Abstract
Tubulobulbar complexes (TBCs) are actin-related endocytic structures that internalize intercellular junctions in the seminiferous epithelium. The structures consist of elongate tubular projections of the attached plasma membranes of two adjacent cells that project into Sertoli cells. This double membrane core is cuffed by a dentritic actin network and is capped at its end by a clathrin-coated pit. Here we explore the possibility that elements of the spectrin cytoskeleton are associated with clusters of tubulobulbar complexes that develop at adhesion junctions between late spermatids and Sertoli cells at the apex of the epithelium, and extend what is known about the distribution of plectin at the sites.
Cryo-sections of perfusion-fixed testes and apical processes of Sertoli cells mechanically dissociated from perfusion-fixed testes were probed for spectrin, EPB41, and actin and analyzed using conventional fluorescence microscopy and confocal microscopy. Data sets from confocal microscopy were analyzed further in three-dimensional reconstructions using computer software. Additional apical Sertoli cell processes were probed for plectin and analyzed using conventional fluorescence microscopy. Antibodies generated against elements of the spectrin cytoskeleton react with material around and between the actin cuffs of tubulobulbar complexes, but appear excluded from the actin cuffs themselves. A similar staining pattern occurs with a probe for plectin. Immunoelectron microscopy confirmed the staining patterns observed by fluourescence microscopy. Based on our results, we suggest that a network of spectrin and plectin forms a scaffold around tubulobulbar complexes that may provide support for the actin network that cuffs each complex and also link adjacent complexes together.
Introduction
In the seminiferous epithelium of the mammalian testis, a dramatic turnover of intercellular junctions occurs during spermatogenesis. At the apex of the epithelium, large adhesion junctions disassemble to free fully developed sperm from their attachment to Sertoli cells.Citation1,Citation2 At the base of the epithelium, between neighboring Sertoli cells, massive belt-like junction complexes disassemble above and re-assemble below the next generation of spermatogenic cells as these cells translocate from basal to adluminal compartments of the epithelium.Citation3,Citation4 Elaborate structures termed tubulobulbar complexes develop at intercellular junctions during junction re-modeling both at apical and at basal sites of attachment in the seminiferous epithelium.Citation5
Tubulobulbar complexes are filament-related membrane protrusions of either a spermatid (apical sites) or a Sertoli cell (basal sites) that extend into invaginations in the adjacent Sertoli cell.Citation5 Each complex consists of an elongate double-membrane core that is surrounded or cuffed by a dendritic actin network and is capped at its end by a clathrin-coated pit.Citation6 As the complex matures, a swelling or bulb develops in the distal third of the structure that is devoid of actin and has a close association with a cistern of endoplasmic reticulum. The bulb “buds” from the complex and enters endocytic compartments of the Sertoli cell.Citation7,Citation8
Tubulobulbar complexes have the molecular signature of clathrin-based endocytosis machinery present generally in cells, and also have some similarities to membrane tubules formed in cell-free systems, and to podosomes that form at specialized sites of attachment between cells and extracellular matrix.Citation9-Citation13 There is now a substantial amount of data indicating that tubulobulbar complexes are subcellular machines that internalize intercellular junctions both at apical sites of attachment between Sertoli cells and spermatids during sperm release, and at basal sites of attachment between neighboring Sertoli cells as part of the mechanism of spermatocyte translocation.Citation14-Citation19
At apical adhesion junctions between Sertoli cells and spermatids in the rat, tubulobulbar complexes are organized in two rows along the concave face of each hook-shaped spermatid head.Citation5 The structures appear uniformly spaced within each row and can number as many as 24 complexes per spermatid head.Citation5 Although numerous components around the membrane core and within the actin cuff have now been identified,Citation6,Citation9,Citation20-Citation22 elements that surround the dense actin networks and relate one tubulobulbar complex to its neighbors are not as well defined. In other systems, elements of the spectrin cytoskeleton and members of the plakin family of proteins have been found to surround actin-rich structures and link one structure to its neighbors.Citation23-Citation26
Spectrin is a tetramer that consists of two identical heterodimers linked head to head with a total length of approximately 200–260 nm.Citation27 Each dimer is composed of an α- and a β-chain that are positioned side-by-side in an anti-parallel orientation.Citation27 The N terminus of β-spectrin has a calponin-homology (CH) domain that binds F-actin.Citation27 The first spectrin repeat of the β-chain also is able to bind to F-actin. EPB41, a spectrin-associated molecule, caps the minus end of actin filaments, promotes the spectrin-EPB41-actin complex, and binds to the plasma membrane.Citation27 The spectrin cytoskeleton formed by spectrin together with its associated proteins ankyrin, adducin, and EPB41, and short actin filaments, is best known as a sub-membrane skeleton where it supports the plasma membrane and stabilizes membrane domains.Citation27,Citation28 Significantly, spectrin also has been found as part of the terminal web of enterocytes where it links the roots of microvilli to each other, and surrounding actin-rich pedestals formed by host cells in response to attaching and effacing bacteria.Citation24-Citation26,Citation29
Plectin is a member of the plakin family of proteins. It is a 300 kDa protein that exists as a homotetramer under physiological conditions and has a dumbbell-like structure that spans 200 nm.Citation30,Citation31 Plectin links intermediate filaments to attachment sites in addition to being a somewhat indiscriminate cytoskeletal crosslinker that can bind to microfilaments, intermediate filaments, and microtubules. In addition to linking the cytoskeleton, particularly intermediate filaments, to junctions, plectin also has been found to surround the actin-rich zone of podosomes and to span the distance between adjacent podosomes.Citation23 Interestingly, plectin and spectrin are found together at podosomes.Citation32,Citation33 Moreover, spectrin and plectin have been reported to bind to each other.Citation34
Although spectrin has been found in spermatogenic cells and in Sertoli cells,Citation35-Citation39 it has not previously been identified at tubulobulbar complexes; however, plectin has been localized to the sites and has been suggested to facilitate development of the tubular form of the complexes.Citation40
Here, we demonstrate that elements of the spectrin cytoskeleton (spectrin and EPB41) are present in regions associated with tubulobulbar complexes. These elements appear to surround each complex and occur around and between the actin cuffs. Plectin has a similar staining pattern. We propose that a spectrin/plectin network surrounds or forms a shell around the actin network of each tubulobulbar complex and may provide a scaffold that supports and facilitates the elongation of the actin cuff. Elements of the spectrin/plectin system that are concentrated between the tubulobulbar complexes may link one complex to the other and participate in determining the spacing pattern.
Results
Spectrin is concentrated at apical tubulobulbar complexes in the seminiferous epithelium
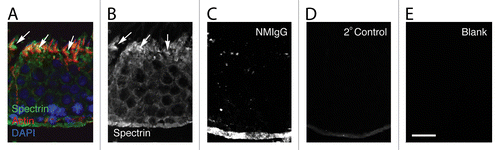
In cryo-sections of fixed testis labeled for actin, DNA, and spectrin, the position of ectoplasmic specializations, tubulobulbar complexes, nuclei, and the localization of spectrin were clearly visualized in the seminiferous epithelium (). Although the spectrin antibody reacted weakly with cells throughout the seminiferous epithelium, it appeared concentrated in apical regions of stage VII epithelium in Sertoli cell regions adjacent to the concave face of the hook-shaped spermatids (arrows in ). These regions are known from previous work and from the actin staining to contain dense clusters of tubulobulbar complexes. This pattern of labeling with the spectrin antibody was absent in controls ().
Figure 1. Spectrin distribution in the seminiferous epithelium. (A) Shown here is a section of stage VII seminiferous epithelium labeled with probes for spectrin, actin, and DNA. Although the spectrin antibody reacts generally throughout the epithelium, it is most concentrated adjacent to the concave face of the hook-shaped late spermatids at the apex of the epithelium (arrows). These regions contain tubulobulbar complexes that also label for actin. The pattern of staining with the spectrin antibody (B) is absent when the primary antibody is replaced with normal mouse IgG (NMIgG) (C), when the primary antibody is replaced with buffer alone (D), or when both the primary and the secondary antibodies are replaced with buffer alone (E). Bar = 20.0 μm.
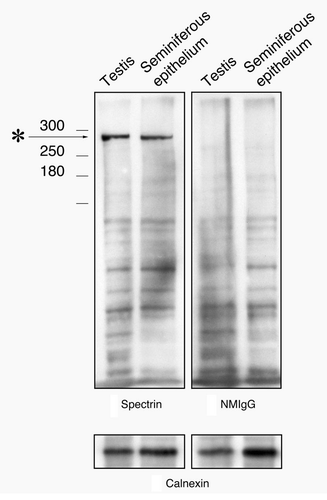
When western blots using the spectrin monoclonal antibodies were performed, one prominent band was observed at the expected 280 kDa molecular weight both in whole testis and seminiferous epithelium lysates (). Blots for normal IgG controls showed no discernable band at that molecular weight.
Figure 2. Western blot of testis and seminiferous epithelium lysates labeled with the spectrin antibody and with normal mouse IgG. A single band at the appropriate molecular weight for spectrin (asterisk) is present in material labeled with the spectrin antibody that is not present in material labeled with normal mouse IgG. To control for protein loading, bots were stripped and re-probed for calnexin.
Spectrin is located around and between apical tubulobulbar complexes
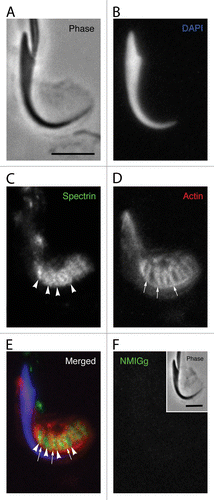
The localization of spectrin in regions containing clusters of tubulobulbar complexes was dramatically evident when apical processes of Sertoli cells that contain mature spermatids were mechanically fragmented away from the seminiferous epithelium () and visualized at high magnification using conventional immunofluorescence microscopy (). In each fragment, the position of the spermatid nucleus was indicated with DAPI staining (), the localization of spectrin was probed with antibodies (), and the exact positions of tubulobulbar complexes were indicated by treating the fragment with fluorescent phalloidin (). The spectrin labeling appeared diffusely localized around the cluster and also appeared to form linear tracts (arrowheads in ) of dense staining that occurred between individual tubulobulbar complexes indicated by the actin staining (arrows in ). This was particularly evident when images of the spectrin, actin, and DAPI channels were merged together (). A similar pattern of spectrin staining was absent when normal mouse IgG was substituted for the primary antibody at the same protein concentration ().
Figure 3. Localization of spectrin in a Sertoli cell apical process containing a late spermatid when analyzed using conventional fluorescence microscopy. The position of the spermatid head is clearly evident in the phase image (A) and when labeled with DAPI for DNA (B). Although the probe for spectrin generally labels in a diffuse pattern in the area adjacent to the concave face of the spermatid head, there are periodic linear tracts where the staining is more intense (arrowheads). These linear tracts appear similar to the labeling pattern for the actin networks of tubulobulbar complexes (arrows) (D); however, when the spectin and actin channels are merged, it is clear that the linear tracts of spectrin concentration actually appear between the actin networks of adjacent tubulobulbar complexes (E). No staining pattern similar to labeling with the spectrin antibody is present when the primary antibody is replaced with normal mouse IgG (F). Bars = 5.0 μm.
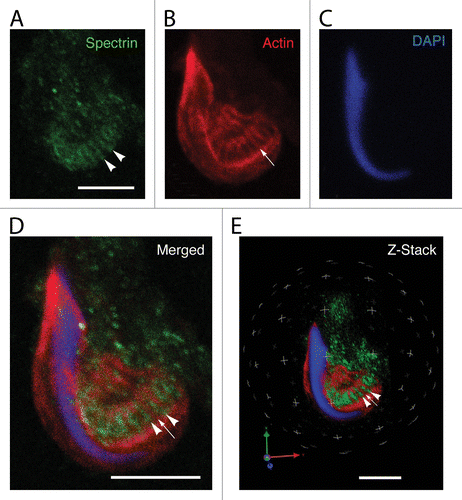
To verify that spectrin was present around and between the actin-rich cuffs of tubulobulbar complexes, we visualized labeled fragments using the confocal microscope, and also analyzed the data sets in three-dimensional reconstructions of the Z-stacks using Velocity software. In spectrin (), actin (), DNA (), and merged projections of Z-stacks, it was evident that spectrin (arrowheads in ) was excluded from the actin zones (arrow in ) of tubulobulbar complexes. Rather, the spectrin staining was found between and around individual tubulobulbar complexes. This was even better visualized in the three-dimensional reconstructions of the same data sets ().
Figure 4. Localization of spectrin in a Sertoli cell apical process containing a late spermatid when analyzed using confocal microscopy. Shown here are projections of an apical process labeled for spectrin (A), actin (B), and DNA (C). The spectrin antibody labels linear tracts (arrowheads in A) that reflect the adjacent positions of actin networks of tubulobulbar complexes (arrow in B). When the projections of the channels are merged, the probe for spectrin is concentrated between the actin networks of adjacent tubulobulbar complexes (D). This is particularly evident when the Z-stacks are analyzed in 3D using Velocity software. A single snapshot of the 3D reconstruction is shown in panel (E). Bars = 5.0 μm.
EPB41 has a similar staining pattern to spectrin
When apical Sertoli cell processes containing late spermatids were mechanically dissociated from fixed seminiferous epithelium and probed for EPB41 (), actin (), and DNA (), the staining pattern for EPB41 resembled the staining pattern for spectrin. When analyzed by confocal microscopy, EPB41 was concentrated in linear tracts (arrow heads in ) located between the dense actin cuffs of tubulobulbar complexes (arrows in ). This pattern was apparent when projections of the Z-stacks of the channels were merged () and when the data sets were analyzed in three dimensions using Velocity software ().
Figure 5. Localization of EPB41 in a Sertoli cell apical process containing a late spermatid when analyzed using confocal microscopy. Shown here are projections of an apical process labeled for EPB41 (A), actin (B), and DNA (C). As with spectrin, EPB41 is present in linear tracts (arrowheads in A) that reflect the positions of actin networks in adjacent tubulobulbar complexes (arrows in B). When the projections of the channels are merged (D) or when the Z-stacks are analyzed in 3D using Velocity software (E), it is evident that the probe for EPB41 is concentrated between adjacent actin networks of tubulobulbar complexes. The staining pattern for EPB41 (F) is absent when normal rabbit IgG is used to replace the primary antibody for EPB41 (G). (H) Western blot of testis and seminiferous epithelium lysates labeled with the EPB41 antibody and with normal rabbit IgG. A prominent band (asterisk) within the molecular weight for EPB41 is present in material labeled with the antibody that is not present in material labeled with normal mouse IgG, as are a couple of weaker bands at a higher molecular weight. Bars = 5.0 μm. To control for protein loading, bots were stripped and re-probed for calnexin.
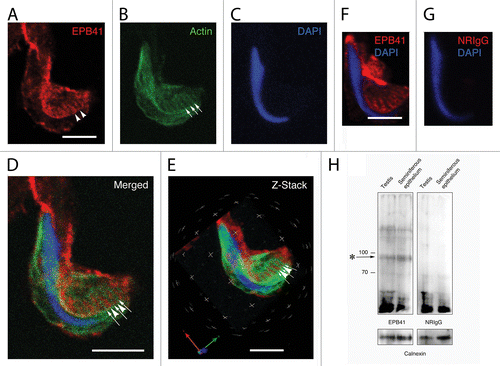
Although a distinct pattern of staining was observed in material treated with the EPB41 antibody (), a similar pattern was absent when the primary antibody was replaced with the same concentration of normal rabbit IgG (). In western blots of testis and seminiferous epithelium, the EPB41 antibody reacted with a band within the predicted molecular weight range for EPB41L2 isoform 3, which is approximately 90 kDa (RefSeq Accession XP_003753194.1). This band was absent in blots treated with normal rabbit IgG (). In addition, there were a couple of weaker bands at a somewhat higher molecular weight that also were not present in the IgG controls.
Plectin is located between and around apical tubulobulbar complexes
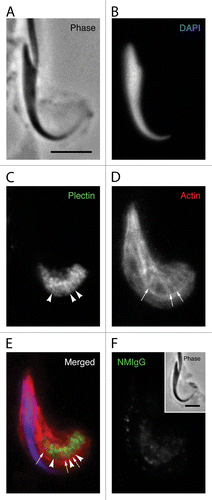
Although the general localization of plectin previously has been described in the seminiferous epitheliumCitation30 and in apical Sertoli cell processes containing tubulobulbar complexes in the rat,Citation40 we were interested in exploring more precisely the pattern of plectin localization at tubulobulbar complexes. In apical Sertoli cell processes mechanically dissociated from fixed seminiferous epithelium (), probed for DNA (), plectin (), and actin (), and evaluated by conventional fluorescence microscopy, plectin staining occurred around and between the actin cuffs () in a pattern reminiscent of the pattern observed when similar material was probed for spectrin. When the plectin antibody was replaced with the same concentration of normal mouse IgG, no specific staining pattern was observed ().
Figure 6. Plectin localization in apical Sertoli cell processes. The position of the spermatid head in the apical process is visible in the phase image (A) and when the DNA is labeled with DAPI (B). The probe for plectin reacts with material in the apical process that appears to form a network adjacent to the concave face of the spermatid head (C). Visible within this network are more concentrated bars of staining (arrowheads in C) that appear to lie between the tubulobulbar complexes as indicated by the actin probe (arrows in D). This alternating actin and plectin pattern is evident when the channels are merged (E). A staining pattern similar to that in material treated with the probe for plectin is absent when the primary antibody is replaced with normal mouse IgG (F). Bars = 5.0 μm.
Immunoelectron microscopy confirms that spectrin and plectin are excluded from the actin cores of tubulobulbar complexes
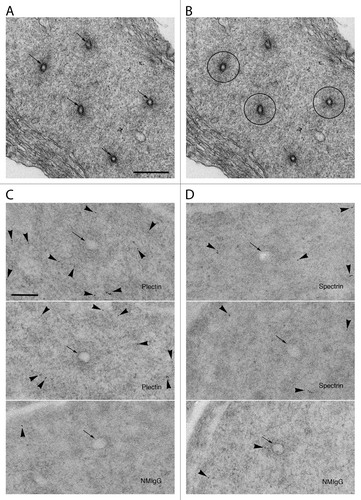
When viewed in tissue processed for conventional electron microscopy, the actin networks of apical tubulobulbar complexes are clearly evident surrounding the double-plasma membrane central elements of the complexes, particularly when tubulobulbar complexes are cross-sectioned (). The actin networks appear slightly greater in density than the material surrounding them. When processed for immunoelectron microscopy, gold particles in cross-sections of tubulobulbar complexes labeled for plectin () and for spectrin () are generally excluded from the actin cores and are concentrated in areas surrounding them. These results are consistent with our immunofluorescence results.
Figure 7. Immunoelectron microscopy of sections labeled for plectin and spectrin. (A) In conventional electron micrographs, the actin network surrounding the central plasma membrane elements (arrows) of tubulobulbar complexes is clearly evident as a dense cuff. (B) This image is the same as in (A), but with the actin rich cuffs circled by the black lines in three of the five complexes. (C) The upper two images are of tubulobulbar complexes labeled for plectin, and the lower image is of the specificity control labeled with normal mouse IgG (NMIgG). The arrows indicate the central membrane elements of each complex. Notice that the gold particles are excluded from the actin cores in material labeled for plectin. (D) The upper two images are of tubulobulbar complexes labeled for spectrin, and the lower image is of the specificity control labeled with normal mouse IgG (NMIgG). The arrows indicate the central membrane elements of each complex. Gold particles are generally excluded from the actin cores. (A and B) Bar = 500 nm. (C and D) Bar = 200 nm.
Discussion
Here, we present evidence that a network formed by plectin and spectrin surround apical tubulobulbar complexes in the rat seminiferous epithelium. We speculate that this network may provide a scaffold that supports the growth and maintenance of the actin cuff around the membrane core of each complex, and may link adjacent complexes to each other.
In other systems, plectin and/or spectrin are known to associate with actin-rich structures. In pedestals formed by host cells beneath the attaching and effacing bacteria Escherichia coli, a spectrin lattice forms a cage-like structure that surrounds the dense actin core of each pedestal.Citation24 In this system, the spectrin cytoskeleton is essential for pedestal formation.Citation41 In the apical cytoplasm of intestinal epithelial cells, a network of spectrin occurs in the terminal web where it links together the actin cores of adjacent microvilli.Citation25,Citation29 Significantly, both plectin and spectrin have been reported to be associated with podosomes, which resemble tubulobulbar complexes. Here, the proteins surround, but are excluded from, the actin-rich cores of those structures.Citation23,Citation33 Plectin also appears to span between adjacent podosomes. Knock-down of plectin results in fewer, smaller, and abnormally distributed podosomes relative to controls.Citation23
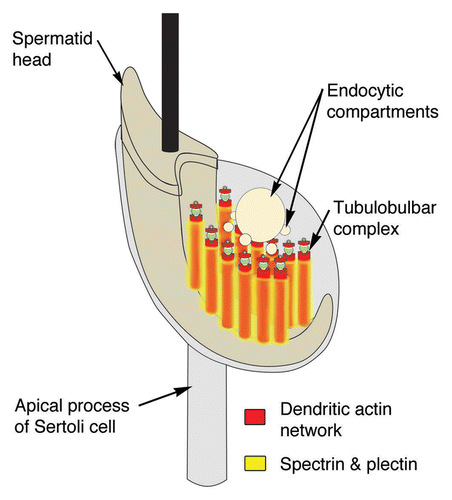
Spectrin (and its associated protein EPB41) and plectin have a pattern of distribution that indicates that, like at podosomes, the proteins are for the most part, excluded from the actin cuffs that surround the membrane cores of tubulobulbar complexes; rather, the proteins form a network or shell that surrounds the actin cuffs and occurs between individual complexes (). The large size both of spectrin and of plectin is consistent with the proteins generally being excluded from the dense actin networks.
Figure 8. Model of the spectrin and plectin distributions around apical tubulobulbar complexes in the rat. Spectrin and plectin together form a network that is excluded from the actin-rich zones of tubulobulbar complexes; rather this network appears to surround individual tubulobulbar complexes and may link adjacent complexes together.
There are a number of possible functions of the spectrin/plectin network associated with tubulobulbar complexes. The network may provide a scaffold that structurally supports the dentritic assembly of the actin cuff and maintains the elongate form of the complexes that can reach up to 2–3 μm in the rat.Citation5 The suggestion that plectin may facilitate the development of the tubular form of tubulobulbar complexes previously has been suggested.Citation40 Other possibilities are that the network may link individual complexes together, participate in regulating the size of the actin cuff around each complex, and determine the spacing between complexes within each cluster. The observations that intermediate filaments are generally absent from Sertoli cell regions surrounding apical clusters of tubulobulbar complexes and that the plasma membrane of the Sertoli cell is not directly associated with regions that label positively for plectin and spectrin suggest that the spectrin/plectin network is indeed primarily related to the actin cuffs of tubulobulbar complexes and not to linking intermediate filaments to the plasma membrane, or to directly supporting the plasma membrane itself.
It is unclear whether or not a spectrin/plectin network is present around basal tubulobulbar complexes that occur as more solitary structures than the dense clusters of complexes that occur at apical sites.
The presence of a network of spectrin and plectin that surrounds the actin cuffs of tubulobulbar complexes that develop between spermatids and Sertoli cells in the seminiferous epithelium adds yet another level of structural detail to our understanding of these complexes. The functions of this spectrin/plectin network remain to be fully elucidated through experimental manipulation.
Materials and Methods
Animals
All animals used in this study were reproductively active adult male Sprague-Dawley rats from obtained from Charles River and housed in animal care facilities at the University of British Columbia. Animal procedures were in accordance with guidelines set by the Canadian Council on Animal Care and as approved by the Animal Care Committee at the University of British Columbia.
Chemicals and reagents
Most chemicals and reagents used were obtained from Sigma-Aldrich, unless otherwise specified. Solid paraformaldehyde (reagent grade) was obtained from Fisher Scientific.
Antibodies
Primary antibodies were obtained from the following sources and used at the indicated working concentrations for immunofluorescence: (1) mouse anti-spectrin (612562, BD Bioscience) used at 5 μg/ml; (2) rabbit anti-EPB41L2 (HPA006642, Sigma-Aldrich) used at 1.7 μg/ml; (3) mouse anti-plectin antibody P9318 (clone 7A8, Sigma-Aldrich) used at 18.2 μg/ml. For westerns of spectrin and EPB41, antibodies were used at 0.1 μg/ml and 0.125 μg/ml, respectively. Westerns with the plectin antibody are presented elsewhere.Citation30
Loading controls for western blots were stained with rabbit anti-calnexin (C4731, Sigma-Aldrich), at 1:40,000 dilution.
Secondary antibodies: (a) For immunofluorescence: Alexa Fluor 488- or 568-conjugated goat anti-mouse IgG (H+L) (Invitrogen) was used at a working concentration of 1:100 (in TPBS/BSA) (b) For immunoblotting: horse radish peroxidase (HRP)-conjugated goat anti-mouse IgG and HRP-conjugated goat anti-rabbit IgG (Bio-Rad) were used at a working concentration of 1:5,000 in 0.1% BSA/TBST.
Actin filaments were labeled with fluorescently labeled Phalloidin-Alexa568 used as indicated by the supplier.
Normal immunoglobulins used in specificity controls were purchased either from Sigama-Aldrich Canada or Jackson immunoResearch Laboratories.
Tissue preparation for fluorescence microscopy
Fixation
Animals were placed under deep anesthesia by isoflurane inhalation, the testes were removed and then the animals immediately euthanized while still under deep anesthesia. Spermatic arteries were cannulated with 26-gauge needles and gravity perfused with warm PBS (150 mM NaCl, 5 mM KCl, 0.8 mM KH2PO4, and 3.2 mM Na2HPO4, pH 7.3 at 33 °C) for 2 min to clear blood and then with warm fixative (3% paraformaldehyde, 150 mM NaCl, 5 mM KCl, 0.8 mM KH2PO4, and 3.2 mM NaHPO4, pH 7.3 at 33 °C) for 30 min. Fixation was followed by a 30 min wash with PBS.
Preparation of cryo-sections
Fixed testes were placed on aluminum stubs with a small amount of optimal cutting temperature compound (OTC) (Sakura Finetek USA, Inc.) between the stub and the tissue and then frozen using liquid nitrogen. Frozen sections (5 μm) were cut, collected on poly-l-lysine-coated slides, and immediately plunged into cold acetone (-20 °C) for 5 min and then air-dried.
Preparation of epithelial fragments
Fixed testes were decapsulated in PBS and the seminiferous tubules were mechanically separated into small pieces (1–2 mm cubes) using scalpels and then transferred into a 50 ml Falcon tube. The material was gently aspirated through an 18-gauge needle and then through a 21-gauge needle to fragment the epithelium. Larger material was allowed to sediment for 10 min and the supernatant was collected. Using low-speed centrifugation, epithelial fragments were pelleted and then re-suspended in fresh PBS. This material, containing apical processes of Sertoli cells with attached late spermatids, was then added to poly-l-lysine-coated slides. The slides were placed in a humidity chamber for 5 min to allow tissue to adhere to the slide. Excess fluid was removed and the slides were immediately placed in cold acetone (-20 °C) for 5 min and then air-dried.
Labeling
Slides with the attached tissue fragments or cryosections were treated with 5% normal goat serum (NGS) in TPBS-BSA (PBS containing 0.05% Tween 20 and 0.1% bovine serum albumin) for 20 min at room temperature. Primary antibodies were added to the experimental slides, made up in TPBS-BSA with 1% NGS, and incubated overnight at 4 °C in a humidity chamber. The material was washed extensively with TPBS-BSA (wash buffer) and incubated for 1 h at 37 °C with secondary antibodies conjugated to fluorochromes. The slides were washed again and then were mounted using Vectashield Mounting Medium (Vector Laboratories, Inc.).
Controls for immunofluorescence included: (1) primary antibodies replaced at the same protein concentration with normal IgG from the host animal in which the antibody was generated (specificity control); (2) primary antibody replaced with buffer alone (secondary antibody control); (3) both primary and secondary antibodies replaced with buffer alone (control for autofluorescence, blank).
Filamentous actin was labeled using phalloidin conjugated to Alexa Fluor® 568 [Life technology Incorporation (Invitrogen)]. For each slide, 12.5 μl stock Alexa phalloidin (in methanol) was placed in a glass test tube, evaporated to dryness, and then rehydrated with 25 μl TPBS-BSA and added to the slide.
Imaging
Labeled samples were viewed and images recorded either on a conventional fluorescence microscope (Zeiss Axiophot) fitted with appropriate filter sets for detecting fluorescence and with the appropriate optics for phase contrast microscopy, or on a confocal microscope (Zeiss LSM 510). In addition to projections of data sets from the confocal microscope, Z-stacks also were analyzed using Velocity software. Snapshots of a single view from the 3D reconstructions of the Z-stacks were collected and are included in the figures presented.
Electron microscopy
Tissue for conventional electron microscopy was fixed and processed exactly as described by Young and coworkers.Citation16
Tissue for immunoelectron microscopy was fixed and processed as described by Vaid and coworkers.Citation20 Primary antibodies were used at working concentrations of 0.81 mg/ml (plectin) and 0.05 mg/ml (spectrin). For sections used as specificity controls, the primary antibodies were replaced with the same concentration of normal mouse IgG. The secondary antibody consisted of goat anti-mouse IgG conjugated to 6 nm colloidal gold (25124, Electron Microscopy Sciences) used at a dilution of 1:25.
All sections were imaged on an FEI Tecnai G2 Spirit Transmission Electron Microscope operated at 120 kV.
Western blotting
Western blot analyses were performed using whole testis and seminiferous epithelium lysates. Testes were removed from animals under deep anesthesia and decapsulated. Seminiferous epithelium was isolated as described elsewhere.Citation20 Both the seminiferous epithelium and whole testis were homogenized in an RIPA lysis buffer (150 mM NaCl, 50 mM Tris, pH 7.4, 5 mM EDTA, 1% Nonidet P-40, 1% deoxycholic acid, and 0.1% SDS) and flash frozen in liquid nitrogen for storage at -80 °C. When ready for use, lysate was thawed at room temperature and the protein concentration determined using a Bradford Assay (Bio-Rad) on a BioPhotometer (Eppendorf). Samples were added to the sample buffer (10% SDS w/v, 0.5M Tris pH 6.8, 30% glycerol v/v, 6% 2-mercaptoethanol, 1.6% bromophenol blue w/v), and heated for 10 min before being loaded onto 1-mm-thick 7% acrylamide gels for SDS-PAGE at equal concentrations as determined by protein assay. Proteins were transferred onto Immobilon-P transfer membrane (Millipore) and then washed for 5 min in 500 mM Tris, pH 7.5, 150 mM NaCl, and 0.1% Tween-20 (TBST). The blots were blocked overnight at 4 °C using TBS containing 4% non-fat milk (sc-2325; Santa Cruz Biotechnologies) to decrease non-specific binding of antibodies. Blots were washed three times with TBST for 10 min each and then incubated at room temperature with relevant primary antibodies and controls at appropriate concentrations. After 60 min, the blots were washed with TBST followed by a 60 min room temperature incubation with a corresponding secondary antibody conjugated to HRP at appropriate concentrations. After washing with TBST, the blots were treated with ECL (Amersham) and bands visualized on Bioflex Econofilm (InterScience). Normal IgG was used at the same concentration as the primary antibody to act as specificity controls. To control for protein loading, the membranes were stripped and re-probed for calnexin.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding
NSERC Discovery Grant #155397-13 awarded to AWV.
References
- Russell LD. Spermiation - the sperm release process: Ultrastructural observations and unresolved problems. In: Van Blerkom J, Motta PM, eds. Ultrastructure of Reproduction. Boston: Martinus Nijhoff, 1984:46-66.
- O’Donnell L, Nicholls PK, O’Bryan MK, McLachlan RI, Stanton PG. Spermiation: The process of sperm release. Spermatogenesis 2011; 1:14 - 35; http://dx.doi.org/10.4161/spmg.1.1.14525; PMID: 21866274
- Russell L. Movement of spermatocytes from the basal to the adluminal compartment of the rat testis. Am J Anat 1977; 148:313 - 28; http://dx.doi.org/10.1002/aja.1001480303; PMID: 857632
- Smith BE, Braun RE. Germ cell migration across Sertoli cell tight junctions. Science 2012; 338:798 - 802; http://dx.doi.org/10.1126/science.1219969; PMID: 22997133
- Russell L, Clermont Y. Anchoring device between Sertoli cells and late spermatids in rat seminiferous tubules. Anat Rec 1976; 185:259 - 78; http://dx.doi.org/10.1002/ar.1091850302; PMID: 937734
- Young JS, Guttman JA, Vaid KS, Shahinian H, Vogl AW. Cortactin (CTTN), N-WASP (WASL), and clathrin (CLTC) are present at podosome-like tubulobulbar complexes in the rat testis. Biol Reprod 2009; 80:153 - 61; http://dx.doi.org/10.1095/biolreprod.108.070615; PMID: 18799755
- Russell LD. Further observations on tubulobulbar complexes formed by late spermatids and Sertoli cells in the rat testis. Anat Rec 1979; 194:213 - 32; http://dx.doi.org/10.1002/ar.1091940204; PMID: 464323
- Russell LD. Observations on the inter-relationships of Sertoli cells at the level of the blood- testis barrier: evidence for formation and resorption of Sertoli-Sertoli tubulobulbar complexes during the spermatogenic cycle of the rat. Am J Anat 1979; 155:259 - 79; http://dx.doi.org/10.1002/aja.1001550208; PMID: 474448
- Vogl AW, Young JS, Du M. New insights into roles of tubulobulbar complexes in sperm release and turnover of blood-testis barrier. Int Rev Cell Mol Biol 2013; 303:319 - 55; http://dx.doi.org/10.1016/B978-0-12-407697-6.00008-8; PMID: 23445814
- Ochoa GC, Slepnev VI, Neff L, Ringstad N, Takei K, Daniell L, et al. A functional link between dynamin and the actin cytoskeleton at podosomes. J Cell Biol 2000; 150:377 - 89; http://dx.doi.org/10.1083/jcb.150.2.377; PMID: 10908579
- Conibear E. Converging views of endocytosis in yeast and mammals. Curr Opin Cell Biol 2010; 22:513 - 8; http://dx.doi.org/10.1016/j.ceb.2010.05.009; PMID: 20538447
- Taylor MJ, Perrais D, Merrifield CJ. A high precision survey of the molecular dynamics of mammalian clathrin-mediated endocytosis. PLoS Biol 2011; 9:e1000604; http://dx.doi.org/10.1371/journal.pbio.1000604; PMID: 21445324
- Wu M, Huang B, Graham M, Raimondi A, Heuser JE, Zhuang X, et al. Coupling between clathrin-dependent endocytic budding and F-BAR-dependent tubulation in a cell-free system. Nat Cell Biol 2010; 12:902 - 8; http://dx.doi.org/10.1038/ncb2094; PMID: 20729836
- Guttman JA, Takai Y, Vogl AW. Evidence that tubulobulbar complexes in the seminiferous epithelium are involved with internalization of adhesion junctions. Biol Reprod 2004; 71:548 - 59; http://dx.doi.org/10.1095/biolreprod.104.028803; PMID: 15084482
- Young JS, Guttman JA, Vaid KS, Vogl AW. Tubulobulbar complexes are intercellular podosome-like structures that internalize intact intercellular junctions during epithelial remodeling events in the rat testis. Biol Reprod 2009; 80:162 - 74; http://dx.doi.org/10.1095/biolreprod.108.070623; PMID: 18799754
- Young JS, de Asis M, Guttman JA, Vogl AW. Cortactin depletion results in short tubulobulbar complexes and spermiation failure in rat testis. Biol Open 2012; 1:1 - 9; http://dx.doi.org/10.1242/bio.20122519; PMID: 23213360
- Young JS, Takai Y, Kojic KL, Vogl AW. Internalization of adhesion junction proteins and their association with recycling endosome marker proteins in rat seminiferous epithelium. Reproduction 2012; 143:347 - 57; http://dx.doi.org/10.1530/REP-11-0317; PMID: 22157319
- Du M, Young J, De Asis M, Cipollone J, Roskelley C, Takai Y, et al. A novel subcellular machine contributes to Basal junction remodeling in the seminiferous epithelium. Biol Reprod 2013; 88:60; http://dx.doi.org/10.1095/biolreprod.112.104851; PMID: 23303684
- D’Souza R, Pathak S, Upadhyay R, Gaonkar R, D’Souza S, Sonawane S, et al. Disruption of tubulobulbar complex by high intratesticular estrogens leading to failed spermiation. Endocrinology 2009; 150:1861 - 9; http://dx.doi.org/10.1210/en.2008-1232; PMID: 19095743
- Vaid KS, Guttman JA, Babyak N, Deng W, McNiven MA, Mochizuki N, et al. The role of dynamin 3 in the testis. J Cell Physiol 2007; 210:644 - 54; http://dx.doi.org/10.1002/jcp.20855; PMID: 17133358
- Guttman JA, Obinata T, Shima J, Griswold M, Vogl AW. Non-muscle cofilin is a component of tubulobulbar complexes in the testis. Biol Reprod 2004; 70:805 - 12; http://dx.doi.org/10.1095/biolreprod.103.022723; PMID: 14627549
- Kusumi N, Watanabe M, Yamada H, Li SA, Kashiwakura Y, Matsukawa T, et al. Implication of amphiphysin 1 and dynamin 2 in tubulobulbar complex formation and spermatid release. Cell Struct Funct 2007; 32:101 - 13; http://dx.doi.org/10.1247/csf.07024; PMID: 17785912
- Gad A, Lach S, Crimaldi L, Gimona M. Plectin deposition at podosome rings requires myosin contractility. Cell Motil Cytoskeleton 2008; 65:614 - 25; http://dx.doi.org/10.1002/cm.20287; PMID: 18553359
- Ruetz TJ, Vogl AW, Guttman JA. Detailed examination of cytoskeletal networks within enteropathogenic Escherichia coli pedestals. Anat Rec (Hoboken) 2012; 295:201 - 7; http://dx.doi.org/10.1002/ar.21544; PMID: 22190417
- Brown JW, McKnight CJ. Molecular model of the microvillar cytoskeleton and organization of the brush border. PLoS One 2010; 5:e9406; http://dx.doi.org/10.1371/journal.pone.0009406; PMID: 20195380
- Mooseker MS. Actin binding proteins of the brush border. Cell 1983; 35:11 - 3; http://dx.doi.org/10.1016/0092-8674(83)90202-7; PMID: 6313218
- Bennett V, Baines AJ. Spectrin and ankyrin-based pathways: metazoan inventions for integrating cells into tissues. Physiol Rev 2001; 81:1353 - 92; PMID: 11427698
- Liu SC, Derick LH, Palek J. Visualization of the hexagonal lattice in the erythrocyte membrane skeleton. J Cell Biol 1987; 104:527 - 36; http://dx.doi.org/10.1083/jcb.104.3.527; PMID: 2434513
- Glenney JR Jr., Glenney P, Weber K. The spectrin-related molecule, TW-260/240, cross-links the actin bundles of the microvillus rootlets in the brush borders of intestinal epithelial cells. J Cell Biol 1983; 96:1491 - 6; http://dx.doi.org/10.1083/jcb.96.5.1491; PMID: 6841456
- Guttman JA, Mulholland DJ, Vogl AW. Plectin is concentrated at intercellular junctions and at the nuclear surface in morphologically differentiated rat Sertoli cells. Anat Rec 1999; 254:418 - 28; http://dx.doi.org/10.1002/(SICI)1097-0185(19990301)254:3<418::AID-AR13>3.0.CO;2-C; PMID: 10096674
- Foisner R, Wiche G. Structure and hydrodynamic properties of plectin molecules. J Mol Biol 1987; 198:515 - 31; http://dx.doi.org/10.1016/0022-2836(87)90297-X; PMID: 3430617
- Gimona M, Buccione R, Courtneidge SA, Linder S. Assembly and biological role of podosomes and invadopodia. Curr Opin Cell Biol 2008; 20:235 - 41; http://dx.doi.org/10.1016/j.ceb.2008.01.005; PMID: 18337078
- Takkunen M, Hukkanen M, Liljeström M, Grenman R, Virtanen I. Podosome-like structures of non-invasive carcinoma cells are replaced in epithelial-mesenchymal transition by actin comet-embedded invadopodia. J Cell Mol Med 2010; 14:6B 1569 - 93; http://dx.doi.org/10.1111/j.1582-4934.2009.00868.x; PMID: 19656240
- Herrmann H, Wiche G. Plectin and IFAP-300K are homologous proteins binding to microtubule-associated proteins 1 and 2 and to the 240-kilodalton subunit of spectrin. J Biol Chem 1987; 262:1320 - 5; PMID: 3027087
- De Cesaris P, Filippini A, Stefanini M, Ziparo E. Spectrin, fodrin and protein 4.1-like proteins in differentiating rat germ cells. Differentiation 1989; 41:216 - 22; http://dx.doi.org/10.1111/j.1432-0436.1989.tb00750.x; PMID: 2612769
- De Cesaris P, Filippini A, Ziparo E, Russo MA, Stefanini M. Distribution of analogues of spectrin, fodrin and protein 4.1 in rat spermatogenic cells. Prog Clin Biol Res 1989; 296:149 - 52; PMID: 2740365
- Damjanov I, Damjanov A, Lehto VP, Virtanen I. Spectrin in mouse gametogenesis and embryogenesis. Dev Biol 1986; 114:132 - 40; http://dx.doi.org/10.1016/0012-1606(86)90389-1; PMID: 3956860
- Ziparo E, Zani BM, Filippini A, Stefanini M, Marchesi VT. Proteins of the membrane skeleton in rat Sertoli cells. J Cell Sci 1986; 86:145 - 54; PMID: 3308927
- Borland K, Osawa S, Kew D, Coleman DB, Goodman SR, Hall PF. Identification of a spectrin-like protein in Sertoli cells. Biol Reprod 1985; 32:1143 - 56; http://dx.doi.org/10.1095/biolreprod32.5.1143; PMID: 2410039
- Upadhyay R, D’Souza R, Sonawane S, Gaonkar R, Pathak S, Jhadav A, et al. Altered phosphorylation and distribution status of vimentin in rat seminiferous epithelium following 17β-estradiol treatment. Histochem Cell Biol 2011; 136:543 - 55; http://dx.doi.org/10.1007/s00418-011-0856-5; PMID: 21915674
- Ruetz TJ, Lin AE, Guttman JA. Enterohaemorrhagic Escherichia coli requires the spectrin cytoskeleton for efficient attachment and pedestal formation on host cells. Microb Pathog 2012; 52:149 - 56; http://dx.doi.org/10.1016/j.micpath.2011.12.001; PMID: 22197999