Abstract
Epithelial cells form organized sheets to protect underlying tissues and maintain the physiological environment by the assembly of tight junctions (TJs) and adherens junctions (AJs), which mainly regulate paracellular molecular passage and selective cell-cell adhesion, respectively. At the cytoplasmic surface, TJs and AJs associate with a specific actomyosin cytoskeletal structure called the perijunctional actomyosin ring (PJAR), which encircles cells in a belt-like manner. ZO family proteins play important roles in regulating TJ and PJAR organization. We recently found that ARHGEF11, a member of the RGS-RhoGEF family of proteins, associates with TJs by binding to ZO-1. ARHGEF11 mediates ZO-1-dependent junction assembly and barrier formation in mammary epithelial cells. Another recent study demonstrated that ARHGEF11-dependent apical actomyosin contraction is coupled to planar cell polarity signaling in neuroepithelial cells for the control of neural tube formation. These findings suggest that ARHGEF11 generally regulates apical junctions and junction-associated actomyosin in various epithelial tissues.
Introduction
For the development and maintenance of multicellular organisms, polarized epithelial cells form sheet-like structures by adhering tightly to each other. The respective organ is protected and controlled by differentiated epithelial cell sheets. The apical junctional complex (AJC), which is composed of tight junctions (TJs) and adherens junctions (AJs) plays crucial roles in the formation and physiological regulation of epithelial cell sheets.Citation1 TJs and AJs are intimately located at the apical region between neighboring cells and the establishment of these two types of junctions is closely linked.Citation2 During the formation of AJC, calcium-dependent homophilic interactions between cadherin molecules initially occur between neighboring cells. Then, clustering of cadherin molecules is promoted and actin filaments are recruited to the cadherin-based cell-cell adhesion sites. The engagement of cadherin with actin filaments via catenin results in the stabilization of cadherin clusters and further accumulation of actin filaments at the junctions, followed by dramatic reorganization of the actomyosin cytoskeleton. The reorganized actomyosin complexes, which encircle cells in a belt-like manner, form a structure defined as the perijunctional actomyosin ring (PJAR).Citation3 The contractility of the PJAR further mediates the transition of cells into a columnar shape and the accumulation of TJ components at the apical surface.Citation4,Citation5 The newly developed TJs are stabilized by their interactions with the PAJR, resulting in a fully polarized epithelial phenotype with distinct apical and basolateral membrane compartments. The established TJs create the primary barrier that prevents the passage of molecules and ions through the paracellular space between cells. In addition, TJs function as the fence that restricts the diffusion of integral membrane proteins and lipids between the apical and basolateral surfaces of the cells to maintain cellular polarity.Citation6 Multiple components of TJs, including the scaffold ZO family proteins (ZO-1, ZO-2, and ZO-3), are reported to interact with the actomyosin cystoskeleton and these interactions are important for the regulation of TJ properties and PJAR organization.Citation7-Citation10 Recent studies have further improved our understanding of the molecular mechanisms underlying the ZO-1-dependent and -independent control of TJs and PJARs.
Rho Signaling in the Control of TJs and PJARs
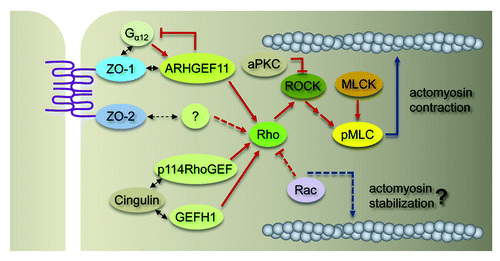
The Rho family of small GTPases are major regulators of the organization of the actin cytoskeleton, and they have been shown to be involved in the establishment and maintenance of TJs as well as PJARs.Citation11 The Rho specific inhibitor, C3 transferase, suppresses junction assembly and increases paracellular permeability.Citation12 Expression of dominant negative Rho in kidney epithelial cells abolishes the fence function of TJs without obvious alterations in the organization of TJs and the expression of their components. Interestingly, constitutively active Rho expressed at relatively low levels perturbs both the barrier and fence functions similar to dominant negative Rho.Citation13 Several other studies have also demonstrated that inhibition of Rho induced the same effects as constitutive activation of Rho,Citation14 suggesting that Rho activity is finely controlled to regulate the structure and function of TJs.
The Rho-associated kinase, ROCK, is a downstream effector of Rho that mainly mediates Rho-dependent control of TJs and PJARs. The inhibition of ROCK induced the reorganization of PJAR structure and enhanced the paracellular permeability of intestinal epithelial cells without affecting the distribution and solubility of TJ components.Citation15 However, the recruitment of ZO-1 to newly forming cell-cell adhesion sites was prevented by the inhibition of ROCK. These observations indicate that ROCK is important for the barrier function and the assembly of TJs, but is not indispensable for maintaining the integrity of the established TJ structure.
ROCK stimulates the activation of myosin-II by regulating the phosphorylation state of myosin light chain (MLC).Citation16 The phosphorylation of MLC is also mediated by myosin light chain kinase (MLCK). The inhibition of MLCK reduces the MLC phosphorylation level and prevents barrier function in response to physiological and pathological stimuli.Citation17 Expression of constitutively active MLCK in confluent intestinal epithelial cells impaired barrier function and increased paracellular permeability.Citation18 These studies indicate that MLC activity is a pivotal element in the regulation of TJs and PJARs downstream of Rho signaling.
Meanwhile, guanine nucleotide-exchange factors (GEFs) are directly responsible for the activation of Rho family GTPases. Of more than 70 GEF family members, two GEF proteins specific for Rho, GEF-H1 and p114RhoGEF, have been reported to localize and function at TJs.Citation19,Citation20 GEF-H1 and p114RhoGEF are recruited to TJs by direct interaction with the cytoplasmic TJ protein cingulin, but they appear to form distinct complexes. GEF-H1 associates with microtubules and 14–3-3,Citation19 while p114RhoGEF binds to the β-gamma subunit of heterotrimeric G-proteins and lulu2.Citation21,Citation22 The association of GEF-H1 with cingulin reduces its GEF activity, and this inactive state is maintained at TJs. The depletion of GEF-H1 attenuated cell spreading but did not affect TJ formation. In contrast, p114RhoGEF depletion caused dissociation of ZO-1 from cell-cell adhesion sites and disrupted TJs in corneal epithelial cells, while it caused the disorganization of PJARs in confluent cultured adenocarcinoma colon cells.Citation20 Because such alterations are not observed in p114RhoGEF-depleted mammary epithelial cells,Citation23 the molecular machinery regulating TJs and PJARs may vary from cell type to cell type.
The RGS-RhoGEF Protein, ARHGEF11, Regulates TJs and PJARs in Epithelial Cells
ZO proteins bind directly to actin and actin regulatory molecules such as cortactin.Citation24 In addition, the depletion of ZO proteins leads to the disorganization not only of TJs but also of PJARs, resulting in impaired barrier function.Citation8,Citation9 These data suggest that ZO proteins play crucial roles in the coordinated regulation of TJs and PJARs.
ARHGEF11 localizes at TJs via direct interaction with ZO-1
To gain further insights into the regulation of TJs and PJARs by ZO proteins, we attempted to identify signaling molecules which work cooperatively with ZO proteins by yeast two-hybrid screening. Using this approach, we identified ARHGEF11 (also named PDZ-RhoGEF) as a novel ZO-1 binding protein.Citation23 ARHGEF11 directly and specifically interacts with ZO-1 via ZO-1’s C-terminal region. ARHGEF11 is a specific GEF for Rho and contains an RGS domain, which regulates the activity of the Gα12/13 subunit of heterotrimeric G proteins.Citation25 Interestingly, it was reported that Gα12 is localized to TJs by binding to ZO-1, and the treatment of cells with LPA, a stimulator of Gα12/13, was shown to modulate PJAR assembly.Citation26,Citation27 Therefore, the ZO-1/ARHGEF11 complex may link G protein-coupled signaling to Rho at TJs.
In epithelial tissues such as the mammary glands and in various epithelial cell lines, ARHGEF11 was co-localized with ZO-1 at TJs. ARHGEF11’s localization at TJs was significantly disturbed in ZO-1-depleted mammary epithelial cells. Almost all ARHGEF11 exhibited a cytoplasmic distribution with a punctuate pattern, but the level of ARHGEF11 expression was not altered by eliminating ZO-1. In contrast, neither the expression nor localization of ZO-1 was markedly affected in the ARHGEF11-depleted cells. These observations suggest that ZO-1 is indispensable for ARHGEF11 to target TJs, while ZO-1 does not require ARHGEF11 for its own TJ targeting.
Participation of ARHGEF11 in junction assembly and maintenance
ARHGEF11 depletion attenuates the assembly of TJs and PJARs as well as establishment of the paracellular barrier. When calcium was depleted from the culture medium of mammary epithelial cells, myosin-IIB was observed diffusely throughout the cytoplasm and at the cell cortex. The reintroduction of calcium induced reorganization of myosin-IIB as thick subcortical bundles that were gradually refined into a thin linear pattern. This process took much longer when ARHGEF11 was depleted. Likewise, the formation of TJs was attenuated and the establishment of the barrier was impaired by ARHGEF11 suppression in this calcium switch assay. ARHGEF11 is localized to a primordial spot-like AJ with the cadherin-catenin complex during the initial phase of epithelial junction assembly similar to ZO-1. Furthermore, in the absence of cell-cell contacts in low calcium culture conditions, ARHGEF11 and ZO-1 were co-localized in intense spots within the cells, indicating that ARHGEF11 is constitutively associated with ZO-1. If that is the case, ARHGEF11 could be quickly recruited to cell-cell adhesion sites and orchestrate the epithelial-type actomyosin architecture required for polarization from the initial step onwards.
In contrast, the organization of TJs and PJARs was not notably compromised when the AHGEF11-depleted mammary epithelial cells were cultured under confluent conditions for several days. Occludin and myosin-IIB exhibited almost the same expression and distribution patterns between control and ARHGEF11-depleted cells. Unexpectedly, however, the lateral distribution and solubility of cadherin-catenin complexes appeared to be increased by ARHGEF11 depletion in confluent cultured mammary epithelial cells. Although the mechanism of and reason for this alteration are not yet known, ARHGEF11 depletion may affect the association between cadherin-catenin complexes and mature PJARs, but the overall organization of established TJs, AJs and PJARs is not appreciably affected by the depletion of ARHGEF11.
ARHGEF11 affects myosin light chain activity at cell-cell contact sites
Of several actomyosin regulators that are downstream targets of Rho, MLC appears to be specifically modulated by ARHGEF11. The phosphorylation level of MLC was significantly downregulated in ARHGEF11-depleted cells compared with control cells in the calcium switch assay, while other actomyosin regulators such as ERM and Src were not affected. Moreover, the accumulation of p-MLC at cell-cell contact sites was retarded in the ARHGEF11-depleted cells. These observations indicate that the depletion of ARHGEF11 led to a reduction in MLC activity at cell-cell adhesion sites involved in PJAR formation.
A previous study demonstrated that the increase in p-MLC level induced by the constitutive activation of MLCK caused TJ barrier dysfunction,Citation18 which seems to be contradictory to our observations. Conversely, another study demonstrated that suppressing the p-MLC level by inhibiting ROCK prevented the formation of TJs and barriers in the calcium switch assay,Citation15 and dominant-negative and constitutively-active Rho exhibited the same effects on TJ properties as noted above.Citation13 A rational interpretation of these diverse results may be that the appropriate contraction force of actomyosin at the cell-cell contact sites is crucial, and that either an excessive or a reduced p-MLC level could lead to inadequate actomyosin contraction and consequently to the impaired formation and function of TJs and PJARs.
ARHGEF11 is specifically required for TJ and PJAR remodeling by ZO-1
The depletion of ZO-1 alone retards junction assembly and barrier establishment similar to the depletion of ARHGEF11.Citation28 In contrast, when ZO-1 and ZO-2 are depleted simultaneously, the organization of TJs and PJARs as well as the epithelial barrier are severely compromised.Citation8,Citation9 The re-expression of either ZO-1 or ZO-2 is sufficient to prevent such defects, suggesting that these proteins play redundant roles in organizing TJ and PJAR architecture. However, it appears that ZO-1 and ZO-2 utilize independent molecular pathways. In the absence of ARHGEF11, re-expressed ZO-1 could not rescue impaired phenotypes in mammary epithelial cells depleted of both ZO-1 and ZO-2. In addition, the defects were not restored by a mutant ZO-1 lacking the ARHGEF11-binding C-terminal domain. This mutant ZO-1 was localized to cell-cell contact sites similar to wild-type ZO-1, but ARHGEF11 was distributed in the cytoplasm.
In contrast, ARHGEF11 depletion did not affect ZO-2-dependent rescue of the impaired TJ and PJAR architecture. Moreover, cells depleted of ARHGEF11 and ZO-2 exhibited aberrant localization of myosin-IIB and occludin, similar to the patterns observed in ZO-1 and ZO-2 depleted cells. This result suggests the possibility that ZO-2 interacts with another RhoGEF protein or utilizes another pathway that regulates TJs and PJARs in parallel with the ZO-1/ARHGEF11 pathway. Although the molecular basis of ZO-2-dependent regulation remains elusive, the existence of redundant molecular pathways to control the integrity of TJs and PJARs seems very likely, because disruption of the epithelial barrier can immediately cause serious problems for the life of multicellular organisms.
ARHGEF11 is indispensable for neural tube formation
Recently, Nishimura et al. reported that ARHGEF11 is involved in polarized actomyosin contraction in the neural plate.Citation29 The neural plate is a specific epithelial sheet which bends along the anterior-posterior axis to form the neural tube in the early embryonic developmental stage. The inward bending of the neural plate depends on actomyosin-mediated constriction of the apical surfaces in the cells specifically located at the region to generate a hinge effect for neural tube formation. Several studies have demonstrated that ROCK plays an important role in the bending of the neural plate and the apical constriction of neuroepithelial cells.Citation30 At the same time, Celsr1, a planar cell polarity (PCP) regulator possessing a seven pass transmembrane nonclassical cadherin structure, was shown to be important for adhesion between neighboring neuroepithelial cells and neural tube formation.Citation31 It has been postulated that there are mediators which link Celsr1-dependent PCP signaling and ROCK-dependent actomyosin activation. Nishimura et al. found that ARHGEF11 is localized at the apical side of neural plates along the pMLC cables. The depletion of ARHGEF11 reduced the p-MLC level and abolished its polarized condensation in neuroepithelial cells. Furthermore, ARHGEF11 depletion caused loss of the polarized contraction of actomyosin and enlargement of the apical surface area of cells, resulting in the prohibition of neural tube closure. Celsr1 functions upstream of ARHGEF11 and ROCK and is not able to regulate the contractility of junction-associated actomyosin in the absence of ARHGEF11. In addition, the junctional accumulation of ARHGEF11 is required to activate ROCK at the junctions. These observations indicate that ARHGEF11 plays critical roles in neural tube formation by integrating PCP signals, cell adhesion and actomyosin contractility in neuroepithelial cells.
Concluding Remarks
The structure and barrier function of TJs are not static, but change dynamically in response to physiological and pathological stimuli. The dynamic regulation of TJs largely depends on Rho signaling and junction-associated actomyosin, and the implicated molecular components and networks connecting these elements appear to be complicated and several pathways may exist (). For example, at least three RhoGEF proteins are localized at TJs and PJARs and implicated in their regulation, but it is not known whether these proteins work cooperatively or independently. Efforts to answer this question by further characterization of the dynamic behavior of these proteins in living cells and in in vivo studies would improve our knowledge of the control mechanisms of epithelial physiology. The coordinated integration of cell-cell junctions and apical actomyosin is a general requirement for the development and maintenance of epithelial tissues, but different tissues could utilize modified molecular machinery according to the need of each tissue. During neural tube formation, apical constriction of neuroepithelial cells needs to be coupled with PCP signals, and the molecules involved in this event are not identical to those involved in TJ formation in mammary epithelial cells, although ARHGEF11-dependent Rho activation plays important roles in both cases. It seems that Rho-mediated MLC activation needs to be finely tuned, and therefore the signals that prevent excess constriction induced by the activation of Rho and MLC are important factors for maintaining the integrity of epithelial cells. It is thought that the activation of Rac antagonizes Rho-mediated actomyosin contraction and contributes to maintaining the appropriate permeability.Citation32 In another study, atypical protein kinase C was shown to prevent ROCK from re-localizing to epithelial junctions from the cytoplasm, thereby inhibiting excess actomyosin contraction and allowing cells to retain normally shaped apical domains.Citation33 In addition, it is possible that other cytoskeletal elements such as microtubules and intermediate filaments are involved in the proper organization of junction-associated actomyosin in epithelial cells, because both cytoskeletons also dramatically change their organization during junction formation. Determining if and how these factors have a connection to the ZO-1/ARHGEF11 pathway and other RhoGEF pathways, and identifying mechanisms that ensure the balance of PJAR constriction, will permit the dissection of the precise molecular mechanisms that regulate the organization of apical junctions and the junction-associated cytoskeleton in multicellular systems.
Figure 1. Molecular pathways regulating TJs and PJARs mediated by Rho. Rho signaling plays essential roles in the establishment and maintenance of TJs and PJARs. Several RhoGEF proteins are possibly implicated in the control of Rho GTPase activity at TJs by their interaction with cytoplasmic components. ARHGEF11 appears to bind ZO-1 constitutively and be indispensable for ZO-1-dependent TJ and PJAR regulation. It is not clear whether connections exist between multiple RhoGEF proteins. The spatiotemporal dynamics of the proteins involved in Rho-mediated TJ and PJAR regulation in response to physiological and pathological stimuli need to be further characterized. See text for details. Two-way arrows indicate physical interactions and dotted lines show hypothetical pathways.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed
References
- Bryant DM, Mostov KE. From cells to organs: building polarized tissue. Nat Rev Mol Cell Biol 2008; 9:887 - 901; http://dx.doi.org/10.1038/nrm2523; PMID: 18946477
- Hartsock A, Nelson WJ. Adherens and tight junctions: structure, function and connections to the actin cytoskeleton. Biochim Biophys Acta 2008; 1778:660 - 9; http://dx.doi.org/10.1016/j.bbamem.2007.07.012; PMID: 17854762
- Ivanov AI. Actin motors that drive formation and disassembly of epithelial apical junctions. Front Biosci 2008; 13:6662 - 81; http://dx.doi.org/10.2741/3180; PMID: 18508686
- Ivanov AI, Hunt D, Utech M, Nusrat A, Parkos CA. Differential roles for actin polymerization and a myosin II motor in assembly of the epithelial apical junctional complex. Mol Biol Cell 2005; 16:2636 - 50; http://dx.doi.org/10.1091/mbc.E05-01-0043; PMID: 15800060
- Ivanov AI, Bachar M, Babbin BA, Adelstein RS, Nusrat A, Parkos CA. A unique role for nonmuscle myosin heavy chain IIA in regulation of epithelial apical junctions. PLoS One 2007; 2:e658; http://dx.doi.org/10.1371/journal.pone.0000658; PMID: 17668046
- Tsukita S, Furuse M, Itoh M. Multifunctional strands in tight junctions. Nat Rev Mol Cell Biol 2001; 2:285 - 93; http://dx.doi.org/10.1038/35067088; PMID: 11283726
- Itoh M, Nagafuchi A, Moroi S, Tsukita S. Involvement of ZO-1 in cadherin-based cell adhesion through its direct binding to alpha catenin and actin filaments. J Cell Biol 1997; 138:181 - 92; http://dx.doi.org/10.1083/jcb.138.1.181; PMID: 9214391
- Umeda K, Ikenouchi J, Katahira-Tayama S, Furuse K, Sasaki H, Nakayama M, et al. ZO-1 and ZO-2 independently determine where claudins are polymerized in tight-junction strand formation. Cell 2006; 126:741 - 54; http://dx.doi.org/10.1016/j.cell.2006.06.043; PMID: 16923393
- Yamazaki Y, Umeda K, Wada M, Nada S, Okada M, Tsukita S, et al. ZO-1- and ZO-2-dependent integration of myosin-2 to epithelial zonula adherens. Mol Biol Cell 2008; 19:3801 - 11; http://dx.doi.org/10.1091/mbc.E08-04-0352; PMID: 18596233
- Van Itallie CM, Fanning AS, Bridges A, Anderson JM. ZO-1 stabilizes the tight junction solute barrier through coupling to the perijunctional cytoskeleton. Mol Biol Cell 2009; 20:3930 - 40; http://dx.doi.org/10.1091/mbc.E09-04-0320; PMID: 19605556
- Samarin S, Nusrat A. Regulation of epithelial apical junctional complex by Rho family GTPases. Front Biosci 2009; 14:1129 - 42; http://dx.doi.org/10.2741/3298; PMID: 19273120
- Nusrat A, Giry M, Turner JR, Colgan SP, Parkos CA, Carnes D, et al. Rho protein regulates tight junctions and perijunctional actin organization in polarized epithelia. Proc Natl Acad Sci U S A 1995; 92:10629 - 33; http://dx.doi.org/10.1073/pnas.92.23.10629; PMID: 7479854
- Jou TS, Schneeberger EE, Nelson WJ. Structural and functional regulation of tight junctions by RhoA and Rac1 small GTPases. J Cell Biol 1998; 142:101 - 15; http://dx.doi.org/10.1083/jcb.142.1.101; PMID: 9660866
- Van Aelst L, Symons M. Role of Rho family GTPases in epithelial morphogenesis. Genes Dev 2002; 16:1032 - 54; http://dx.doi.org/10.1101/gad.978802; PMID: 12000787
- Walsh SV, Hopkins AM, Chen J, Narumiya S, Parkos CA, Nusrat A. Rho kinase regulates tight junction function and is necessary for tight junction assembly in polarized intestinal epithelia. Gastroenterology 2001; 121:566 - 79; http://dx.doi.org/10.1053/gast.2001.27060; PMID: 11522741
- Amano M, Fukata Y, Kaibuchi K. Regulation and functions of Rho-associated kinase. Exp Cell Res 2000; 261:44 - 51; http://dx.doi.org/10.1006/excr.2000.5046; PMID: 11082274
- Cunningham KE, Turner JR. Myosin light chain kinase: pulling the strings of epithelial tight junction function. Ann N Y Acad Sci 2012; 1258:34 - 42; http://dx.doi.org/10.1111/j.1749-6632.2012.06526.x; PMID: 22731713
- Shen L, Black ED, Witkowski ED, Lencer WI, Guerriero V, Schneeberger EE, et al. Myosin light chain phosphorylation regulates barrier function by remodeling tight junction structure. J Cell Sci 2006; 119:2095 - 106; http://dx.doi.org/10.1242/jcs.02915; PMID: 16638813
- Aijaz S, D’Atri F, Citi S, Balda MS, Matter K. Binding of GEF-H1 to the tight junction-associated adaptor cingulin results in inhibition of Rho signaling and G1/S phase transition. Dev Cell 2005; 8:777 - 86; http://dx.doi.org/10.1016/j.devcel.2005.03.003; PMID: 15866167
- Terry SJ, Zihni C, Elbediwy A, Vitiello E, Leefa Chong San IV, Balda MS, et al. Spatially restricted activation of RhoA signalling at epithelial junctions by p114RhoGEF drives junction formation and morphogenesis. Nat Cell Biol 2011; 13:159 - 66; http://dx.doi.org/10.1038/ncb2156; PMID: 21258369
- Niu J, Profirovic J, Pan H, Vaiskunaite R, Voyno-Yasenetskaya T. G Protein betagamma subunits stimulate p114RhoGEF, a guanine nucleotide exchange factor for RhoA and Rac1: regulation of cell shape and reactive oxygen species production. Circ Res 2003; 93:848 - 56; http://dx.doi.org/10.1161/01.RES.0000097607.14733.0C; PMID: 14512443
- Nakajima H, Tanoue T. Lulu2 regulates the circumferential actomyosin tensile system in epithelial cells through p114RhoGEF. J Cell Biol 2011; 195:245 - 61; http://dx.doi.org/10.1083/jcb.201104118; PMID: 22006950
- Itoh M, Tsukita S, Yamazaki Y, Sugimoto H. Rho GTP exchange factor ARHGEF11 regulates the integrity of epithelial junctions by connecting ZO-1 and RhoA-myosin II signaling. Proc Natl Acad Sci U S A 2012; 109:9905 - 10; http://dx.doi.org/10.1073/pnas.1115063109; PMID: 22665792
- Katsube T, Takahisa M, Ueda R, Hashimoto N, Kobayashi M, Togashi S. Cortactin associates with the cell-cell junction protein ZO-1 in both Drosophila and mouse. J Biol Chem 1998; 273:29672 - 7; http://dx.doi.org/10.1074/jbc.273.45.29672; PMID: 9792678
- Siehler S. Regulation of RhoGEF proteins by G12/13-coupled receptors. Br J Pharmacol 2009; 158:41 - 9; http://dx.doi.org/10.1111/j.1476-5381.2009.00121.x; PMID: 19226283
- Meyer TN, Schwesinger C, Denker BM. Zonula occludens-1 is a scaffolding protein for signaling molecules. Galpha(12) directly binds to the Src homology 3 domain and regulates paracellular permeability in epithelial cells. J Biol Chem 2002; 277:24855 - 8; http://dx.doi.org/10.1074/jbc.C200240200; PMID: 12023272
- van Nieuw Amerongen GP, Vermeer MA, van Hinsbergh VW. Role of RhoA and Rho kinase in lysophosphatidic acid-induced endothelial barrier dysfunction. Arterioscler Thromb Vasc Biol 2000; 20:E127 - 33; http://dx.doi.org/10.1161/01.ATV.20.12.e127; PMID: 11116077
- Umeda K, Matsui T, Nakayama M, Furuse K, Sasaki H, Furuse M, et al. Establishment and characterization of cultured epithelial cells lacking expression of ZO-1. J Biol Chem 2004; 279:44785 - 94; http://dx.doi.org/10.1074/jbc.M406563200; PMID: 15292177
- Nishimura T, Honda H, Takeichi M. Planar cell polarity links axes of spatial dynamics in neural-tube closure. Cell 2012; 149:1084 - 97; http://dx.doi.org/10.1016/j.cell.2012.04.021; PMID: 22632972
- Suzuki M, Morita H, Ueno N. Molecular mechanisms of cell shape changes that contribute to vertebrate neural tube closure. Dev Growth Differ 2012; 54:266 - 76; http://dx.doi.org/10.1111/j.1440-169X.2012.01346.x; PMID: 22524600
- Curtin JA, Quint E, Tsipouri V, Arkell RM, Cattanach B, Copp AJ, et al. Mutation of Celsr1 disrupts planar polarity of inner ear hair cells and causes severe neural tube defects in the mouse. Curr Biol 2003; 13:1129 - 33; http://dx.doi.org/10.1016/S0960-9822(03)00374-9; PMID: 12842012
- Wojciak-Stothard B, Tsang LY, Haworth SG. Rac and Rho play opposing roles in the regulation of hypoxia/reoxygenation-induced permeability changes in pulmonary artery endothelial cells. Am J Physiol Lung Cell Mol Physiol 2005; 288:L749 - 60; http://dx.doi.org/10.1152/ajplung.00361.2004; PMID: 15591411
- Ishiuchi T, Takeichi M. Willin and Par3 cooperatively regulate epithelial apical constriction through aPKC-mediated ROCK phosphorylation. Nat Cell Biol 2011; 13:860 - 6; http://dx.doi.org/10.1038/ncb2274; PMID: 21685893