulobulbar complexes during the spermatogenic cycle of the rat. Am J Anat 1979; 155:259 - 79; http://dx.doi.org/10.1002/aja.1001550208; PMID: 474448
- Russell LD, Clermont Y. Anchoring device between Sertoli cells and late spermatids in rat seminiferous tubules. Anat Rec 1976; 185:259 - 78; http://dx.doi.org/10.1002/ar.1091850302; PMID: 937734
- Wolski KM, Perrault C, Tran-Son-Tay R, Cameron DF. Strength measurement of the Sertoli-spermatid junctional complex. J Androl 2005; 26:354 - 9; http://dx.doi.org/10.2164/jandrol.04142; PMID: 15867003
- Russell LD, Goh JC, Rashed RMA, Vogl AW. The consequences of actin disruption at Sertoli ectoplasmic specialization sites facing spermatids after in vivo exposure of rat testis to cytochalasin D. Biol Reprod 1988; 39:105 - 18; http://dx.doi.org/10.1095/biolreprod39.1.105; PMID: 3207792
- Hess RA, Renato de Franca L. Spermatogenesis and cycle of the seminiferous epithelium. Adv Exp Med Biol 2008; 636:1 - 15; http://dx.doi.org/10.1007/978-0-387-09597-4_1; PMID: 19856159
- Mruk DD, Silvestrini B, Cheng CY. Anchoring junctions as drug targets: role in contraceptive development. Pharmacol Rev 2008; 60:146 - 80; http://dx.doi.org/10.1124/pr.107.07105; PMID: 18483144
- O’Donnell L, Nicholls PK, O’Bryan MK, McLachlan RI, Stanton PG. Spermiation: The process of sperm release. Spermatogenesis 2011; 1:14 - 35; http://dx.doi.org/10.4161/spmg.1.1.14525; PMID: 21866274
- Cheng CY, Mruk DD. A local autocrine axis in the testes that regulates spermatogenesis. Nat Rev Endocrinol 2010; 6:380 - 95; http://dx.doi.org/10.1038/nrendo.2010.71; PMID: 20571538
- Hanks SK. Messenger ribonucleic acid encoding an apparent isoform of phosphorylase kinase catalytic subunit is abundant in the adult testis. Mol Endocrinol 1989; 3:110 - 6; http://dx.doi.org/10.1210/mend-3-1-110; PMID: 2915644
- Siu MKY, Mruk DD, Lee WM, Cheng CY. Adhering junction dynamics in the testis are regulated by an interplay of β 1-integrin and focal adhesion complex-associated proteins. Endocrinology 2003; 144:2141 - 63; http://dx.doi.org/10.1210/en.2002-221035; PMID: 12697723
- André E, Becker-André M. Expression of an N-terminally truncated form of human focal adhesion kinase in brain. Biochem Biophys Res Commun 1993; 190:140 - 7; http://dx.doi.org/10.1006/bbrc.1993.1022; PMID: 8422239
- Whitney GS, Chan PY, Blake J, Cosand WL, Neubauer MG, Aruffo A, et al. Human T and B lymphocytes express a structurally conserved focal adhesion kinase, pp125FAK. DNA Cell Biol 1993; 12:823 - 30; http://dx.doi.org/10.1089/dna.1993.12.823; PMID: 7692878
- Wine RN, Chapin RE. Adhesion and signaling proteins spatiotemporally associated with spermiation in the rat. J Androl 1999; 20:198 - 213; PMID: 10232655
- Mulholland DJ, Dedhar S, Vogl AW. Rat seminiferous epithelium contains a unique junction (Ectoplasmic specialization) with signaling properties both of cell/cell and cell/matrix junctions. Biol Reprod 2001; 64:396 - 407; http://dx.doi.org/10.1095/biolreprod64.1.396; PMID: 11133699
- Hall JE, Fu W, Schaller MD. Focal adhesion kinase: exploring Fak structure to gain insight into function. Int Rev Cell Mol Biol 2011; 288:185 - 225; http://dx.doi.org/10.1016/B978-0-12-386041-5.00005-4; PMID: 21482413
- Lim ST, Mikolon D, Stupack DG, Schlaepfer DD. FERM control of FAK function: implications for cancer therapy. Cell Cycle 2008; 7:2306 - 14; PMID: 18677107
- Dym M. Basement membrane regulation of Sertoli cells. Endocr Rev 1994; 15:102 - 15; PMID: 8156935
- Siu MKY, Cheng CY. Dynamic cross-talk between cells and the extracellular matrix in the testis. Bioessays 2004; 26:978 - 92; http://dx.doi.org/10.1002/bies.20099; PMID: 15351968
- Beardsley A, Robertson DM, O’Donnell L. A complex containing α6β1-integrin and phosphorylated focal adhesion kinase between Sertoli cells and elongated spermatids during spermatid release from the seminiferous epithelium. J Endocrinol 2006; 190:759 - 70; http://dx.doi.org/10.1677/joe.1.06867; PMID: 17003277
- Lie PPY, Mruk DD, Mok KW, Su L, Lee WM, Cheng CY. Focal adhesion kinase-Tyr407 and -Tyr397 exhibit antagonistic effects on blood-testis barrier dynamics in the rat. Proc Natl Acad Sci USA 2012; 109:12562 - 7; http://dx.doi.org/10.1073/pnas.1202316109; PMID: 22797892
- Schlaepfer DD, Hanks SK, Hunter T, van der Geer P. Integrin-mediated signal transduction linked to Ras pathway by GRB2 binding to focal adhesion kinase. Nature 1994; 372:786 - 91; PMID: 7997267
- Schlaepfer DD, Hunter T. Integrin signalling and tyrosine phosphorylation: just the FAKs?. Trends Cell Biol 1998; 8:151 - 7; http://dx.doi.org/10.1016/S0962-8924(97)01172-0; PMID: 9695829
- Boutros T, Chevet E, Metrakos P. Mitogen-activated protein (MAP) kinase/MAP kinase phosphatase regulation: roles in cell growth, death, and cancer. Pharmacol Rev 2008; 60:261 - 310; http://dx.doi.org/10.1124/pr.107.00106; PMID: 18922965
- Tomar A, Schlaepfer DD. Focal adhesion kinase: switching between GAPs and GEFs in the regulation of cell motility. Curr Opin Cell Biol 2009; 21:676 - 83; http://dx.doi.org/10.1016/j.ceb.2009.05.006; PMID: 19525103
- Owens LV, Xu L, Craven RJ, Dent GA, Weiner TM, Kornberg L, et al. Overexpression of the focal adhesion kinase (p125FAK) in invasive human tumors. Cancer Res 1995; 55:2752 - 5; PMID: 7796399
- Sanchez AM, Flamini MI, Baldacci C, Goglia L, Genazzani AR, Simoncini T. Estrogen receptor-α promotes breast cancer cell motility and invasion via focal adhesion kinase and N-WASP. Mol Endocrinol 2010; 24:2114 - 25; http://dx.doi.org/10.1210/me.2010-0252; PMID: 20880986
- Slack JK, Adams RB, Rovin JD, Bissonette EA, Stoker CE, Parsons JT. Alterations in the focal adhesion kinase/Src signal transduction pathway correlate with increased migratory capacity of prostate carcinoma cells. Oncogene 2001; 20:1152 - 63; http://dx.doi.org/10.1038/sj.onc.1204208; PMID: 11313859
- Wang S, Basson MD. Protein kinase B/AKT and focal adhesion kinase: two close signaling partners in cancer. Anticancer Agents Med Chem 2011; 11:993 - 1002; http://dx.doi.org/10.2174/187152011797927661; PMID: 22023045
- Ma WW. Development of focal adhesion kinase inhibitors in cancer therapy. Anticancer Agents Med Chem 2011; 11:638 - 42; http://dx.doi.org/10.2174/187152011796817628; PMID: 21787276
- Wehrle-Haller B. Assembly and disassembly of cell matrix adhesions. Curr Opin Cell Biol 2012; 24:569 - 81; http://dx.doi.org/10.1016/j.ceb.2012.06.010; PMID: 22819514
- Cobb BS, Schaller MD, Leu TH, Parsons JT. Stable association of pp60src and pp59fyn with the focal adhesion-associated protein tyrosine kinase, pp125FAK. Mol Cell Biol 1994; 14:147 - 55; PMID: 7505391
- Xing Z, Chen HC, Nowlen JK, Taylor SJ, Shalloway D, Guan JL. Direct interaction of v-Src with the focal adhesion kinase mediated by the Src SH2 domain. Mol Biol Cell 1994; 5:413 - 21; PMID: 8054685
- Bolós V, Gasent JM, López-Tarruella S, Grande E. The dual kinase complex FAK-Src as a promising therapeutic target in cancer. Onco Targets Ther 2010; 3:83 - 97; http://dx.doi.org/10.2147/OTT.S6909; PMID: 20616959
- Bianchi M, De Lucchini S, Marin O, Turner DL, Hanks SK, Villa-Moruzzi E. Regulation of FAK Ser-722 phosphorylation and kinase activity by GSK3 and PP1 during cell spreading and migration. Biochem J 2005; 391:359 - 70; http://dx.doi.org/10.1042/BJ20050282; PMID: 15975092
- Xie Z, Sanada K, Samuels BA, Shih H, Tsai LH. Serine 732 phosphorylation of FAK by Cdk5 is important for microtubule organization, nuclear movement, and neuronal migration. Cell 2003; 114:469 - 82; http://dx.doi.org/10.1016/S0092-8674(03)00605-6; PMID: 12941275
- Siu MKY, Wong CH, Xia W, Mruk DD, Lee WM, Cheng CY. The β1-integrin-p-FAK-p130Cas-DOCK180-RhoA-vinculin is a novel regulatory protein complex at the apical ectoplasmic specialization in adult rat testes. Spermatogenesis 2011; 1:73 - 86; http://dx.doi.org/10.4161/spmg.1.1.15452; PMID: 21866278
- Siu MKY, Wong CH, Lee WM, Cheng CY. Sertoli-germ cell anchoring junction dynamics in the testis are regulated by an interplay of lipid and protein kinases. J Biol Chem 2005; 280:25029 - 47; http://dx.doi.org/10.1074/jbc.M501049200; PMID: 15870075
- Cheng CY, Mruk DD. Regulation of spermiogenesis, spermiation and blood-testis barrier dynamics: novel insights from studies on Eps8 and Arp3. Biochem J 2011; 435:553 - 62; http://dx.doi.org/10.1042/BJ20102121; PMID: 21486226
- Cheng CY, Mruk DD. Actin binding proteins and spermiogenesis: Some unexpected findings. Spermatogenesis 2011; 1:99 - 104; http://dx.doi.org/10.4161/spmg.1.2.16913; PMID: 22319657
- Cheng CY, Lie PPY, Wong EWP, Mruk DD, Silvestrini B. Adjudin disrupts spermatogenesis via the action of some unlikely partners: Eps8, Arp2/3 complex, drebrin E, PAR6 and 14-3-3. Spermatogenesis 2011; 1:291 - 7; http://dx.doi.org/10.4161/spmg.1.4.18393; PMID: 22332112
- Vogl AW, Young JS, Du M. New insights into roles of tubulobulbar complexes in sperm release and turnover of blood-testis barrier. Int Rev Cell Mol Biol 2013; 303:319 - 55; http://dx.doi.org/10.1016/B978-0-12-407697-6.00008-8; PMID: 23445814
- Lie PPY, Mruk DD, Lee WM, Cheng CY. Epidermal growth factor receptor pathway substrate 8 (Eps8) is a novel regulator of cell adhesion and the blood-testis barrier integrity in the seminiferous epithelium. FASEB J 2009; 23:2555 - 67; http://dx.doi.org/10.1096/fj.06-070573; PMID: 19293393
- Qian X, Mruk DD, Wong EWP, Lie PPY, Cheng CY. Palladin is a regulator of actin filament bundles at the ectoplasmic specialization in adult rat testes. Endocrinology 2013; 154:1907 - 20; http://dx.doi.org/10.1210/en.2012-2269; PMID: 23546604
- Lie PPY, Chan AYN, Mruk DD, Lee WM, Cheng CY. Restricted Arp3 expression in the testis prevents blood-testis barrier disruption during junction restructuring at spermatogenesis. Proc Natl Acad Sci USA 2010; 107:11411 - 6; http://dx.doi.org/10.1073/pnas.1001823107; PMID: 20534520
- Xiao X, Mruk DD, Cheng CY. c-Yes regulates cell adhesion at the apical ectoplasmic specialization-blood-testis barrier axis via its effects on protein recruitment and distribution. Am J Physiol Endocrinol Metab 2013; 304:E145 - 59; http://dx.doi.org/10.1152/ajpendo.00422.2012; PMID: 23169788
- Campbell M, Humphries P. The blood-retina barrier: tight junctions and barrier modulation. Adv Exp Med Biol 2012; 763:70 - 84; PMID: 23397619
- Easton AS. Regulation of permeability across the blood-brain barrier. Adv Exp Med Biol 2012; 763:1 - 19; PMID: 23397617
- Ashraf T, Kis O, Banerjee N, Bendayan R. Drug transporters at brain barriers: expression and regulation by neurological disorders. Adv Exp Med Biol 2012; 763:20 - 69; PMID: 23397618
- Russell LD, Peterson RN. Sertoli cell junctions: morphological and functional correlates. Int Rev Cytol 1985; 94:177 - 211; http://dx.doi.org/10.1016/S0074-7696(08)60397-6; PMID: 3894273
- Mruk DD, Cheng CY. Sertoli-Sertoli and Sertoli-germ cell interactions and their significance in germ cell movement in the seminiferous epithelium during spermatogenesis. Endocr Rev 2004; 25:747 - 806; http://dx.doi.org/10.1210/er.2003-0022; PMID: 15466940
- Dym M, Fawcett DW. The blood-testis barrier in the rat and the physiological compartmentation of the seminiferous epithelium. Biol Reprod 1970; 3:308 - 26; PMID: 4108372
- Cheng CY, Mruk DD. Cell junction dynamics in the testis: Sertoli-germ cell interactions and male contraceptive development. Physiol Rev 2002; 82:825 - 74; PMID: 12270945
- Siu ER, Wong EW, Mruk DD, Sze KL, Porto CS, Cheng CY. An occludin-focal adhesion kinase protein complex at the blood-testis barrier: a study using the cadmium model. Endocrinology 2009; 150:3336 - 44; http://dx.doi.org/10.1210/en.2008-1741; PMID: 19213829
- Siu ER, Wong EWP, Mruk DD, Porto CS, Cheng CY. Focal adhesion kinase is a blood-testis barrier regulator. Proc Natl Acad Sci USA 2009; 106:9298 - 303; http://dx.doi.org/10.1073/pnas.0813113106; PMID: 19470647
- Puri P, Walker WH. The tyrosine phosphatase SHP2 regulates Sertoli cell junction complexes. Biol Reprod 2013; 88:59; http://dx.doi.org/10.1095/biolreprod.112.104414; PMID: 23325809
- Leblond CP, Clermont Y. Spermiogenesis of rat, mouse, hamster and guinea pig as revealed by the periodic acid-fuchsin sulfurous acid technique. Am J Anat 1952; 90:167 - 215; http://dx.doi.org/10.1002/aja.1000900202; PMID: 14923625
- Clermont Y. Kinetics of spermatogenesis in mammals: seminiferous epithelium cycle and spermatogonial renewal. Physiol Rev 1972; 52:198 - 236; PMID: 4621362
- Clermont Y. The cycle of the seminiferous epithelium in man. Am J Anat 1963; 112:35 - 51; http://dx.doi.org/10.1002/aja.1001120103; PMID: 14021715
- Clermont Y, Leblond CP, Messier B. Duree du cycle de l'epithelium seminal du rat. Arch Anat Microsc Morphol Exp 1959; 48:37 - 56; PMID: 13810668
- Parvinen M. Regulation of the seminiferous epithelium. Endocr Rev 1982; 3:404 - 17; http://dx.doi.org/10.1210/edrv-3-4-404; PMID: 6295753
- Wong EWP, Cheng CY. Polarity proteins and cell-cell interactions in the testis. Int Rev Cell Mol Biol 2009; 278:309 - 53; http://dx.doi.org/10.1016/S1937-6448(09)78007-4; PMID: 19815182
- Yan HHN, Mruk DD, Wong EWP, Lee WM, Cheng CY. An autocrine axis in the testis that coordinates spermiation and blood-testis barrier restructuring during spermatogenesis. Proc Natl Acad Sci USA 2008; 105:8950 - 5; http://dx.doi.org/10.1073/pnas.0711264105; PMID: 18579774
- Yan HHN, Cheng CY. Laminin α 3 forms a complex with β3 and γ3 chains that serves as the ligand for α 6β1-integrin at the apical ectoplasmic specialization in adult rat testes. J Biol Chem 2006; 281:17286 - 303; http://dx.doi.org/10.1074/jbc.M513218200; PMID: 16608848
- Siu MKY, Cheng CY. Interactions of proteases, protease inhibitors, and the β1 integrin/laminin γ3 protein complex in the regulation of ectoplasmic specialization dynamics in the rat testis. Biol Reprod 2004; 70:945 - 64; http://dx.doi.org/10.1095/biolreprod.103.023606; PMID: 14645107
- Koch M, Olson PF, Albus A, Jin W, Hunter DD, Brunken WJ, et al. Characterization and expression of the laminin γ3 chain: a novel, non-basement membrane-associated, laminin chain. J Cell Biol 1999; 145:605 - 18; http://dx.doi.org/10.1083/jcb.145.3.605; PMID: 10225960
- Wong EWP, Cheng CY. NC1 domain of collagen α3(IV) derived from the basement membrane regulates Sertoli cell blood-testis barrier dynamics. Spermatogenesis 2013; 3:e25465; http://dx.doi.org/10.4161/spmg.25465
- Su L, Mruk DD, Lie PPY, Silvestrini B, Cheng CY. A peptide derived from laminin-γ3 reversibly impairs spermatogenesis in rats. Nat Communs 2012; 3:1185; http://dx.doi.org/10.1038/ncomms2171
- Yao PL, Lin YC, Richburg JH. TNF α-mediated disruption of spermatogenesis in response to Sertoli cell injury in rodents is partially regulated by MMP2. Biol Reprod 2009; 80:581 - 9; http://dx.doi.org/10.1095/biolreprod.108.073122; PMID: 19038859
- Yao PL, Lin YC, Richburg JH. Mono-(2-ethylhexyl) phthalate-induced disruption of junctional complexes in the seminiferous epithelium of the rodent testis is mediated by MMP2. Biol Reprod 2010; 82:516 - 27; http://dx.doi.org/10.1095/biolreprod.109.080374; PMID: 19828778
- Mazaud-Guittot S. Dissecting the phthalate-induced Sertoli cell injury: the fragile balance of proteases and their inhibitors. Biol Reprod 2011; 85:1091 - 3; http://dx.doi.org/10.1095/biolreprod.111.095976; PMID: 21900678
- Wan HT, Mruk DD, Wong CKC, Cheng CY. The apical ES-BTB-BM functional axis is an emerging target for toxicant-induced infertility. Trends Mol Med 2013; In press http://dx.doi.org/10.1016/j.molmed.2013.03.006; PMID: 23643465
- Lui WY, Wong CH, Mruk DD, Cheng CY. TGF-β3 regulates the blood-testis barrier dynamics via the p38 mitogen activated protein (MAP) kinase pathway: an in vivo study. Endocrinology 2003; 144:1139 - 42; http://dx.doi.org/10.1210/en.2002-0211; PMID: 12639893
- Wong CH, Mruk DD, Lui WY, Cheng CY. Regulation of blood-testis barrier dynamics: an in vivo study. J Cell Sci 2004; 117:783 - 98; http://dx.doi.org/10.1242/jcs.00900; PMID: 14734653
- Wong EWP, Lee WM, Cheng CY. Secreted Frizzled-related protein 1 (sFRP1) regulates spermatid adhesion in the testis via dephosphorylation of focal adhesion kinase and the nectin-3 adhesion protein complex. FASEB J 2013; 27:464 - 77; http://dx.doi.org/10.1096/fj.12-212514; PMID: 23073828
- Xiao X, Mruk DD, Lee WM, Cheng CY. c-Yes regulates cell adhesion at the blood-testis barrier and the apical ectoplasmic specialization in the seminiferous epithelium of rat testes. Int J Biochem Cell Biol 2011; 43:651 - 65; http://dx.doi.org/10.1016/j.biocel.2011.01.008; PMID: 21256972